Abstract
Killifish (Fundulus heteroclitus) has been extensively used as a model for ion regulation by euryhaline fishes. Na+ and Cl− dynamics have been well studied in killifish, but few studies have addressed that of Ca2+. Therefore, this study aimed to characterize Ca2+ fluxes in freshwater (FW) and seawater (SW)-acclimated killifish, their response to salinity transfer, and to elucidate the mechanisms of Ca2+ influx in FW and SW. SW killifish displayed a significantly higher Ca2+ influx rate than that of FW fish, while Ca2+ efflux rates were comparable in both salinities. Ca2+ influx was saturable in FW (Km = 78 ± 19 µmol/L; Jmax = 53 ± 3 nmol/g/h) and influx by SW killifish was linear up to 7 mmol/L Ca2+. In SW-acclimated fish, 36% of Ca2+ influx was attributed to “intestinal Ca2+ intake”, likely caused by drinking, whereas intestinal Ca2+ intake in FW contributed to < 2% of total. Throughout the study, results suggested that “cation competition” in SW modulates Ca2+ influx. Therefore, we hypothesized that SW-acclimated fish actually have a higher affinity Ca2+ influx system than FW-acclimated fish but that it is competitively inhibited by competing SW cations. In agreement with this cation competition hypothesis, we demonstrated for the first time that “extra-intestinal” Ca2+ influx was inhibited by Mg2+ in both FW and SW-acclimated killifish. Following acute salinity transfer, extra-intestinal Ca2+ influx was rapidly regulated within 12–24 h, similar to Na+ and Cl−. Ca2+ influx in FW was inhibited by La3+, an epithelial Ca2+ channel blocker, whereas La3+ had no significant effect in SW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In fishes, Ca2+ is obtained from the surrounding aquatic environment and diet via the gills, integument, and gastrointestinal tract. In typical freshwater (FW) and seawater (SW) fishes, the gills represent the dominant site of Ca2+ uptake (Marshall 2002; Evans et al. 2005). Drinking in SW, an osmoregulatory strategy used to counteract osmotic water loss, may also provide a route for Ca2+ influx via the intestine; however, the magnitude of intestinal Ca2+ influx from imbibed water appears to vary depending on species (Sundell and Bjornsson 1988; Schoenmakers et al. 1993). At the gills, Ca2+ influx is an active process in both FW and SW fishes (Flik et al. 1996) and is regulated by mitochondrion-rich, ion-transporting cells termed ionocytes (see Perry 1997; Dymowska et al. 2012 for review). A general model for Ca2+ uptake has been established whereby Ca2+ entry across the apical membrane of ionocytes occurs via facilitated diffusion mediated by epithelial Ca2+ channels (ECaC) (Qiu and Hogstrand 2004; Pan et al. 2005; Shahsavarani and Perry 2006; Shahsavarani et al. 2006; Lin et al. 2016), while basolateral movement of Ca2+ from the cytosol to the blood is facilitated by high-affinity plasma membrane Ca2+-ATPases (PMCA; Flik et al. 1983, 1985a, b; Perry and Flik 1988; Liao et al. 2007) and/or Na+/Ca2+-exchangers (NCX; Flik et al. 1993, 1997; Verbost et al. 1994, 1997; Liao et al. 2007). In general, it is believed that Ca2+ entry across the apical membrane is the rate-limiting step to transepithelial Ca2+ uptake in fishes (see Flik et al. 1993, 1996; Perry 1997; Marshall 2002; Perry et al. 2003; Evans et al. 2005; Hwang and Lee 2007; Dymowska et al. 2012; Lin and Hwang 2016 for reviews).
The euryhaline killifish (Fundulus heteroclitus) has been used as a model for understanding ion regulation in euryhaline fishes (Wood and Marshall 1994; Burnett et al. 2007). However, only a few studies have examined the mechanisms of Ca2+ influx in F. heteroclitus, despite the fact that this species has been used extensively to study the endocrine regulation of Ca2+ balance in fishes (Pang and Pang 1986). One of the first studies to examine Ca2+ uptake in vivo in killifish found that extra-intestinal Ca2+ uptake was greater in SW-acclimated fish than in FW-acclimated fish when assayed in their native ambient [Ca2+] (Mayer-Gostan et al. 1983). Similarly, when killifish were treated acutely with progressive increases in salinity, whole-body Ca2+ influx generally increased as a function of increasing salinity (Prodocimo et al. 2007), demonstrating a positive correlation between salinity and Ca2+ influx rate. However, at low ambient [Ca2+], FW fish had a greater extra-intestinal Ca2+ influx than SW fish, potentially indicating a greater affinity for Ca2+ in FW (Mayer-Gostan et al. 1983). Ca2+ influx in FW was saturated at 2–4 mmol/L [Ca2+], while Ca2+ influx was linear up to 12 mmol/L [Ca2+] in SW acclimated fish (Mayer-Gostan et al. 1983). In a later study, Marshall et al. (1995) examined the characteristics of Ca2+ flux across isolated opercular epithelia, a tissue known to express ionocytes and to be involved in ion transport (Wood and Marshall 1994), from FW-acclimated killifish using an Ussing chamber approach. The Km of Ca2+ influx across the opercular epithelium was 0.348 mmol/L (Marshall et al. 1995), markedly higher than the Km (0.0625 mmol/L) observed in whole-body experiments with FW-acclimated fish in another study (Patrick et al. 1997), indicating a lower affinity Ca2+ uptake system by the opercular epithelium in vitro than the whole fish in vivo. In this latter study, Jmax for Ca2+ influx was 15.5 nmol/g/h in fish acclimated to 0.1 mmol/L Ca2+ (Patrick et al. 1997), much lower than the maximal value of approximately 50 nmol/g/h observed in FW killifish acclimated to a similar ambient [Ca2+] in an earlier study (Mayer-Gostan et al. 1983). The overall mechanism of Ca2+ uptake in killifish appears to be similar to that of other fish species. It was demonstrated to be La3+-sensitive in FW-acclimated killifish (Marshall et al. 1995; Patrick et al. 1997), similar to rainbow trout (Perry and Flik 1988), and basolateral movement of Ca2+ was found to be driven in part by Na+/Ca2+ exchange in isolated opercular epithelia (Verbost et al. 1997).
Killifish are an excellent model for ionoregulation in euryhaline fish species because they are very tolerant of salinity extremes and can rapidly adjust ionoregulatory mechanisms in response to acute salinity challenges (Wood and Marshall 1994). For example, when acutely transferred from a 10% SW acclimation to FW or SW, Na+ and Cl− flux rates typical of FW- or SW-acclimated fish, respectively, were established within 12 h post-transfer (Wood and Laurent 2003; Wood 2011). Similarly, transepithelial potential (TEP), intimately tied to branchial ionoregulatory mechanisms, is rapidly regulated within 24 h following transfer from SW to FW in killifish (Wood and Grosell 2008). To date, no study has addressed temporal changes in Ca2+ regulation following acute salinity transfer and only the handful of studies listed above have attempted to elucidate the mechanisms of Ca2+ influx utilized by FW- and SW-acclimated killifish.
The aim of the present study was to better characterize the mechanisms of Ca2+ influx in FW- and SW-acclimated killifish and to understand the factors that influence these mechanisms following salinity transfer. Moreover, several of the results obtained from our initial experiments indirectly suggested the possibility that cation competition by Mg2+ and/or Na+, the two most abundant cations in SW, reduced Ca2+ influx rates in SW-acclimated killifish, potentially competitively inhibiting a high affinity/capacity Ca2+ uptake system. Cation competition refers to the competitive binding of two or more cations in the ambient environment to the same uptake site on ionoregulatory epithelia, resulting in a competitive reduction in the uptake of one or more of the cations. For example, cation competition is the mechanism by which some toxic metal ions (e.g. Pb2+ and Cd2+) disrupt calcium balance in fishes. Both Pb2+ and Cd2+ are known to competitively inhibit the entry of Ca2+ across the gill epithelium by binding to the same sites of uptake, thereby reducing whole-body Ca2+ uptake (Niyogi and Wood 2004; Rogers and Wood 2004). Mg2+ has been shown to have variable effects on Ca2+ influx in FW-acclimated killifish, slightly reducing in vitro Ca2+ influx across the opercular epithelium at a concentration of 1 mmol/L (Marshall et al. 1995), but having no effect on whole-body Ca2+ influx at a concentration of 0.1 mmol/L in intact killifish (Patrick et al. 1997). To our knowledge, no study to date has addressed the effect of Mg2+ or Na+ on Ca2+ influx by FW and SW-acclimated killifish at concentrations relevant to the SW environment. Therefore, based on our initial results, we hypothesized that in the absence of competing SW cations, SW-acclimated killifish would have a greater Ca2+ influx than FW-acclimated fish and that the presence of Mg2+ and/or Na+ would reduce Ca2+ influx. We also hypothesized that changes in Ca2+ influx rates would occur rapidly following acute salinity transfer, as has been observed with Na+ and Cl− fluxes in previous work. Using a suite of inhibitors, we also aimed to determine the specific mechanisms of Ca2+ influx in FW and SW-acclimated killifish. We hypothesized that mechanisms would be similar in both groups, being sensitive to the ECaC blocker La3+ and the NCX blocker KB-R7934, based on previous work discussed above. We further tested the contribution of the gut to whole-body Ca2+ influx in FW and SW fish. Overall, our study provides further insight into the mechanisms of Ca2+ influx in fasted FW and SW-acclimated killifish, demonstrates that Ca2+ uptake mechanisms change rapidly following salinity transfer, and, for the first time, shows that cation competition by Mg2+ plays a large role in modulating Ca2+ uptake in SW.
Materials and methods
Animals
Adult killifish (Fundulus heteroclitus) were obtained from Aquatic Research Organisms (ARO) Ltd. (Hampton, New Hampshire, USA) and were maintained in 10% SW (3.2 ppt) at 18–20 °C in animal holding facilities at McMaster University, Hamilton, ON, Canada and at the University of British Columbia (UBC), Vancouver, BC, Canada. As described below, some experiments were performed at McMaster University and others at UBC. In both instances, fish were maintained in aquaria receiving recirculating water passing through charcoal filters. Depending on experimental series (see below), fish were acclimated for 2 weeks to fresh water (McMaster University: Na+ = 0.5, Cl− = 0.7, Ca2+ = 0.8, Mg2+ = 0.15, K+ = 0.05 mmol/L, hardness = 140 mg/L CaCO3, pH = 8.0; UBC: Na+ = 0.09, Cl− = 0.10, Ca2+ = 0.10, Mg2+ = 0.011, K+ = 0.004 mmol/L, hardness = 3.3 mg/L CaCO3, pH = 7.0) or to various concentrations of sea water (SW; 32 ppt; Na+ = 450, Cl− = 600, Ca2+ = 10, Mg2+ = 50 mmol/L) ranging from 10 to 100% SW that were achieved by mixing distilled water with commercial sea salt (Instant Ocean™, Spectrum Brands, Blacksburg, VA, USA). Note that for clarity, the FW from Hamilton, ON will henceforth be referred to as hFW and that from Vancouver, BC will be referred to as vFW. In both facilities, fish were maintained at room temperature (22–25 °C) on a 12 h light:12 h dark photoperiod and were fed to satiation once daily with a mix of commercial flakes (Wardley Total Tropical Gourmet Flake Blend, Hartz Mountain Corp., Secaucus, NJ, USA) and frozen brine shrimp (San Francisco Bay Brand, Newark, CA, USA). Fish were fasted for 48 h prior to and during all experimentation as previous research has demonstrated that dietary Ca2+ can have a profound impact on extra-intestinal routes of Ca2+ uptake (Baldisserotto et al. 2005; Franklin et al. 2005). Experimental procedures and protocols were approved by the McMaster University Animal Research Ethics Board (AUP 12-12-45) and by the University of British Columbia Animal Care Committee (certificate A14-0251) and adhered to the guidelines of the Canadian Council on Animal Care.
Experimental series
Series 1: effects of salinity acclimation on Ca2+ influx and efflux rates
This series of experiments was performed at McMaster University. Adult killifish were acclimated to hFW (dechlorinated Hamilton, ON tap water) or to 10, 20, 30, or 100% SW (commercial sea salt dissolved in distilled water) for 2 weeks. To assess Ca2+ influx at these acclimation salinities, fasted fish from each acclimation condition were placed individually into plastic containers fitted with aeration devices and containing 400 mL of their respective acclimation water for overnight settling (n = 6 per treatment). The following day, water was completely replaced and fish were allowed to settle for an additional 1 h. After this period, 45Ca2+ (Perkin Elmer, Waltham, MA, USA) was added to each container which marked the beginning of the flux period. In order to maintain specific activity (the proportion of total Ca2+ that is radiolabeled; see Analytical Techniques) relatively constant across all treatments, different amounts of radioisotope were added to each container depending on salinity (hFW = 3 µCi; 10% SW = 3.6 µCi; 20% SW = 4.2 µCi; 30% SW = 4.8 µCi; 100% SW = 10 µCi). After radioisotope addition, water samples (5.5 mL) were taken at 0, 3, and 6 h. Following collection of final water samples at 6 h, fish were rinsed 3 times in water of the respective salinity to which 100 mmol/L Ca(NO3)2 was added to displace any loosely bound 45Ca2+, and were then euthanized with an overdose of neutralized MS-222. Fish were weighed and then digested in approximately 3 volumes of 1 N HNO3 (exact volume noted) in sealed vials for 48 h at 65 °C. Radioactivity (β emissions) of Ca2+ in water and digest samples, and [Ca2+] of water samples were measured as described below in Analytical Techniques and Calculations.
In a separate experiment, Ca2+ efflux was assessed in hFW and 100% SW-acclimated killifish. Fasted fish (n = 6) were placed in 1 L of aerated hFW or 100% SW containing 200 or 600 µCi 45Ca2+, respectively, and were allowed to accumulate the radiotracer for 24 h. Following this initial accumulation period, fish were removed, rinsed 3 times in 100 mmol/L Ca(NO3)2 (in respective hFW or 100% SW), and then placed individually into containers with 250 mL of hFW or 100% SW. Following a 1-h settling period, 5.5-mL water samples were collected at 1-h intervals for 6 h. Water samples were processed as described below in Analytical Techniques and Calculations. At the end of the 6-h flux period, fish were euthanized, rinsed 3 times in 100 mmol/L Ca(NO3)2 and 3 times with distilled water, and a blood sample was collected by caudal puncture. Blood samples were centrifuged and plasma was collected and later sampled for [Ca2+] and radioactivity (see Analytical Techniques and Calculations).
Ca2+ influx kinetics were determined for hFW and SW-acclimated killifish. Following a 2-week acclimation to hFW or 100% SW, fasted fish (n = 6) were placed individually into plastic containers containing 400 mL of aerated, respective acclimation water. The following day, water was replaced with 250 mL of reconstituted, Ca2+-free FW (Table 1) or Ca2+-free SW (Kester et al. 1967; Table 1) containing nominal 50, 100, 200, 400, 800, or 1600 µmol/L Ca2+. Ca2+ levels were achieved by the addition of Ca(NO3)2; pH was maintained constant (pH = 8) across all treatments via manual titration with 0.1 mol/L KOH or HCl. Fish were allowed to settle for 1 h, after which 45Ca2+ was added to each container, marking the beginning of the flux period. Specific activity was maintained relatively constant across all ambient Ca2+ concentrations (2, 2, 2.5, 3, 3.5, or 4.5 µCi, respectively). Water samples (5.5 mL) were collected at 0, 3, and 6 h following radioisotope addition. Following collection of final water samples at 6 h, fish were processed as described above in the first experiment of this series.
The final experiment of this series was aimed at determining the effect of ambient salinity on Ca2+ uptake at an ambient [Ca2+] more closely resembling that of SW. Killifish were acclimated to hFW, 50% SW, or 100% SW for 2 weeks. Fasted fish (n = 6) were then placed individually into plastic containers containing aerated water (400 mL) of the respective salinity and left overnight. The following day, water was replaced with 250 mL of reconstituted Ca2+-free FW, 50% SW, or 100% SW (Table 1) containing nominally 1, 2.5, 5, or 7 mmol/L Ca2+ (as Ca(NO3)2) and fish were allowed to adjust for 1 h. 45Ca2+ was then added to each container (3, 3, 4, or 4 µCi, respectively), marking the beginning of the flux period. Water samples (5.5 mL) were collected at 0, 3, and 6 h following radioisotope addition. Following collection of final water samples at 6 h, fish were processed as described above in the first experiment of this series.
Series 2: contribution of intestine to Ca2+ intake
This series of experiments was performed at McMaster University. In order to determine whether drinking by SW-acclimated killifish played a significant role in overall Ca2+ intake, fasted hFW and SW-acclimated fish (n = 8) were placed into plastic containers containing 1 L of aerated, respective acclimation water. After overnight settling and water change, fish were allowed to settle for 1 h, after which 45Ca2+ (7.5 µCi in hFW, 25 µCi in SW) was added to each container, marking the beginning of the flux period. 5.5-mL water samples were collected at 0 and 3 h. Following the collection of final water samples at 3 h, fish were rinsed in water of the respective salinity to which 100 mmol/L Ca(NO3)2 was added and were then euthanized with an overdose of neutralized MS-222. The entire gut of each fish was then carefully ligated at esophagus and rectum, excised, taking care to avoid the loss of any accumulated fluid, and the gut and the rest of the fish were weighed and digested separately in approximately 3 volumes (exact volume noted) of 1 N HNO3 for 48 h at 65 °C. The β radioactivity of Ca2+ in water and digest samples and [Ca2+] of water samples were measured as described below in Analytical Techniques and Calculations. “Extra-intestinal Ca2+ influx” and “Intestinal Ca2+ intake” were determined using the calculations described below in Analytical Techniques and Calculations.
Series 3: effects of cation competition and salinity transfer on Ca2+ influx
This series of experiments was performed at the University of British Columbia. The first experiment of this series was designed to test the effects of cation competition by Mg2+ and Na+ on extra-intestinal Ca2+ influx and intestinal Ca2+ intake in vFW-acclimated killifish (dechlorinated Vancouver, BC tap water) and SW-acclimated killifish exposed acutely to the same ambient [Ca2+] (1 mmol/L as Ca(NO3)2) in the same medium, vFW. Killifish were acclimated to vFW or 100% SW for 2 weeks, after which fasted fish from each salinity were transferred individually to plastic containers containing 250 mL of exposure media. All exposure media contained 1 mmol/L Ca2+ added to vFW in order to directly test whether SW-acclimated killifish would exhibit higher Ca2+ influx rates than vFW-acclimated fish assayed in the same condition, and fish from each salinity were exposed individually to one of five different treatments: control (background vFW ion concentrations), 25 mmol/L MgCl2, 50 mmol/L MgCl2, 225 mmol/L NaCl, and 450 mmol/L NaCl. Once fish were transferred to the container, 4 µCi of 45Ca2+ was added, marking the beginning of the flux period. Water samples (5.5 mL) were collected at 0 and 3 h after which the fish were rinsed three times in 100 mmol/L Ca(NO3)2 and the gut was removed following the procedure described above in Series 2. The gut and fish samples were then processed in the same manner described above.
In the second experiment of this series, we aimed to determine how Ca2+ influx rates responded to acute salinity transfer from vFW to SW (vFW → SW) and from SW to vFW (SW → vFW). Moreover, based on our results that indicated an inhibitory effect of competing cations on Ca2+ influx in SW (see “Results”), Ca2+ influx in both salinity transfer groups was measured in a common background of vFW ([Ca2+] = 0.1 mmol/L) in order to remove the confounding effect of cation competition. Killifish were acclimated to vFW or 100% SW for 2 weeks, after which fasted fish from each salinity (n = 6–7) were removed for the measurement of “pre-transfer” Ca2+ influx rates of vFW and SW-acclimated fish (measured in a vFW background in both groups); the remaining fish were then transferred to the opposite salinity. Ca2+ influx rates were assessed in vFW and SW-acclimated fish (pre-transfer) and in fish from the vFW → SW and SW → vFW treatments (n = 6) at 3, 9, 24, and 48 h post-transfer following the same protocol described above for the first experiment in this series. In all cases, fish were transferred to vFW for 15 min before the flux measurement was started.
Series 4: effects of La3+ and pharmacological agents on Ca2+ influx in FW and SW
This series of experiments was performed at McMaster University. Adult killifish acclimated to hFW or 100% SW for 2 weeks were placed individually (n = 7–8) into plastic containers containing 400 mL of aerated, respective acclimation water and left overnight. The following day, water was completely replaced and various blockers targeting specific pathways of the Ca2+ uptake system were added to the water to the following final concentrations: La3+ (targeting ECaC) = 10−4 mol/L; nifedipine (targeting voltage-gated Ca2+ channels) = 10−5 mol/L; verapamil (targeting voltage-gated Ca2+ channels) = 10−5 mol/L; KB-R7943 (targeting NCX) = 3.3 × 10−6 mol/L. Nifedipine, verapamil, and KB-R7943 were suspended in 0.1% DMSO in order to achieve complete dissolution and, therefore, in addition to a regular control group (no blocker added), a group exposed to only 0.1% DMSO was included as a carrier control. Following the addition of the blockers, fish were allowed to settle for 1 h, after which 45Ca2+ was added to each container (3 µCi for hFW; 10 µCi for SW), marking the beginning of the flux period. After radioisotope addition, water samples (5.5 mL) were taken at 0 and 6 h. Following collection of final water samples at 6 h, fish were rinsed 3 times in water of the respective salinity to which 100 mmol/L Ca(NO3)2 was added, and were then euthanized with an overdose of neutralized MS-222. Fish were weighed and then digested in approximately 3 volumes of 1 N HNO3 (exact volume noted) in sealed vials for 48 h at 65 °C. Radioactivity of Ca2+ in water and digest samples and [Ca2+] of water samples were measured as described below in Analytical Techniques and Calculations.
Analytical techniques and calculations
The β-radioactivity from 45Ca2+ in water, plasma, and digest samples was measured using a scintillation counter (Tri-Carb 2900TR Liquid Scintillation Analyzer, Perkin Elmer) after mixing 5 mL of water sample, 10 µL of plasma, or 0.1–2 mL digest with 10 mL of scintillation cocktail (Ultima Gold AB, Perkin Elmer) and incubating in the dark for 3 h to minimize chemiluminescence. The counting efficiency of digest samples was corrected to be the same as that of water samples using a quench curve constructed from various amounts of digest. [Ca2+] in water and plasma samples was measured via atomic absorption spectrophotometry (SpectrAA 220FS, Varian, Agilent, Santa Clara, CA, USA).
In Series 1 and 4, Ca2+ influx rate (nmol/g/h) was calculated using the following equation:
where Rfish is the radioactivity (counts per minute; cpm) in the entire fish digest, SAwater is the average specific activity (cpm/nmol) of the water over the period of the experiment, wt is the weight of the fish (g), and t is time (h).
In Series 2 and 3, extra-intestinal Ca2+ influx rate (nmol/g/h) was calculated using the following equation:
where Rfish-intestine is the radioactivity (cpm) in the entire digest of the fish from which the intestine had been removed and wt is the weight (g) of the whole fish. As described below in the Results and Discussion, the radioactivity in the gut digest most likely represented 45Ca2+ that was simply imbibed by the fish during the flux period and was trapped in the gut. Therefore, in Series 2 and 3, rather than referring to this rate as an “influx” since this 45Ca2+ was likely not absorbed into the fish tissue, we have adopted the term “intestinal Ca2+ intake” (i.e., not “influx”) (nmol/g/h) which was calculated using the following equation:
where Rintestine is the radioactivity (cpm) in the entire intestine digest and wt is the weight (g) of the whole fish. Total Ca2+ influx (nmol/g/h) was calculated as the sum of Eqs. 2 and 3.
Ca2+ efflux rate (nmol/g/h) in Series 1 was calculated using the following equation:
where Rf and Ri are the radioactivity (cpm) of the water at the end and beginning of the flux period, respectively, and SAplasma is the specific activity (cpm/nmol) of the plasma at the end of the flux period.
Statistical analyses
Data are presented as means ± SEM and statistical significance was accepted at the P < 0.05 level. In general, comparisons between two means were performed using a Student’s two-tailed unpaired t test. Comparisons among multiple means were performed using a one-way ANOVA (for single factor comparisons) or a two-way ANOVA (for two factor comparisons). In the case of failed normality or equal variance that could not be normalized by log, natural log, square-root, square, or arcsine square-root transformations, ANOVA analyses were performed on rank-transformed data. Details on data transformations and post-hoc tests are included in corresponding figure captions. Statistical analyses and linear regressions were performed using SigmaStat software (Systat Software Inc., San Jose, CA, USA).
Results
Series 1: effects of acclimation salinity on whole-body Ca2+ influx and efflux
Killifish were acclimated to hFW or to 10, 20, 30, or 100% SW for 2 weeks. The lowest rates of whole-body Ca2+ influx were observed in hFW-acclimated fish whereas SW-acclimated fish had the highest influx rates, approximately threefold greater than those observed in hFW (Fig. 1). Influx rates in 10, 20, and 30% SW-acclimated fish were similar to one another and not significantly different than those observed in hFW or SW (Fig. 1). Whole-body Ca2+ efflux rates, which were measured in a separate group of fish, were not significantly different between hFW and SW-acclimated fish (Fig. 2).
Whole-body Ca2+ influx rates in killifish acclimated to Hamilton, ON freshwater (hFW), or to 10, 20, 30, or 100% seawater (SW) for 2 weeks. Means not sharing the same letter are significantly different from one another as determined by a one-way ANOVA followed by a Holm–Sidak post-hoc test (n = 6; P = 0.015)
Ca2+ influx kinetics were assessed in hFW and SW-acclimated fish. Importantly, all other ions in the reconstituted FW and SW media were kept constant within acclimation treatments and ambient Ca2+ was adjusted via the addition of Ca(NO3)2. hFW-acclimated killifish demonstrated a saturable Ca2+ influx as a function of ambient [Ca2+], modeled by a Michaelis–Menten relationship (Km = 78 ± 19 µmol/L; Jmax = 53 ± 3 nmol/g/h) (Fig. 3). In SW-acclimated fish, Ca2+ influx was linear up to 1.5 mmol/L ambient [Ca2+] and rates were lower at all ambient Ca2+ concentrations than those observed in FW over this range of ambient Ca2+ (Fig. 3). When assayed at higher ambient [Ca2+] (up to 7 mmol/L), the Ca2+ influx rate of hFW-acclimated fish showed no further significant change as it had essentially already saturated (Fig. 4). However, in this same range, the Ca2+ influx rate of SW-acclimated fish continued to increase linearly, such that by 4 and 7 mmol/L [Ca2+], it significantly exceeded the influx rate of hFW-acclimated fish (Fig. 4). Interestingly, when 50% SW-acclimated killifish were assayed at the same concentrations of ambient Ca2+, but in a medium representative of 50% SW, the Ca2+ influx rate was significantly greater than those of either hFW-acclimated or SW-acclimated killifish at 1 mmol/L ambient [Ca2+] (Fig. 4). However, there were no significant differences between 50% and 100% SW-acclimated animals at higher Ca2+ concentrations (2.5–7 mmol/L), and both 50 and 100% SW treatment groups exhibited Ca2+ influx rates significantly higher than those of hFW-acclimated animals (Fig. 4).
Whole-body Ca2+ influx rates as a function of ambient Ca2+ in killifish acclimated to Hamilton, ON freshwater (hFW; closed circles) or seawater (SW; open circles) for 2 weeks. In both salinity treatments, flux measurements were performed in reconstituted FW or SW in which the level of [Ca2+] was adjusted by the addition of Ca(NO3)2. The relationships between Ca2+ influx and ambient [Ca2+] were described by the Michaelis–Menten equation y = 52.55 × x/(78.19 + x) [R2 = 0.9174] for hFW-acclimated fish and the linear equation y = 0.021x + 0.198 [R2 = 0.9687] for SW-acclimated fish (n = 6)
Whole-body Ca2+ influx rates as a function of ambient [Ca2+] in killifish acclimated to Hamilton, ON freshwater (hFW; closed circles) 50% seawater (50% SW; open circles) or 100% seawater (100% SW; closed triangles) for 2 weeks. In both salinity treatments, fluxes were performed in reconstituted 50% or 100% SW in which the level of Ca2+ was adjusted by the addition of Ca(NO3)2 Means within the hFW (abc), 50% SW (xyz) or 100% SW (αβγ) treatments not sharing the same letter represent a statistically significant effect of ambient [Ca2+] (P < 0.001); asterisks denote a statistically significant effect of salinity within an ambient Ca2+ concentration (P < 0.001) as determined by a two-way ANOVA on rank-transformed data followed by a Tukey post-hoc test. There was a significant interaction between salinity acclimation and ambient [Ca2+] (P = 0.02) (n = 6)
Series 2: contribution of intestine to Ca2+ intake
In hFW-acclimated killifish assayed in hFW, intestinal Ca2+ intake accounted for only 2% of total Ca2+ influx (Fig. 5). However, in SW-acclimated killifish assayed in SW, 36% of total Ca2+ influx could be attributed to intestinal Ca2+ intake (Fig. 5).
Contribution of extra-intestinal influx and intestinal Ca2+ intake to total Ca2+ influx in killifish acclimated to Hamilton, ON freshwater (hFW) or seawater (SW) for 2 weeks. Means within extra-intestinal (ab) or intestinal (xy) groups not sharing the same letter represent a statistically significant effect of acclimation salinity (P = 0.016), asterisks denote a statistically significant difference between extra-intestinal and intestinal means within a salinity acclimation (P < 0.001) as determined by a two-way ANOVA on ranked arcsine square-root transformed data followed by a Tukey post-hoc test. There was a statistically significant interaction between salinity acclimation and pathway (extra-intestinal, intestinal) (P < 0.001) (n = 8)
Series 3: effects of cation competition and salinity transfer on Ca2+ influx
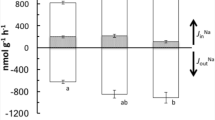
Killifish were acclimated to vFW or SW for 2 weeks and Ca2+ influx was measured in a common medium consisting of 1 mmol/L Ca2+ in vFW. Under these conditions, SW-acclimated fish exhibited total Ca2+ influx rates (sum of extra-intestinal influx and intestinal intake rates) which were more than double those of vFW-acclimated fish assayed under the same conditions (Fig. 6a). This difference in total Ca2+ influx was driven entirely by a greater extra-intestinal influx rate in SW killifish (Fig. 6b), given that intestinal Ca2+ intake was not significantly different between both groups under the control condition (Fig. 6c). Extra-intestinal Ca2+ influx in vFW-acclimated killifish was significantly inhibited in response to 50 mmol/L Mg2+, which reduced influx by 70% (Fig. 6b), but the small reduction in 25 mmol/L Mg2+ was not significant. In SW-acclimated fish, exposure to both 25 and 50 mmol/L Mg2+ resulted in significant 75% reductions in extra-intestinal Ca2+ influx (Fig. 6b). Exposure to 225 or 450 mmol/L Na+ had no effect on total or extra-intestinal Ca2+ influx in either salinity acclimation. Note that intestinal Ca2+ intake was not significantly inhibited by any of the competition treatments, but was increased by high [Na+] (225 or 450 mmol/L Na+) in both vFW-acclimated and SW-acclimated fish (Fig. 6c).
Total whole-body Ca2+ influx (a), extra-intestinal Ca2+ influx (b), and intestinal Ca2+ intake (c) rates in response to differing concentrations of ambient Mg2+ or Na+ in killifish acclimated to Vancouver, BC freshwater (vFW) or seawater (SW) measured in 1 mmol/L Ca2+ in Vancouver, BC FW (vFW). In each panel, means within the vFW group (abc) or SW group (xyz) not sharing the same letter represent a statistically significant effect of ambient ion treatment; asterisks denote a statistically significant effect of salinity acclimation within a given ion treatment as determined by a two-way ANOVA on log-transformed data followed by a Holm–Sidak post-hoc test. Total: salinity, P = 0.004; ion treatment, P < 0.001; interaction, P = 0.021. Extra-intestinal: salinity, P < 0.001; ion treatment, P < 0.001; interaction, P = 0.029. Intestinal: salinity, P = 0.595; ion treatment, P < 0.001; interaction, P = 0.974
In the second part of this series, fish were assayed both initially and throughout the experiment in common vFW background (0.1 mmol/L Ca2+) which had only 10% of the [Ca2+] (1.0 mmol/L) used in the first part of this series. Prior to salinity transfer (“pre-transfer”), SW-acclimated fish (open symbols) displayed a total Ca2+ influx that was eightfold greater than that of “pre-transfer” vFW-acclimated fish (closed symbols) when Ca2+ influx was measured in both groups in this common vFW background (0.1 mmol/L Ca2+) (Fig. 7a). Under these conditions, both extra-intestinal Ca2+ influx (by eightfold) and intestinal Ca2+ intake rates (by sevenfold) were significantly greater in SW fish than in vFW fish (Fig. 7b, c). By 24 h post-transfer, these patterns had reversed; vFW → SW fish had a significantly greater total Ca2+ influx rate than did the SW → vFW group, a difference that persisted throughout the rest of the experimental period (Fig. 6a). By 48 h post-transfer, FW → SW killifish displayed an extra-intestinal Ca2+ influx rate that was not statistically different (P = 0.236, unpaired Student’s t test) from that of SW-acclimated fish prior to transfer (22.5 ± 3.5 versus 16.8 ± 2.6 nmol/g/h, respectively; Fig. 7b). Similarly, SW → vFW fish displayed extra-intestinal Ca2+ influx rates at 48 h post-transfer that were similar to, though still statistically higher (P = 0.013, unpaired Student’s t test) than, those displayed by vFW-acclimated killifish prior to transfer (3.6 ± 0.4 versus 2.1 ± 0.4 nmol/g/h, respectively; Fig. 7b). Interestingly, however, intestinal Ca2+ intake in the vFW → SW group increased significantly following transfer and remained elevated such that by 48 h post-transfer, intestinal Ca2+ intake in vFW → SW fish was fivefold greater than that of SW fish prior to salinity transfer (Fig. 7c). On the other hand, intestinal Ca2+ intake by SW → vFW fish decreased by 48 h post-transfer to match the rates observed in vFW-acclimated fish prior to transfer (Fig. 7c).
Total whole-body Ca2+ influx (a), extra-intestinal Ca2+ influx (b), and intestinal Ca2+ intake (c) rates measured in Vancouver, BC freshwater (vFW) in killifish that were acclimated to seawater (SW) for 2 weeks and transferred acutely to vFW (SW → vFW; closed circles) or in killifish that were acclimated to vFW for 2 weeks and transferred acutely to SW (vFW → SW; open circles). In each panel, means within SW → vFW (abc) or vFW → SW (xyz) treatments not sharing the same letter represent a statistically significant effect of time; asterisks denote a statistically significant effect of salinity treatment within time as determined by a two-way ANOVA on rank-transformed data followed by a Tukey post-hoc test. Total: salinity, P < 0.001; time, P = 0.013; interaction, P < 0.001. Extra-intestinal: salinity, P = 0.767; time, P = 0.040; interaction, P < 0.001. Intestinal: salinity, P < 0.001; time, P < 0.001; interaction, P < 0.001 (n = 6–7)
Series 4: effects of inhibitors of Ca2+ uptake pathways on Ca2+ influx in FW and SW
Exposure to La3+ (10− 4 mol/L), a general blocker of Ca2+ channels, caused a significant reduction in whole-body Ca2+ uptake in hFW-acclimated fish but the slight decrease in SW-acclimated fish in response to La3+ treatment was not significant (Fig. 8). Exposure to verapamil (10− 5 mol/L), a voltage-gated Ca2+ channel blocker, stimulated Ca2+ influx in hFW and SW relative to the carrier (DMSO) control (Fig. 8). Exposure to nifedipine (10− 5 mol/L), a more general Ca2+ channel blocker, and the NCX blocker KB-R7934 (3.3 × 10− 6 mol/L) had no effect on Ca2+ influx in either salinity acclimation (Fig. 8).
Whole-body Ca2+ influx rates in response to various pharmacological blockers in killifish acclimated to Hamilton, ON freshwater (hFW; a) or seawater (SW; b) for 2 weeks. Asterisks denote a statistically significant difference between lanthanum and control treatments as determined by a two-tailed unpaired Student’s t test (FW: P = 0.042; SW: P = 0.269). Daggers denote a significant difference between carrier control and nifedipine, verapamil, or KB-R7934 treatments as determined by a one-way ANOVA followed by a Holm–Sidak post-hoc test (FW: P < 0.001; SW: P < 0.001) (n = 7–8)
Discussion
Overview
In the present study, we identified several features of Ca2+ influx by killifish acclimated to FW or SW and their response to acute salinity transfer. (1) Ca2+ influx increased with increasing salinity acclimation; however, Ca2+ efflux rates were the same between FW and SW-acclimated killifish. (2) Ca2+ influx rates were significantly greater in FW-acclimated killifish than in SW fish at Ca2+ concentrations < 2.5 mmol/L when fish were assayed in an ionic background equal to their native salinity acclimation. (3) Ca2+ influx rates in FW killifish were saturable while influx in SW fish was linear up to the highest ambient [Ca2+] tested (7 mmol/L) when fish were assayed in an ionic background equal to their native salinity acclimation. (4) Through several experiments, we found that cation competition in SW (specifically by Mg2+ rather than Na+) reduced Ca2+ influx and, moreover, likely inhibited a high affinity Ca2+ uptake system in SW-acclimated killifish. (5) Intestinal Ca2+ intake contributed 36% of total influx in SW killifish but only 2% of total Ca2+ influx in FW fish that are known to drink much less. Importantly, intestinal Ca2+ intake likely represents Ca2+ that was trapped within the intestinal fluid but not absorbed by the fish and is therefore probably an overestimation of the contribution of the intestine to whole-body Ca2+ influx. (6) Extra-intestinal Ca2+ influx rates were rapidly regulated following acute transfer from FW → SW and from SW → FW, contributing to a greater understanding of the regulation of Ca2+ transport in response to salinity challenges in this species (Wood and Marshall 1994; Wood and Laurent 2003; Wood and Grosell 2008; Wood 2011). (7) Ca2+ influx in FW killifish was inhibited by La3+, indicating a role for ECaC. La3+ had no effect on Ca2+ influx by SW killifish, although this lack of inhibitory effect could have been masked by competition from Mg2+ in SW. Overall, the present study adds to our current understanding of Ca2+ regulation by FW and SW killifish, demonstrating for the first time that cation competition modulates Ca2+ influx in SW, and provides the first evidence of the rapid regulation of Ca2+ influx following acute salinity transfer challenges in killifish.
Effects of salinity acclimation and the kinetics of Ca2+ influx
Ca2+ influx in killifish increased as a function of acclimation salinity; however, the only significant differences observed in the present study were between hFW and 100% SW acclimation groups (Fig. 1). Our findings are similar to those of Prodocimo et al. (2007) where Ca2+ fluxes were measured in killifish experiencing acute, progressive increases in salinity. In their work, a threshold for an increase in Ca2+ influx was observed at 80% SW, whereas Ca2+ influx below 80% SW was not significantly different from rates measured in 10% SW, the lowest salinity tested (Prodocimo et al. 2007). These researchers also found that Ca2+ efflux was not significantly different across all salinities (Prodocimo et al. 2007), similar to what we observed in hFW and 100% SW acclimated killifish (Fig. 2). Mayer-Gostan et al. (1983) similarly demonstrated that extra-intestinal Ca2+ uptake rate was higher in SW-acclimated fish than FW-acclimated fish when assayed in their native salinity acclimation, supporting our results and those of Prodocimo et al. (2007).
The concentration-dependent kinetics of Ca2+ influx in FW-acclimated killifish has been determined previously using in vivo (Patrick et al. 1997; Km = 0.0625 mmol/L) and in vitro (Marshall et al. 1995; Km = 0.35 mmol/L) approaches, which both demonstrated a relatively high affinity Ca2+ uptake system. Our in vivo findings are similar to those of Patrick et al. (1997), with hFW killifish possessing a high-affinity Ca2+ uptake system (Km = 0.078 mmol/L; Fig. 3). This low Km for Ca2+ uptake in FW killifish is comparable to or even lower than those of other freshwater fishes acclimated to ~ 1 mmol/L [Ca2+] [adult rainbow trout (Oncorhynchus mykiss), Km = 0.140 mmol/L (Perry and Wood 1985); juvenile rainbow trout, Km = 0.038–0.244 (Hogstrand et al. 1994; Niyogi and Wood 2004), juvenile yellow perch (Perca flavescens), Km = 0.092 mmol/L (Niyogi and Wood 2004)]. In addition, our findings support those of Mayer Gostan et al. (1983) in that Ca2+ influx in low ambient [Ca2+] (< 2.5 mmol/L) was greater in FW-acclimated fish than in SW-acclimated fish (Figs. 3, 4).
Ca2+ influx in SW-acclimated killifish was essentially linear up to 7 mmol/L ambient [Ca2+] (Figs. 3, 4). Notably, we did not exclude intestinal Ca2+ intake in these experiments which may have influenced the apparent rates of Ca2+ influx. In previous work, a component of extra-intestinal Ca2+ uptake was found to saturate in SW at approximately 2–4 mmol/L ambient [Ca2+] (Mayer-Gostan et al. 1983), indicating that intestinal Ca2+ intake in our experiment may have masked a saturable component of Ca2+ influx; we discuss the contribution of intestinal Ca2+ intake in SW-acclimated fish in more detail below. Saturable Ca2+ influx in SW would fit more logically within our current understanding of Ca2+ uptake in SW fishes given that passive paracellular entry of Ca2+ across the gill is probably limited by a large, inside-positive TEP in SW-acclimated killifish (Wood and Grosell 2008; see; Flik et al. 1996 for review).
Intestinal contribution to apparent Ca2+ influx
Drinking by SW-acclimated killifish likely contributed to apparent whole-body Ca2+ influx. In FW, where drinking is low (Potts and Evans 1967; Malvin et al. 1980; Scott et al. 2006; Blewett et al. 2013), intestinal Ca2+ intake contributed to only 2% of total influx (Fig. 5). However, intestinal Ca2+ intake in SW comprised 36% of total apparent influx (Fig. 5). This intestinal component most likely represents imbibed 45Ca2+ that was trapped in the intestine rather than absorbed into the bloodstream. In killifish, Pang et al. (1980) demonstrated that in a 3-h flux, calcium did not cross the gut barrier and that intestinal Ca2+ accumulation gave the same drinking rate as that determined by 125I-polyvinylpyrrolidone (PVP) which is not absorbed across the intestine epithelium. Therefore, it unlikely that our “extra-intestinal” Ca2+ influx estimates were substantially influenced by intestinal routes of Ca2+ intake.
Intestinal Ca2+ intake by SW-acclimated fish could potentially affect the interpretation of our whole-body Ca2+ influx results (Figs. 1, 3, 4, and 8). For example, as described above, it is possible that the linearity of Ca2+ influx up to 7 mmol/L [Ca2+] observed in SW (Fig. 3) was due to drinking of water with a high 45Ca2+ specific activity, masking a potential saturation in extra-intestinal Ca2+ influx. Based on this observation, we opted to account for intestinal Ca2+ intake in our cation competition experiment (Fig. 6) and our salinity transfer experiment (Fig. 7), which we predicted would influence drinking rates (Scott et al. 2006, 2008).
While intestinal Ca2+ intake was much greater in SW-acclimated fish than hFW-acclimated fish when measured in their native salinities (Fig. 5), intestinal Ca2+ intake was the same in SW and vFW-acclimated fish when assayed in 1 mmol/L Ca2+ in vFW (Fig. 6c). In both acclimation groups, addition of NaCl up to 450 mmol/L significantly stimulated intestinal Ca2+ intake (Fig. 6c), demonstrating the known role of these ions in stimulating drinking in killifish (Grosell 2010).
Intestinal Ca2+ intake was also altered significantly by acute salinity transfer. Following transfer from vFW to SW (vFW → SW treatment), intestinal Ca2+ intake rates (measured in vFW) increased by over 20-fold within 3 h post-transfer (Fig. 6c). This increase in intestinal Ca2+ intake was most certainly a result of drinking upon exposure to SW. Following transfer from brackish water (10% SW) to 100% SW, drinking rates approximately doubled in killifish within 12 h (Scott et al. 2008), though to our knowledge, no study has examined drinking rates in killifish following a full FW to 100% SW transfer. If intestinal Ca2+ intake rates are any indication, the onset of drinking following transfer to SW is extremely rapid, and this should be examined in future studies. Following transfer to vFW, SW-acclimated killifish (SW → vFW treatment) demonstrated decreases in intestinal Ca2+ intake rates over time (Fig. 6c). Again, this was most likely related to decreases in drinking rate over time, which have been observed in killifish transferred from 10% SW to FW (Scott et al. 2006).
Effects of cation competition on Ca2+ influx in SW
Several experiments in the present study demonstrated that cation competition in SW inhibits Ca2+ influx. In the kinetic analyses, hFW-acclimated fish had a much greater Ca2+ uptake rate than SW-acclimated fish at Ca2+ concentrations below approximately 2.5 mmol/L when fish were assayed in reconstituted FW and SW media, respectively (Figs. 3, 4). Notably, a similar response was observed previously for extra-intestinal Ca2+ uptake in FW and SW-acclimated killifish (Mayer-Gostan et al. 1983), suggesting that FW-acclimated fish have a higher affinity for Ca2+ influx than SW-acclimated killifish. However, this conclusion does not take into account the background ionic composition of the acclimation medium. In fact, when fish were acclimated to hFW, 50% SW or 100% SW and Ca2+ influx was measured in a range of ambient [Ca2+], Ca2+ influx at 1 mmol/L ambient [Ca2+] was significantly greater in 50% SW compared to 100% SW or FW, with a similar pattern in 2.5 mmol/L [Ca2+] (Fig. 4), indicating that reducing ambient cations by 50% increased Ca2+ influx in the 50% SW group. At higher concentrations of ambient Ca2+, cation competition was apparently overcome, and Ca2+ influx rates were not significantly different between 50 and 100% SW killifish (Fig. 4).
This cation competition hypothesis was directly tested by measuring Ca2+ influx in vFW and SW-acclimated killifish in a common [Ca2+] (1 mmol/L Ca2+) in the absence of competing cations (background vFW ions) or in the presence of 25 or 50 mmol/L Mg2+, or 225 or 450 mmol/L Na+. In agreement with the cation competition hypothesis, the presence of Mg2+ at 50 mmol/L in vFW killifish or at 25 and 50 mmol/L Mg2+ in SW killifish significantly reduced total and extra-intestinal Ca2+ influx by 70–75% (Fig. 6a, b). Previous work has demonstrated that Mg2+ can inhibit in vitro Ca2+ influx across the opercular epithelium when [Mg2+] (1 mmol/L) was tenfold greater than [Ca2+] (0.1 mmol/L) (Marshall et al. 1995), but that Mg2+ had no effect on in vivo whole-body Ca2+ influx when both ions were present at 0.1 mmol/L (Patrick et al. 1997). Future studies should, therefore, aim to determine the inhibition constant for Mg2+ on Ca2+ influx at different levels of ambient [Ca2+] to better understand its role in modulating Ca2+ influx in different environments. Moreover, when killifish were assayed at 1 mmol/L Ca2+ in the absence of competing cations, Ca2+ influx by SW-acclimated fish was more than double that of vFW-acclimated fish (Fig. 6a, b), suggesting a higher affinity and/or capacity Ca2+ uptake system in SW-acclimated killifish. Most importantly, this experiment directly demonstrated that Ca2+ influx rates of SW-acclimated killifish can appear to be lower than those of their FW-acclimated counterparts when fish are assayed in native background ion concentrations.
The effect of cation competition was also apparent in our salinity transfer experiment which was designed to assess the regulation of Ca2+ influx in the absence of the confounding effect of cation competition by measuring Ca2+ influx in vFW in both salinity acclimation groups. Prior to salinity transfer (pre-transfer), SW-acclimated killifish had an eightfold greater extra-intestinal Ca2+ influx rate than that of vFW-acclimated fish when both groups were assayed in vFW (0.1 mmol/L Ca2+) (Fig. 7b). Therefore, in the absence of competing SW ions, SW-acclimated fish had greater total/extra-intestinal Ca2+ influx rates at 0.1 mmol/L Ca2+ (Fig. 7a, b) and 1 mmol/L Ca2+ (Fig. 6a, b), likely indicating that the affinity for Ca2+ is in fact higher in SW-acclimated killifish than in FW-acclimated killifish, contrary to the initial indication of our kinetic analyses (Fig. 3) and the conclusion of Mayer-Gostan et al. (1983). A higher affinity system in SW-acclimated killifish was also supported by the fact that Ca2+ influx in SW was more sensitive to ambient Mg2+ than it was in FW (Fig. 6), an effect that would not likely result from a simple difference in Ca2+ influx capacity (i.e., an increase in sites available for uptake).
Ca2+ influx following acute salinity transfer
Killifish rapidly alter osmoregulatory mechanisms in response to changes in ambient salinity (Marshall et al. 1999; Scott et al. 2004, 2005, 2006, 2008; Wood and Grosell 2008; Wood 2011). However, no study to date has examined how Ca2+ influx is regulated following acute salinity transfer. Therefore, we acutely transferred vFW-acclimated killifish to SW (vFW → SW) and SW-acclimated fish to vFW (SW → vFW) and measured Ca2+ uptake in vFW (to avoid the confounding effect of cation competition) at different times post-transfer. vFW fish initially had very low extra-intestinal Ca2+ influx rates but these rates increased dramatically following transfer to SW (Fig. 7a). This rapid increase (3.5-fold increase within 3–6 h post-transfer) in extra-intestinal Ca2+ rates observed in vFW → SW fish was likely a function of neuroendocrine modulation of Ca2+ uptake mechanisms. In fishes, Ca2+ influx is known to be stimulated by various factors including prolactin and cortisol (see Pang and Pang 1986; Flik et al. 1996; Kwong et al. 2016; Lin and Hwang 2016 for reviews). SW → vFW fish also demonstrated rapid regulation of Ca2+ influx whereby extra-intestinal influx rates (assayed in vFW) decreased by 60% within the first 3–6 h post-transfer (Fig. 7a). The progressive decrease in extra-intestinal Ca2+ uptake over time could have been regulated by neuroendocrine factors such as stanniocalcin (STC) which is released from the corpuscles of Stannius (CS) in response to increases in environmental or plasma Ca2+ in fishes (Hanssen et al. 1991; Wendelaar Bonga and Pang 1991).
Mechanisms of Ca2+ influx in FW and SW-acclimated killifish
In hFW, the ECaC inhibitor La3+ reduced Ca2+ influx by 35% but there was no significant effect of La3+ treatment in SW-acclimated killifish (Fig. 7). The inhibitory effect of La3+ on Ca2+ influx in FW-acclimated killifish is consistent with previous reports (Marshall et al. 1995; Patrick et al. 1997). The lack of effect in SW may be explained by the fact that La3+ acts by competitively inhibiting Ca2+ influx via ECaC, and therefore a higher dose is needed in SW both because of the much higher [Ca2+] and to overcome competition from high [Mg2+] in SW (Fig. 6). It is also possible that the role of ECaC in Ca2+ uptake is diminished in SW as was demonstrated in medaka (Oryzias latipes) larvae where ECaC mRNA expression was downregulated in response to SW acclimation (Hsu et al. 2014). Aside from La3+, none of the inhibitors used in the present study had an inhibitory effect on Ca2+ uptake. Interestingly, the voltage-gated Ca2+ channel blockers nifedipine and verapamil had divergent effects on Ca2+ influx. While nifedipine had no effect, verapamil stimulated Ca2+ influx in both hFW and SW-acclimated killifish (Fig. 7). These results are in contrast with results from isolated opercular membrane preparations where verapamil had no effect on Ca2+ transport (Marshall et al. 1995). It is possible that verapamil treatment could have altered intracellular Ca2+ levels in ionocytes, leading to alterations in transmembrane Ca2+ transport given that intracellular Ca2+ is believed to regulate its own transport (Marshall et al. 1995) or that whole-body Ca2+ influx responds to verapamil in a way that is fundamentally different from that observed in vitro. It was also surprising that the Na+/Ca2+ exchanger (NCX) blocker KB-R7934 had no effect on Ca2+ influx since Verbost et al. (1997) suggested that the majority of basolateral Ca2+ transport in killifish, at least in the opercular epithelium, occurs via Na+/Ca2+ exchange. Overall, we can conclude only that ECaC plays a role in Ca2+ influx in hFW-acclimated killifish.
References
Baldisserotto B, Chowdhury MJ, Wood CM (2005) Effects of dietary calcium and cadmium on cadmium accumulation, calcium and cadmium uptake from the water, and their interactions in juvenile rainbow trout. Aquat Toxicol 72:99–117
Blewett T, MacLatchy DL, Wood CM (2013) The effects of temperature and salinity on 17-α-ethynylestradiol uptake and its relationship to oxygen consumption in the model euryhaline teleost (Fundulus heteroclitus). Aquat Toxicol 127:61–71
Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gómez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL (2007) Fundulus as the premier teleost model in environmental biology: opportunities for new insights using genomics. Comp Biochem Physiol D 2:257–286
Dymowska AK, Hwang PP, Goss GG (2012) Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol 184:282–292
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Flik G, Wendelaar Bonga SE, Fenwick JC (1983) Ca2+-dependent phosphatase and ATPase activities in eel gill plasma membranes-I. Identification of Ca2+-activated ATPase activities with non-specific phosphatase activities. Comp Biochem Physiol B 76:745–754
Flik G, van Rijs JH, Wendelaar Bonga SE (1985a) Evidence for high-affinity Ca2+-ATPase activity and ATP-driven Ca2+-transport in membrane preparations of the gill epithelium of the cichlid fish Oreochromis mossambicus. J Exp Biol 119:335–347
Flik G, Wendelaar Bonga SE, Fenwick JC (1985b) Active Ca2+ transport in plasma membranes of branchial epithelium of the North-American eel, Anguilla rostrata LeSueur. Biol Cell 55:265–272
Flik G, Van Der Velden JA, Dechering KJ, Verbost PM, Schoenmakers TJM, Kolar ZI, Bonga SEW (1993) Ca2+ and Mg2+ transport in gills and gut of tilapia, Oreochromis mossambicus: a review. J Exp Zool 265:356–365
Flik G, Klaren PHM, Schoenmakers TJM, Bijvelds MJC, Verbost PM, Wendelaar Bonga SE (1996) Cellular calcium transport in fish: unique and universal mechanisms. Physiol Zool 69:403–417
Flik G, Kaneko T, Greco AM, Li J, Fenwick JC (1997) Sodium dependent ion transporters in trout gills. Fish Physiol Biochem 17:385–396
Franklin NM, Glover CN, Nicol JA, Wood CM (2005) Calcium/cadmium interactions at uptake surfaces in rainbow trout: Waterborne versus dietary routes of exposure. Environ Toxicol Chem 24:2954–2964
Grosell M (2010) The role of the gastrointestinal tract in salt and water balance. In: Grosell M, Farrell AP, Brauner CJ (eds) Fish physiology, vol 30. Academic Press, Amsterdam, pp 135–164
Hanssen RGJM, Aarden EM, van der Venne WPHG, Pang PKT, Wendelaar Bonga SE (1991) Regulation of secretion of the teleost fish hormone stanniocalcin: effects of extracellular calcium. Gen Comp Endocrinol 163:155–163
Hogstrand C, Wilson RW, Polgar D, Wood CM (1994) Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J Exp Biol 186:55–73
Hsu HH, Lin LY, Tseng YC, Horng JL, Hwang PP (2014) A new model for fish ion regulation: identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes). Cell Tissue Res 357:225–243
Hwang PP, Lee TH (2007) New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A 148:479–497
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–179
Kwong RWM, Kumai Y, Perry SF (2016) Neuroendocrine control of ionic balance in zebrafish. Gen Comp Endocrinol 234:40–46
Liao BK, Deng AN, Chen SC, Chou MY, Hwang PP (2007) Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genom 8:1–13
Lin C-H, Hwang P-P (2016) The control of calcium metabolism in zebrafish (Danio rerio). Int J Mol Sci 17:1783
Lin CH, Kuan WC, Liao BK, Deng AN, Tseng DY, Hwang PP (2016) Environmental and cortisol-mediated control of Ca2+ uptake in tilapia (Oreochromis mossambicus). J Comp Physiol B 186:323–332
Malvin RL, Schiff D, Eiger S (1980) Angiotensin and drinking rates in the euryhaline killifish. Am J Physiol Regul Integr Comp Physiol 239:R31–R34
Marshall WS (2002) Na+, Cl–, Ca2+ and Zn2+ transport by fish gills: retrospective review and prospective synthesis. J Exp Zool 293:264–283
Marshall WS, Bryson SE, Burghardt JS, Verbost PM (1995) Ca2+ transport by opercular epithelium of the fresh water adapted euryhaline teleost, Fundulus heteroclitus. J Comp Physiol B 165:268–277
Marshall W, Emberley T, Singer T, Bryson S, Mccormick S (1999) Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: a multivariable approach. J Exp Biol 202:1535–1544
Mayer-Gostan N, Bornancin M, DeRenzis G, Naon R, Yee JA, Shew RL, Pang PK (1983) Extraintestinal calcium uptake in the killifish, Fundulus heteroclitus. J Exp Zool 227:329–338
Niyogi S, Wood CM (2004) Kinetic analyses of waterborne Ca and Cd transport and their interactions in the gills of rainbow trout (Oncorhynchus mykiss) and yellow perch (Perca flavescens), two species differing greatly in acute waterborne Cd sensitivity. J Comp Physiol B 174:243–253
Pan T-C, Liao B-K, Huang C-J, Lin L-Y, Hwang P-P (2005) Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am J Physiol Regul Integr Comp Physiol 289:R1202–R1211
Pang PKT, Pang RK (1986) Hormones and calcium regulation in Fundulus heteroclitus. Am Zool 234:225–234
Pang PKT, Griffith RW, Maetz J, Pic R (1980) Calcium uptake in fishes. In: Lahlou B (ed) Epithelial transport in lower vertebrates. Cambridge University Press, Cambridge, pp 122–132
Patrick ML, Wood CM, Marshall WS (1997) Calcium regulation in the freshwater-adapted mummichog. J Fish Biol 51:135–145
Perry SF (1997) The chloride cell: structure and function in the gills of freshwater fishes. Annu Rev Physiol 59:325–347
Perry SF, Flik G (1988) Characterization of branchial transepithelial calcium fluxes in freshwater trout, Salmo gairdneri. Am J Physiol Regul Integr Comp Physiol 254:R491–R498
Perry SF, Wood CM (1985) Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J Exp Biol 116:411–433
Perry SF, Shahsavarani A, Georgalis T, Bayaa M, Furimsky M, Thomas SLY (2003) Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid–base regulation. J Exp Zool 300A:53–62
Potts WTW, Evans DH (1967) Sodium and chloride balance in the killifish Fundulus heteroclitus. Biol Bull 133:411–425
Prodocimo V, Galvez F, Freire CA, Wood CM (2007) Unidirectional Na+ and Ca2+ fluxes in two euryhaline teleost fishes, Fundulus heteroclitus and Oncorhynchus mykiss, acutely submitted to a progressive salinity increase. J Comp Physiol B 177:519–528
Qiu A, Hogstrand C (2004) Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene 342:113–123
Rogers JT, Wood CM (2004) Characterization of branchial lead-calcium interaction in the freshwater rainbow trout Oncorhynchus mykiss. J Exp Biol 207:813–825
Schoenmakers TJM, Verbost PM, Flik G, Wendelaar Bonga SE (1993) Transcellaular intestinal calcium transport in freshwater and seawater fish and its dependence on sodium/calcium exchange. J Exp Biol 176:195–206
Scott GR, Richards JG, Forbush B, Isenring P, Schulte PM (2004) Changes in gene expression in gills of the euryhaline killifish Fundulus heteroclitus after abrupt salinity transfer. Am J Physiol Cell Physiol 287:C300–C309
Scott GR, Claiborne JB, Edwards SL, Schulte PM, Wood CM (2005) Gene expression after freshwater transfer in gills and opercular epithelia of killifish: insight into divergent mechanisms of ion transport. J Exp Biol 208:2719–2729
Scott GR, Schulte PM, Wood CM (2006) Plasticity of osmoregulatory function in the killifish intestine: drinking rates, salt and water transport, and gene expression after freshwater transfer. J Exp Biol 209:4040–4050
Scott GR, Baker DW, Schulte PM, Wood CM (2008) Physiological and molecular mechanisms of osmoregulatory plasticity in killifish after seawater transfer. J Exp Biol 211:2450–2459
Shahsavarani A, Perry SF (2006) Hormonal and environmental regulation of epithelial calcium channel in gill of rainbow trout (Oncorhynchus mykiss). Am J Physiol Regul Integr Comp Physiol 291:1490–1498
Shahsavarani A, McNeil B, Galvez F, Wood CM, Goss GG, Hwang P-P, Perry SF (2006) Characterization of a branchial epithelial calcium channel (ECaC) in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 209:1928–1943
Sundell K, Bjornsson BT (1988) Kinetics of calcium fluxes across the intestinal mucosa of the marine teleost, Gadus morhua, measured using an in vitro perfusion method. J Exp Biol 140:171–186
Verbost PM, Schoenmakers TJ, Flik G, Wendelaar Bonga SE (1994) Kinetics of ATP- and Na+-gradient driven Ca2+ transport in basolateral membranes from gills of freshwater- and seawater-adapted tilapia. J Exp Biol 186:95–108
Verbost PM, Bryson SE, Wendelaar Bonga SE, Marshall WS (1997) Na+-dependent Ca2+ uptake in isolated opercular epithelium of Fundulus heteroclitus. J Comp Physiol B 167:205–212
Wendelaar Bonga SE, Pang PKT (1991) Control of calcium regulating hormones in the vertebrates: parathyroid hormone, calcitonin, prolactin, and stanniocalcin. Int Rev Cytol 128:139–213
Wood CM (2011) Rapid regulation of Na+ and Cl− flux rates in killifish after acute salinity challenge. J Exp Mar Biol Ecol 409:62–69
Wood CM, Grosell M (2008) A critical analysis of transepithelial potential in intact killifish (Fundulus heteroclitus) subjected to acute and chronic changes in salinity. J Comp Physiol B 178:713–727
Wood CM, Laurent P (2003) Na+ versus Cl− transport in the intact killifish after rapid salinity transfer. Biochim Biophys Acta Biomembr 1618:106–119
Wood CM, Marshall WS (1994) Ion balance, acid-base regulation, and chloride cell function in the common killifish, Fundulus heteroclitus—a euryhaline estuarine teleost. Estuaries 17:34–52
Acknowledgements
Special thanks are given to Linda Diao who conducted many of the experiments and analyses at McMaster University, to Sunita Nadella who also helped with experiments and analyses, and to Marina Giacomin who assisted with experiments at UBC. Constructive comments from four anonymous reviewers improved the MS. Funded by a NSERC Discovery Grant to CMW. CMW was supported by the Canada Research Chairs Program. KVB was supported by a NSF Post-Doctoral Fellowship (DBI-1306452) and by a NSERC Discovery Grant to CMW. AMZ is supported by a NSERC Post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Zimmer, A.M., Brix, K.V. & Wood, C.M. Mechanisms of Ca2+ uptake in freshwater and seawater-acclimated killifish, Fundulus heteroclitus, and their response to acute salinity transfer. J Comp Physiol B 189, 47–60 (2019). https://doi.org/10.1007/s00360-018-1192-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-018-1192-z