Abstract
Summary
The effect of cortisol on calcium (Ca2+) transport across cultured rainbow trout gill epithelia composed of both pavement cells (PVCs) and mitochondria-rich cells (MRCs) was examined. Under symmetrical culture conditions (L15 media apical/L15 media basolateral), cortisol had subtle effects on gill epithelial preparations. Both control and cortisol treated epithelia exhibited Ca2+ influx and efflux rates (measured radioisotopically using 45Ca) that were approximately balanced, with a slight inwardly directed net Ca2+ flux. Ussing flux ratio analysis indicated active Ca2+ transport in the inward direction across epithelia bathed symmetrically regardless of hormone treatment. In contrast, under asymmetrical conditions (freshwater apical/L15 media basolateral) control epithelia exhibited active Ca2+ transport in the outward direction (basolateral to apical) throughout experiments conducted over a 24-h period, whereas cortisol-treated preparations exhibited active transport in the inward direction (apical to basolateral) during the early stages of an asymmetrical culture period (e.g., T0–6 h) and passive transport during the later stages (e.g., T18–24 h). When soft freshwater (with tenfold lower [Ca2+]) was used for asymmetrical culture instead of freshwater, control epithelia developed outwardly directed active Ca2+ transport properties, whereas cortisol-treated preparations did not. The results of this study support a hypercalcemic role for cortisol in rainbow trout and demonstrate that treating cultured gill epithelia composed of both PVCs and MRCs with cortisol can stimulate active Ca2+ uptake under circumstances that more closely resemble natural conditions for fish gills (i.e., freshwater bathing the apical side of the epithelium).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcium (Ca2+) homeostasis in freshwater (FW) teleost fishes occurs, in part, through Ca2+ acquisition from the surrounding environment. Of particular importance is trans-branchial Ca2+ movement, which is a well-studied phenomenon believed to take place primarily across the mitochondria-rich cells (MRCs) of fish gills (for reviews, see Perry 1997; Marshall 2002; Evans et al. 2005). Ca2+ influx rates have been demonstrated to alter as a result of various environmental perturbations, with a particularly effective stimulus being acclimatization of fishes to Ca2+ poor water (e.g., Perry and Wood 1985; McCormick et al. 1992). Diminishing the environmental Ca2+ pool increases MRC abundance and apical exposure (Perry and Wood 1985; McCormick et al. 1992), a response that seems to be at least partly mediated by elevated circulating cortisol levels (Perry and Wood 1985). Since these early observations, the hypercalcemic properties of cortisol have been variously exploited to examine, among other things, intraspecific relationships between Ca2+ uptake and MRC exposure at the surface of the gill as well as the regulation of epithelial proteins believed to be involved in Ca2+ movement across the apical membrane of the gill (Perry et al. 1992; Shahsavarani and Perry 2006). To the best of our knowledge, the direct effects of cortisol on Ca2+ transport across gill epithelia have yet to be explored.
Recently developed techniques for the preparation and culture of FW fish gill epithelia on permeable filter supports have provided new opportunities for the examination of gill epithelia properties in vitro (Wood and Pärt 1997; Fletcher et al. 2000; Kelly and Wood 2002a). Cultured freshwater gill preparations can be “reconstructed” either as pavement cell (PVC) epithelia (Wood and Pärt 1997; Kelly and Wood 2002a) or as epithelia containing both PVCs and MRCs (Fletcher et al. 2000). The latter of these preparations, thus far developed only for rainbow trout gills, has been demonstrated capable of actively transporting Ca2+ in the correct vectorial direction (i.e., apical to basolateral uptake) under symmetrical culture conditions (L15 media bathing both the apical and basolateral side of the epithelium). Trout gill preparations containing PVCs only do not exhibit the same properties (Fletcher et al. 2000). It would, therefore, appear that the presence of MRCs in cultured gill epithelia confers Ca2+ transporting properties, and this observation is consistent with current models of Ca2+ movement across the FW gill (Perry 1997; Marshall 2002; Evans et al. 2005). However, cultured preparations do not actively transport Ca2+ in the inward direction when epithelia are exposed to more “natural” conditions—i.e., bathed on the apical side with FW (Fletcher et al. 2000). We have recently reported the sensitivity of trout epithelia to various hormone treatments (Kelly and Wood 2001a, b; 2002a, b; Zhou et al. 2003, 2004), including cortisol, which has marked and dose-dependent effects on the electrophysiological properties and ion movement across cultured preparations (Kelly and Wood 2001a, 2002a). The aforementioned studies not only offer us insight into the role of these various signaling factors on gill epithelial characteristics, but also allow us to augment the properties of cultured gill preparations by stimulating ion transport and enhancing epithelia integrity under both symmetrical and, importantly, asymmetrical culture conditions (for review, see Wood et al. 2002).
Therefore, the objectives of these studies were to examine the Ca2+-transporting properties of cultured gill epithelia composed of both PVCs and MRCs in the presence of cortisol, a hypercalcemic hormone, under several culture conditions as follows: (1) symmetrical conditions (L15 apical/L15 basolateral); (2) regular asymmetrical (FW apical/L15 basolateral); and (3) soft freshwater (SFW) asymmetrical (SFW apical/L15 basolateral).
Materials and Methods
Preparation of cultured rainbow trout gill epithelia.
Cultured gill epithelia were prepared using stock rainbow trout held in flow-through Hamilton dechlorinated tap water (approximate composition in mM: [Na+] = 0.55, [Cl−] = 0.70, [Ca2+] = 0.90, [Mg2+] = 0.15, [K+] = 0.05, pH range 7.8–8.0) at ~12° C. Fish size ranged from 200 to 250 g. The epithelia prepared and used in these studies were of the variety originally developed and described by Fletcher et al. (2000). These preparations are composed of both PVCs and MRCs. Full details for the preparation of gill epithelia have been outlined in Kelly et al. (2000). Falcon cell culture inserts, incorporating track-etched polyethylene terephthalate membranes with a pore size of 0.4 μm and a pore density of 1.6 × 106 pores/cm2, of 0.9 cm2 surface area were employed. Cortisol treatment of cultured gill epithelia has previously been outlined by Kelly and Wood (2001a). A single concentration of 500 ng/ml cortisol (hydrocortisone hemisuccinate, Sigma, on the basolateral side only) was used for all experiments. This dose was selected based on previous observations of the response of cultured trout epithelia treated with cortisol (see Kelly and Wood, 2001a; Zhou et al. 2003). Epithelia were cultured under symmetrical culture conditions (i.e., Leibovitz’s L-15 supplemented with 2 mmol l−1 glutamine and 6% fetal bovine serum bathing both apical and basolateral surfaces of the preparation) for ~ 6 d before experimental manipulation, and experiments were conducted under symmetrical (L15 apical/L15 basolateral) and asymmetrical (sterile FW apical/L15 basolateral) conditions. Cortisol was present in culture media from the beginning of the 6-d culture period and throughout all experiments. Total [Ca2+] in supplemented L15 media was 1.37 ± 0.02 mM (n = 80). In addition to “regular” asymmetrical conditions with FW bathing the apical side of epithelia, a second set of asymmetrical experiments were run using soft FW (SFW) so that the conditions were SFW apical/L15 basolateral. Sterile SFW was prepared by mixing regular FW (composition as above) with reverse osmosis water in a 95:5 ratio (i.e., 95% reverse osmosis water and 5% regular freshwater). The resulting [Ca2+] was ~89 μM.
Electrophysiological measurements of cultured rainbow trout gill epithelia.
A custom modified voltohmmeter (World Precision Instruments, Sarasota, FL) fitted with chopstick electrodes (STX-2) were used to measure transepithelial resistance (TER) across trout epithelia. All reported TER measurements were background-corrected for the resistance of the cell culture insert and appropriate solutions alone, following which TER was expressed as a function of epithelial (insert cup) surface area (cm2). To monitor the development of cultured epithelia, TER measurements are recorded daily from 24 h after the last seeding of cells in to the insert cups. Unidirectional Ca2+ flux experiments were initiated to coincide with a plateau of TER that regularly occurs 6–8 d after a final seeding of cells (for details, see Kelly et al. 2000). Transepithelial potential (TEP) measurements across cultured trout epithelia were performed using agar-salt (3 M KCl in 4% agar) bridges connected to a high-impedance electrometer (Radiometer pHM 84, Copenhagen, Denmark) through Ag/AgCl electrodes (World Precision Instruments). TEP measurements were expressed relative to the apical side as 0 mV after correction for junction potential.
Unidirectional Ca2+ flux measurements across cultured rainbow trout gill epithelia.
Unidirectional flux measurements using a 45Ca radiotracer (NEN-PerkinElmer—Waltham, MA) were conducted according to methods previously outlined for unidirectional Na+ and Cl− flux measurements by Wood et al. (1998). Measurements worked on the principle of radioisotope appearance from a “hot” to a “cold” side of the cultured epithelial preparation. For influx (apical to basolateral isotope movement: positive sign) and efflux (basolateral to apical isotope movement: negative sign) studies, 0.5–1.0 μCi of 45Ca was added to a solution designated the “hot” side. As with previous studies, epithelial preparations used for unidirectional flux measurements were employed for influx or efflux study only and matched for calculations of the Ussing flux ratio (see next section) based on electrophysiological similarity. Under symmetrical culture conditions (L15 apical/L15 basolateral) TER and TEP measurements were recorded at the beginning and at the end of the flux period. Under asymmetrical conditions, when FW or SFW was bathing the apical side of epithelia, TER and TEP measurements were recorded at the beginning (0 h), middle (3 h), and end (6 h) of each flux period. Unidirectional Ca2+ flux rates were examined under three experimental conditions as follows: (1) control versus cortisol-treated epithelia, symmetrical (L15 apical/L15 basolateral); (2) control versus cortisol-treated epithelia, regular asymmetrical (FW apical/L15 basolateral) culture conditions; and (3) control versus cortisol-treated epithelia, SFW asymmetrical (SFW apical/L15 basolateral) culture conditions. Ca2+ fluxes under asymmetrical conditions were first examined immediately after the introduction of FW or SFW (i.e., T0–6 h). A second flux was subsequently run 18 h after initial exposure to asymmetrical conditions (i.e., T18–24 h).
Ussing flux ratio criterion and active Ca2+ transport across cultured trout gill epithelia.
As an indication of active Ca2+ transport across cultured trout epithelia, we used the Ussing flux ratio criterion, which examined for disagreement between the passive flux ratio predicted from the measured electrochemical gradient and the actual measured flux ratio (J in/J out), as outlined by Kirschner (1970). The predicted flux ratio was calculated as a function of ionic activities, valence, measured TEP, and several thermodynamic constants as follows:
where A Ap and A Bl are Ca2+ ion activities on the apical and basolateral side of the epithelium respectively, z is Ca2+ valence, V is the measured TEP (average for matched inserts, see above) and the usual thermodynamic values are given to F, R, and T. In media, A Ca was 86% of measured concentrations and under asymmetrical conditions A Ca in apical freshwater (both regular and soft freshwater) was equal to the measured concentrations.
Statistical analysis.
All data are expressed as mean values ± SEM (n), where n represents the number of filter inserts. Significant differences (P ≤ 0.05) between groups were determined using either a one-way analysis of variance (ANOVA) followed by a Student–Newman–Kuels test or Student’s unpaired or paired t tests as appropriate (Sigmastat Software, Jandel Scientific-San Rafael, CA).
Results
Symmetrical (L15 apical/L15 basolateral) culture conditions.
At the outset of the flux period, the TER values of control and cortisol-treated epithelia were 31.15 ± 0.73 and 33.04 ± 0.23 kΩ cm2, respectively. These values were not significantly different. In contrast, there was a significant difference (P ≤ 0.05) between TEP values of control (+20.47 ± 2.55 mV) and cortisol-treated (+30.20 ± 0.95 mV) epithelia. With the exception of an ∼5-mV increase in the TEP measured across both treatment groups, the electrophysiological characteristics of epithelia for the duration of the flux period varied little from values recorded at the outset of the experiment (Table 1).
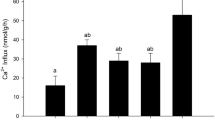
In control epithelia, radioisotopically determined unidirectional Ca2+ flux measurements under symmetrical culture conditions revealed influx rates (apical to basolateral) that were slightly greater than efflux rates (basolateral to apical), resulting in a net flux rate that was in the inward direction (Fig. 1). Epithelia that had been treated with cortisol exhibited approximately equal movement of ions in both directions. A slight observable difference between the net flux rates of control and cortisol-treated preparations can be attributed to decreased Ca2+ influx across cortisol-treated epithelia as a significant (P ≤ 0.05) decline in Ca2+ influx rate was evident (Fig. 1). A significant (P ≤ 0.05) and robust disagreement between the predicted and observed Ussing flux ratio criterion for both control and cortisol-treated epithelia was evident (Table 1), strongly suggesting active Ca2+ uptake across these preparations.
Effect of cortisol (500 ng/ml, basolateral side only) on unidirectional Ca2+ flux across cultured gill epithelia under symmetrical culture conditions (L15 apical/L15 basolateral). Data are expressed as mean values ± SEM (n = 7). An asterisk denotes significant difference (P≤ 0.05) between flux rates of control and cortisol treated preparations.
Asymmetrical (FW apical/L15 basolateral) culture conditions.
Unidirectional Ca2+ flux experiments under asymmetrical culture conditions were conducted twice over a 24-h period using the same insert preparations. During the first flux period (T0–6 h), TER was slightly higher in control preparations when compared to cortisol-treated epithelia (Table 2). The TEP across control epithelia was −1.36 ± 0.48 mV. The TEP across cortisol-treated epithelia was measured at +8.33 ± 2.12 mV (Table 2). Relative to symmetrical culture conditions, efflux rates across cultured epithelia under asymmetrical conditions (T0–6 h) were approximately tenfold greater, whereas influx rates were approximately sixfold greater (Fig. 2). Therefore, unidirectional influx rates were approximately 60% of the efflux rates, resulting in a net flux in the outward (basolateral to apical) direction (Fig. 2). No significant differences between efflux, influx, or net flux rates between control and cortisol-treated epithelia during the T0–6 h asymmetrical flux period were observed. Statistically significant disagreements with the relevant Ussing flux ratio criteria indicated active Ca2+ transport in the outward direction (basolateral to apical) across control preparations and active Ca2+ transport in the inward direction (apical to basolateral) across cortisol-treated epithelia (Table 2). During the second asymmetrical flux period (T18–24 h), TER had declined relative to T0–6 h and no significant difference was observed between control and cortisol-treated epithelia (Table 2). In contrast, a significant difference between TEP values between treatments persisted, with both treatments now exhibiting a negative TEP relative to the apical side (control = −6.24 ± 0.30 mV and cortisol-treated = −1.83 ± 0.66 mV). Unidirectional Ca2+ influx rates across control epithelia moderately, but not significantly, increased after an 18-h culture under asymmetrical conditions (Fig. 2). However, Ca2+ efflux rates significantly increased (P ≤ 0.05) and, accordingly, net flux rates significantly increased in the outward (basolateral to apical) direction. In cortisol- treated preparations, a significant increase (P ≤ 0.05) in Ca2+ influx rates was observed relative to control values during the same time period and relative to influx rates during the first 6 h of asymmetrical culture conditions (Fig. 2). Although Ca2+ efflux also increased in cortisol-treated preparations, the extent of the change was not as substantial as that observed in control epithelia, resulting in a net flux rate that was not significantly different from the T0–6 h net flux rate (Fig. 2). Disagreement between the predicted and observed flux ratio continued to suggest that active Ca2+ transport was occurring in the outward direction across control epithelia. No significant difference between the observed and predicted flux ratio was evident in cortisol-treated epithelia, indicating passive movement of Ca2+ (Table 2).
Unidirectional Ca2+ flux across control (0 ng/ml cortisol) and cortisol treated (500 ng/ml, basolateral side only) cultured gill epithelia under asymmetrical culture conditions (freshwater apical/L15 basolateral). Data are expressed as mean values ± SEM (n = 7). An asterisk denotes significant difference (P ≤ 0.05) between flux rates of control and cortisol treated preparations within a specific time period. Dagger denotes a significant difference (P ≤ 0.05) between either control or cortisol-treated preparations between different time periods.
Asymmetrical (SFW apical/L15 basolateral) culture conditions.
Before the start of unidirectional Ca2+ flux experiments under asymmetrical conditions with SFW bathing the apical side of the preparations, control and cortisol-treated epithelia exhibited TER values of 27.33 ± 2.49 kΩ cm2 and 24.82 ± 2.79 kΩ cm2, respectively. These values were not significantly different. TEP across the same preparations did significantly differ (P ≤ 0.05) with control epithelia exhibiting a TEP of +15.67 ± 2.04 mV and cortisol-treated epithelia exhibiting a greater TEP of +25.60 ± 4.98 mV. During the first flux period (T0–6 h) under asymmetrical conditions (SFW apical/L15 basolateral), TER values increased across both treatment groups, but were not significantly different between groups. TEP in control epithelia exhibited a negative value of −9.29 ± 0.73 mV. The TEP of cortisol-treated epithelia was marginally positive at +0.63 ± 0.80 mV (Table 2). These values significantly differed (P ≤ 0.05). During the T0- to T6-h flux period, Ca2+ influx across both preparations was considerably lower than that found across preparations in regular freshwater, with influx rates from SFW ranging from ~ 0.050 to 0.060 nmol cm−2 h−1. Cortisol treatment had no significant effect on Ca2+ influx measured at this time period under these conditions (Fig. 3). Cortisol also had no significant effect on efflux or net flux rates during the T0- to T6-h flux period. There was no statistically significant disagreement between the predicted and observed flux ratios for either treatment, suggesting passive Ca2+ movement across the preparations (Table 2). However, it should be noted that there was an obvious trend that paralleled the results observed at T0–6 h under regular FW apical/L15 basolateral conditions. That is, the observed flux ratios suggested active Ca2+ extrusion across control epithelia and active Ca2+ uptake across cortisol-treated epithelia (Table 2).
The effect of soft freshwater (SFW) asymmetrical culture conditions (SFW apical/L15 basolateral) on unidirectional Ca2+ flux across control (0 ng/ml cortisol) and cortisol-treated (500 ng/ml, basolateral side only) cultured gill epithelia. Data are expressed as mean values ± SEM (n = 5–7). No significant differences (P ≥ 0.05) were found between groups or time periods.
During the second flux period (T18–24 h), TER remained high across epithelia (Table 2) and cortisol-treated inserts exhibited a significantly (P ≤ 0.05) elevated TER relative to control epithelia. TEP values were also significantly different (Table 2). Statistically significant disagreement between the predicted and observed flux ratios for control preparations indicated active Ca2+ transport in the outward direction. There was no such disagreement between predicted and observed flux ratios for cortisol-treated epithelia (Table 2).
Discussion
Overview.
The results of this study confirm the ability of cultured trout gill preparations composed of a heterogeneous cell population, i.e., preparations composed of both pavement cells (PVCs) and mitochondria rich cells (MRCs), to actively transport Ca2+ in the inward direction under symmetrical culture conditions (L15 apical/L15 basolateral) (see Fletcher et al. 2000). The current study also confirms the curious observation that active Ca2+ transport occurs in the outward direction under asymmetrical (FW apical/L15 basolateral) conditions (see Fletcher et al. 2000). The differences observed in absolute Ca2+ flux rates between the present study and that of Fletcher et al. (2000) likely originates from differences in epithelial “tightness”—~ 10 kΩ cm2 in Fletcher et al. (2000) versus ~32 kΩ cm2 in the present study. Under asymmetrical conditions, epithelial tightness (as measured by TER) did not exhibit the same disparity between the two studies.
To date, we have not tested the potential modulatory effects of any hormone or hormone combination on Ca2+ movement across cultured gill preparations, although we have examined the effects of cortisol, prolactin, and triiodo-l-thyronine (either alone or in combination) on Na+ and Cl− transporting properties of cultured gill epithelia (Kelly and Wood 2001a, b; Kelly and Wood 2002a, b; Zhou et al. 2003, 2004). Of the hormone treatments already tested in isolation, cortisol elicits the most robust response from cultured gill epithelia, regardless of epithelial cellular composition (Kelly and Wood 2001a, b; Kelly and Wood 2002a, b; Zhou et al. 2003, 2004). Under symmetrical culture conditions, cortisol treatment has been demonstrated to significantly tighten cultured PVC epithelial preparations, at least in part by reducing paracellular permeability, and stimulate active, inwardly directed Na+ and Cl− transport (Kelly and Wood 2001a, 2002a). Cortisol treatment has also been demonstrated to stimulate active Na+ uptake under asymmetrical culture conditions in preparations composed of both PVCs and MRCs (Zhou et al. 2003). Here we demonstrate for the first time that cortisol treatment of cultured trout epithelia stimulates Ca2+ uptake under asymmetrical culture conditions (i.e., FW apical/L15 basolateral). These observations are consistent with the hypercalcemic properties of cortisol in rainbow trout (Perry and Wood 1985; Flik and Perry 1989; Laurent and Perry 1990; Shahsavarani and Perry 2006).
Cortisol and Ca2+ transport across cultured trout epithelia—symmetrical and asymmetrical (FW apical/L15 basolateral) culture conditions.
The current working model for Ca2+ movement across the freshwater fish branchial epithelium involves apical entry of the cation through an epithelial Ca2+ channel (ECaC) and basolateral extrusion by either a Ca2+-ATPase and/or a Na+ /Ca2+ exchanger (see Shahsavarani et al. 2006). It is presumed that apical entry occurs down an electrochemical diffusion gradient and correlative evidence suggests that movement across the apical membrane is the rate limiting step in trans-branchial uptake (for review, see Evans et al. 2005). In addition to the mechanistic details, the favored model for Ca2+ transport also recognizes the branchial MRC as being primarily responsible for Ca2+ transport. There is a substantial amount of support for this contention. Ishihara and Mugiya (1987) have demonstrated the localization of electron-opaque calcium salt precipitates to MRCs, and in more recent experiments utilizing isolated gill MRCs and PVCs, Galvez et al. (2006) reported Ca2+ uptake rates in MRCs that were threefold higher than those found in PVCs. However, the bulk of the evidence that supports the localization of Ca2+ transport to MRCs comes from the close relationship between Ca2+ uptake and MRC density/exposure (e.g., Perry and Wood 1985; Marshall et al. 1992, 1995; McCormick et al. 1992; Perry et al. 1992). Nevertheless, both the study of Galvez et al. (2006) and immunohistochemical work of Shahsavarani et al. (2006) on the cell-specific localization of ECaC suggest that the PVCs may also contribute to Ca2+ uptake to a lesser degree.
Several of the studies reporting a relationship between Ca2+ uptake and MRC density/exposure utilize cortisol treatment as a means to alter MRC abundance in the gill epithelium (Perry and Wood 1985; Marshall et al. 1992, 1995; Perry et al. 1992). As Fletcher et al. (2000) have demonstrated that cultured gill epithelia composed of both PVCs and MRCs exhibit active Ca2+ uptake (a phenomenon confirmed in this study), whereas preparations composed of PVCs alone do not, it would be expected that treatment of the former with cortisol would positively alter the dynamics of Ca2+ transport. Under symmetrical culture conditions, the effects of cortisol on Ca2+ transport are subtle and both control and cortisol-treated epithelia exhibit qualitatively similar Ca2+ transport properties. However, under asymmetrical culture conditions, when FW is first introduced to the apical side of the culture preparations (i.e., T0–6 h), cortisol reverses the direction of active Ca2+ transport, counteracting a “maladaptive” property of cultured preparations whereby control epithelia exhibit outwardly directed (basolateral to apical) active Ca2+ transport when exposed to conditions that more closely resemble a natural environment (i.e., FW bathing the apical side of the gill epithelium). Over a more prolonged period of asymmetrical culture (i.e., between 18 and 24 h), Ca2+ influx rates across control epithelia did not significantly alter, whereas efflux rates significantly increased resulting in net flux rates approximately doubling in the outward direction. Qualitatively similar results across “untreated” epithelia composed of both PVCs and MRCs can be observed when examining temporal alterations in Na+ and Cl− movement (Kelly and Wood 2002b). That is, a disproportionate increase in ion efflux rate relative to influx rate results in an increased, outwardly directed, net flux rate. However, in the current experiments, Ca2+ influx rates across cortisol-treated preparations during the T18- to T24-h flux period were approximately double those observed at T0–6 h, whereas Ca2+ efflux rates increased only 1.4-fold. The overall result was that net flux rates did not appreciably change over the 24-h exposure period. Despite these observations, the flux ratio analysis indicated that inwardly directed active Ca2+ transport had now been replaced with passive transport mechanisms. Nevertheless, Ca2+ transport was not active in the outward direction. Taken together, the above results support the contention that the hypercalcemic properties of cortisol positively influence Ca2+ transport across cultured epithelia, especially under asymmetrical culture conditions.
Cortisol and Ca2+ transport across cultured trout epithelia—asymmetrical (SFW apical/L15 basolateral) culture conditions.
Exposure to lowered environmental Ca2+ has been demonstrated to increase Ca2+ transport capacity in rainbow trout (Perry and Wood 1985) and other fishes (McCormick et al. 1992). Furthermore, lowering environmental ion content has also been demonstrated to increase circulating cortisol levels (Perry and Wood 1985, Perry and Laurent 1989). It is generally accepted that these two phenomena are linked, in that cortisol contributes to the maintenance of Ca2+ balance by mediating, at least in part, an upregulation of transport mechanisms required for Ca2+ homeostasis. Given the close relationship between Ca2+ balance and cortisol in fish acclimated to ion-poor water, we were interested in examining this association in vitro. An ideal situation would be to culture gill epithelial cells that exhibit an ion poor water phenotype; however, after isolation of gill cells from fish acclimated to ion-poor water, the required extensive culture period in apical L15 media under conditions resembling the mineral content of blood would likely diminish the desired transport characteristics. Instead, we rationalized that treatment with cortisol and subsequent exposure to ion-poor conditions (soft freshwater, SFW) would offer insight into the benefits of cortisol in such an environment.
When epithelia were initially exposed to SFW, Ca2+ influx rates ranged from 0.05 to 0.06 nmol cm−2 h−1. These values were approximately tenfold lower than influx rates across epithelia exposed to regular FW (i.e., 0.06 nmol cm−2 h−1 in SFW versus ~0.60 nmol cm−2 h−1 in regular FW) and are in accordance with an approximate tenfold reduction in water [Ca2+] (i.e. ~90 μM versus ~900 μM in SFW and FW, respectively). Ca2+ efflux rates across epithelia exposed to apical SFW were approximately ~56% of those observed across epithelia exposed to apical FW, and due to the low Ca2+ influx rates, net Ca2+ flux rates were not found to be that different when comparing regular FW- and SFW-exposed preparations. When compared to control epithelia, cortisol had no significant effect on flux rates measured across epithelia exposed to apical SFW either during the T0- to T6-h or T18- to T24-h time periods. The flux ratio analysis also indicated passive movement of Ca2+ across both control and cortisol-treated preparations during the T0- to T6-h flux period, although the numbers exhibited a trend that mimicked the flux ratio characteristics observed under normal FW conditions. In contrast, during the T18- to T 24-h flux period, the flux ratio analysis indicated active Ca2+ transport in the outward direction across control epithelia and passive transport in cortisol-treated preparations.
A particularly interesting observation was an ~8 kΩ cm2 increase in the TER of cortisol-treated preparations between the first (T0–6 h) and second (T18–24 h) flux periods. This increase in TER contrasts with the ~7 kΩ cm2 decrease observed in control epithelia. This is the first time we have observed an increase in TER across cultured gill epithelia exposed to asymmetrical culture conditions for an extended period of time. Under normal circumstances, the TER of epithelial preparations will decrease to varying degrees over an asymmetrical culture period of this duration (see Kelly and Wood 2002b; Wood et al. 2002; this study), presumably as epithelial integrity is slightly compromised. Cortisol generally attenuates a decline in TER across cultured epithelia under asymmetrical conditions (Kelly and Wood 2001a; Wood et al. 2002), and has been shown to reduce diffusive Na+ and Cl− movement in the outward direction across epithelia (Kelly and Wood 2001a). In cortisol-treated epithelia exhibiting, an increased TER during T18–24 h, net Ca2+ flux is reduced relative to control preparations, but not significantly. Nevertheless, electrophysiological data suggest that the integrity of cortisol-treated epithelia is enhanced and in a manner consistent with acclimation to the asymmetrical conditions rather than simple tolerance.
Conclusion.
The current study confirms the ability of cultured gill epithelia composed of both PVCs and MRCs to actively transport Ca2+ in the inward direction (apical to basolateral) under symmetrical culture conditions (L15 apical/L15 basolateral). We also report, for the first time, that cortisol can stimulate active Ca2+ uptake under asymmetrical conditions, and thus reverse the outwardly directed active Ca2+ transport that is seen in the absence of hormone supplementation. These observations are consistent with the hypercalcemic actions of cortisol and the general consensus that cortisol helps mediate Ca2+ acquisition across freshwater fish gill epithelia. These observations are also consistent with the presence of membrane proteins such as ECaC in cultured gill epithelia, the dominant localization of such proteins to MRCs in cultured preparations (Shahsavarani et al. 2006), and the modulation of ECaC by cortisol treatment in vivo (Shahsavarani and Perry 2006). However, it is noteworthy that ECaC has also been localized to PVCs both in vivo and in vitro (e.g., in cultured gill preparations containing PVCs only) which, together with the isolated cell results of Galvez et al. (2006), challenges the generally accepted gill Ca2+ transport paradigm, at least in terms of the exclusivity of Ca2+ transport across MR cells (Shahsavarani et al. 2006). It is currently assumed that active Ca2+ uptake does not occur across cultured PVC (see Fletcher et al. 2000). However, given the above outlined observations, a reevaluation of the Ca2+ transport capacity of PVCs (PVC epithelia) in the presence of hormones such as cortisol is warranted.
References
Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 85:97–177; 2005.
Fletcher, M.; Kelly, S.P.; Pärt, P.; O’Donnell, M.; Wood, C.M. Transport properties of cultured branchial epithelia from freshwater rainbow trout: a novel preparation with mitochondria-rich cells. J. Exp. Biol. 203:1523–1537; 2000.
Flik, G.; Perry, S.F. Cortisol stimulates whole body calcium uptake and the branchial calcium pump in freshwater rainbow trout. J. Endocrinol. 120:75–82; 1989.
Galvez, F.; Wong, D.; Wood, C.M. Cadmium and calcium uptake in isolated mitochondria-rich cell populations from the gills of the freshwater rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291:R170–R176; 2006.
Ishihara, A.; Mugiya, Y. Ultrastructural evidence of calcium uptake by chloride cells in the gills of goldfish, Carassius auratus. J. Exp. Zool. 242:121–129; 1987.
Kelly, S.P.; Fletcher, M.; Pärt, P.; Wood, C.M. Procedures for the preparation and culture of “reconstructed” rainbow trout branchial epithelia. Methods Cell Sci. 22:153–163; 2000.
Kelly, S.P.; Wood, C.M. Effect of cortisol on the physiology of cultured pavement cell epithelia from freshwater trout gills. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R811–R820; 2001a.
Kelly, S.P.; Wood, C.M. The physiological effects of 3’,5’,3’-triiodo-L-thyronine alone or combined with cortisol on cultured pavement cell epithelia from freshwater rainbow trout gills. Gen. Comp. Endocrinol. 123:280–299; 2001b.
Kelly, S.P.; Wood, C.M. Cultured gill epithelia from freshwater tilapia (Oreochromis niloticus): Effect of cortisol and homologous serum supplements from stressed and unstressed fish. J. Membrane Biol. 190:29–42; 2002a.
Kelly, S.P.; Wood, C.M. Prolactin effects on cultured pavement cell epithelia and pavement cell plus mitochondria-rich cell epithelia from freshwater rainbow trout gills. Gen. Comp. Endocrinol. 128:44–56; 2002b.
Kirschner, L.B. The study of NaCl transport in aquatic animals. Am. Zool. 10:365–376; 1970.
Laurent, P.; Perry, S.F. Effects of cortisol on gill chloride cell morphology and ionic uptake in the freshwater rainbow trout, Salmo gairdneri. Cell Tissue Res. 259:429–442; 1990.
Marshall, W.S. Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J. Exp. Zool. 293:264–283; 2002.
Marshall, W.S.; Bryson, S.E.; Wood, C.M. Calcium transport by isolated skin of rainbow trout. J. Exp. Biol. 166:297–316; 1992.
Marshall, W.S.; Bryson, S.E.; Burghardt, J.S.; Verbost, P.M. Ca2+ transport by the opercular epithelium of the fresh water adapted euryhaline teleost, Fundulus heteroclitus. J. Comp. Physiol. B. 165:268–277; 1995.
McCormick, S.D.; Hasegawa, S.; Hirano, T. Calcium uptake in the skin of a freshwater teleost. Proc. Natl. Acad. Sci. 89:3635–3638; 1992.
Perry, S.F. The chloride cell: structure and function in the gills of freshwater fishes. Ann. Rev. Physiol. 59:325–347; 1997.
Perry, S.F.; Goss, G.G.; Fenwick, J.C. Interrelationships between gill chloride cell morphology and calcium uptake in freshwater teleosts. Fish Physiol. Biochem. 10:327–337; 1992.
Perry, S.F.; Laurent, P. Adaptational responses of rainbow trout to lowered external NaCl concentration-contribution of the branchial chloride cell. J. Exp. Biol. 147:147–168; 1989.
Perry, S.F.; Wood, C.M. Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J. Exp. Biol. 116:411–433; 1985.
Shahsavarani, A.; McNeill, B.; Glavez, F.; Wood, C.M.; Goss, G.G.; Hwang, P.-P.; Perry, S.F. Characterization of a branchial epithelial calcium channel (ECaC) in freshwater rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 209:1928–1943; 2006.
Shahsavarani, A.; Perry, S.F. Hormonal and environmental regulation of epithelial calcium channel in gill of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291:R1490–R1498; 2006.
Wood, C.M.; Gilmour, K.M.; Pärt, P. Passive and active transport properties of a gill model, the cultured branchial epithelium of the freshwater rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. 119A:87–96; 1998.
Wood, C.M.; Kelly, S.P.; Zhou, B.; Fletcher, M.; O’Donnell, M.; Eletti, B.; Pärt, P. Cultured gill epithelia as models for the freshwater fish gill. Biochim. Biophys. Acta. 1566:72–83; 2002.
Wood, C.M.; Pärt, P. Cultured branchial epithelia from freshwater fish gills. J. Exp. Biol. 200:1047–1059; 1997.
Zhou, B.; Kelly, S.P.; Ianowski, J.; Wood, C.M. Effects of cortisol and prolactin on Na+ and Cl− transport in cultured branchial epithelia from freshwater rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285:R1305–R1330; 2003.
Zhou, B.; Kelly, S.P.; Wood, C.M. Physiological effects of gradual apical media dilution during development and hormone supplementation on cultured branchial epithelia from freshwater rainbow trout. J. Exp. Zool. 301A:867–881; 2004.
Acknowledgments
This work was supported by NSERC Discovery Grants to CMW and SPK. CMW is supported by the Canada Research Chair Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Kelly, S.P., Wood, C.M. Cortisol stimulates calcium transport across cultured gill epithelia from freshwater rainbow trout. In Vitro Cell.Dev.Biol.-Animal 44, 96–104 (2008). https://doi.org/10.1007/s11626-007-9077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-007-9077-6