Abstract
Background
Although older adults are disproportionately affected by painful musculoskeletal conditions and receive more opioid analgesics than persons in other age groups, insufficient evidence is available regarding opioid harms in this age group.
Objective
To examine longitudinal relationships between opioid use and falls, clinical fractures, and changes in physical performance. We hypothesized that opioid use would be associated with greater risks of falling and incident clinical fractures and greater declines in physical performance.
Design
We analyzed data from the Osteoporotic Fractures in Men Study (MrOS), a large prospective longitudinal cohort study. Participants completed baseline visits from 2000 to 2002 and were followed for 9.1 (SD 4.0) years.
Participants
MrOS enrolled 5994 community-dwelling men ≥ 65 years of age. The present study included 2902 participants with back, hip, or knee pain most or all of the time at baseline.
Main Measures
The exposure of interest was opioid use, defined at each visit as participant-reported daily or near-daily use of any opioid-containing analgesic. Among patients, 309 (13.4 %) reported opioid use at one or more visits. Participants were queried every 4 months about falls and fractures. Physical performance scores were derived from tests of grip strength, chair stands, gait speed, and dynamic balance.
Key Results
In the main analysis, the adjusted risk of falling did not differ significantly between opioid use and non-use groups (RR 1.10, 95 % CI 0.99, 1.24). Similarly, adjusted rates of incident clinical fracture did not differ between groups (HR 1.13, 95 % CI 0.94, 1.36). Physical performance was worse at baseline for the opioid use group, but annualized change in physical performance scores did not differ between groups (−0.022, 95 % CI −0.138, 0.093).
Conclusions
Additional research is needed to determine whether opioid use is a marker of risk or a cause of falls, fractures, and progressive impairment among older adults with persistent pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Older adults are disproportionately affected by painful musculoskeletal conditions and also receive more opioid analgesics than persons in other age groups.1,2 Potential harms of opioid therapy in this age group may differ from those affecting younger adults. Evidence suggests that some opioid-related harms, including addiction, are more prevalent among younger than older age groups.3,4 Other harms, especially falls and fractures, may be more common and worrisome among older adults.5
Falls are an important mechanism of serious trauma and the most common cause of injury-related emergency visits among older adults.6,7 Fracture is a serious potential consequence of falling, which among older adults can lead to significant morbidity, loss of independence, or death, especially in the case of hip fracture.8,9 Most prior studies have found positive associations between the use of opioid analgesics and fractures in older adults,5,10–12 but evidence for an association between opioid use and falls has been inconsistent.13,14 Importantly, chronic musculoskeletal pain is also associated with declines in physical function14–18 and increased risk of falling.19
We used data from the Osteoporotic Fractures in Men Study (MrOS), a large prospective cohort study of community-dwelling older men, to examine longitudinal associations of opioid analgesic use with fracture and fall outcomes among older men with persistent musculoskeletal pain. We also examined the longitudinal association of opioid use with changes in physical performance over time. We hypothesized that participants who reported opioid use would have a greater risk of falling and incident clinical fractures and would experience greater declines in physical performance compared with those who reported no opioid use.
METHODS
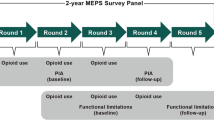
MrOS enrolled 5994 men ≥ 65 years of age from six sites in the United States. Participants completed baseline visits from March 2000 to April 2002. MrOS study design, recruitment methods, and cohort characteristics have been previously reported.20,21 The present study included 2902 MrOS participants who had persistent musculoskeletal pain at baseline (Fig. 1); inclusion criteria for this analysis were 1) participant-reported back, hip, or knee pain most or all of the time at baseline, and 2) non-missing analgesic data at ≥ 1 visit.
Pain Measures
Pain was assessed by three questions focused on the back, hips, and knees. The back question asked about the frequency of pain within the past 12 months; participants who reported pain most or all of the time were considered as having persistent pain. The hip and knee questions asked whether pain was present “on most days for at least a month;” for both questions, participants who answered yes were considered as having persistent pain.
Medication Exposure
Medication exposure and covariate data were collected from participants at baseline and two follow-up visits, completed a mean of 4.6 years (visit 2) and 6.9 years (visit 3) after the baseline visit. Participants were asked to bring all current medications to each visit. Interviewers recorded medication names and frequency of use, but not dose, for each medication. The active ingredients of medications were coded according to the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).22
The exposure of interest was opioid use, defined as participant-reported daily or near-daily use of any opioid analgesic (including tramadol) or opioid-containing combination analgesic product (e.g., hydrocodone-acetaminophen). Opioid formulations indicated for reasons other than pain (e.g., cough) were excluded. Participants who reported use of non-opioid analgesics in addition to opioid analgesics or opioid-containing combination analgesic were included in the opioid use group. Opioid use was expressed in models as a time-varying categorical variable.
Outcomes
Participants were sent questionnaires every 4 months to collect self-reported fall and fracture event data; these contacts were > 99 % complete. The fall outcome was a repeated measure of one or more falls reported during each 4-month period, which therefore accounted for multiple falls within individuals over time. The primary fracture outcome was any incident clinical fracture, defined as non-vertebral fracture or clinically recognized vertebral fracture. Hip fracture was evaluated as a secondary fracture outcome. All fractures were centrally confirmed by x-ray or review of imaging reports). Incident clinical vertebral fractures were those reported by participants and confirmed by the study radiologist, who used the visual semi-quantitative method to establish that the community imaging study showed a new deformity of higher grade than was present in the same vertebra on the baseline study film.
Physical performance was assessed at baseline and follow-up visits using tests of grip strength, chair stands, gait speed, and dynamic balance. Each individual test was scored from 0 (unable to complete) to 5 (best) and converted to quintiles based on score distributions. The four individual test scores were summed to create an overall physical performance score with a possible range of 0 to 20, where lower scores indicate worse performance.23
Covariates
Age, body mass index (BMI; kg/m2), and total hip bone mineral density (BMD; g/cm2) were assessed as continuous variables. Categorical variables included self-reported race/ethnicity (non-Hispanic white or non-white), smoking status (current, former, never), current alcohol use (<1 drink/week, 1–13 drinks/week, and ≥14 drinks/week), and self-reported health status (very poor/poor/fair or very good/excellent). Chronic conditions were evaluated by self-report and categorized (0, 1–2, or ≥3 conditions). Cognitive function was assessed with the Modified Mini-Mental State (3MS) examination24 (continuous) and Part B of the Trail Making Test25 (quintiles), and mental health was assessed with the Medical Outcomes Study 12-Item Short-Form Survey (SF-12)26 mental component summary score (continuous). The continuous Physical Activity Scale for the Elderly (PASE) score was included as a measure of participation in physical activity.27 Participants were categorized as robust, intermediate, or frail using the Study of Osteoporotic Fractures frailty index.28 Baseline values were included in models, except where specified otherwise.
Propensity Scores
We used a propensity score (PS) approach to adjust for between-group differences in covariates while minimizing the dimensionality of the models. The PS, representing the conditional probability of receiving opioids at a given visit, was estimated for each visit using logistic regression models that included covariates described above. Participants were then sub-classified by quintiles of propensity score, and covariates were compared between opioid use and non-use groups within each quintile to determine the adequacy of the model in balancing measured confounders.29,30 To achieve adequate balance of covariates between participants with and without opioid use in the lowest PS quintiles,31 men with PS < 0.03 (meaning < 3 % conditional probability of receiving opioids) for a given visit were excluded from the analytic cohort at that visit. They were not excluded from the overall cohort, and contributed data to other visits. In this PS-restricted cohort, covariates other than smoking and back pain were well-balanced between the opioid use and non-use groups within all quintiles at each visit. For all outcomes, the primary analysis is the adjusted model in the PS-restricted cohort. Unadjusted and adjusted models using the unrestricted cohort are also reported.
Statistical Analysis
We included time-varying PS quintile, baseline smoking status, and time-varying back pain in all adjusted models. Participants were excluded from analyses at a given visit if they were missing information on opioid use at that visit. To model longitudinal associations of opioid use with repeated measures of falling over time, we used generalized estimating equations (GEE) with a binomial distribution, log link function, and an auto-regressive correlation structure. Similarly, to model longitudinal associations of opioid use with physical performance over time, we used GEE with a normal distribution, identity link function, and an auto-regressive correlation structure. To model associations between opioid use and incident fracture outcomes, we used Cox proportional survival analyses. In primary models for all outcomes, any change in opioid use status between visits was assumed to occur at the midpoint between visits, and all outcomes occurring during the follow-up time period were included. We used separate models to conduct sensitivity analyses in which a) any change in opioid use status was assumed to occur at the time of the visit at which the change was reported, and b) outcomes were limited to the 12 months following each visit.
We conducted secondary analyses to examine potential effects of the competing risk of mortality.32 Based on findings from prior studies,5,33 we expected any alteration in risk for death and for fracture to be in the same direction (i.e., increased with opioid use). Cox proportional hazards models were constructed, as for the main analyses, to examine three separate outcomes: death, death or clinical fracture composite, and death or hip fracture composite.
RESULTS
The cohort for this study included 2902 men with persistent pain at baseline and non-missing analgesic data for at least one visit; of these, 390 (13.4 %) reported opioid use at one or more visits. Opioid use increased over time from 4.7 % at visit 1 to 7.2 % at visit 2 and 10.5 % at visit 3. Changes in patterns of opioid use were observed between visit 1 and visit 3 (average interval between visits 6.9 years); most notably in the proportion of participants with opioid use who reported using hydrocodone (10.6 % at baseline to 35.3 % at visit 3), oxycodone (4.5 % to 13.0 %), or propoxyphene (23.9 % to 12.3 %).
Table 1 shows the baseline characteristics of participants with and without opioid use at that visit. The two groups differed on numerous characteristics; for example, participants with opioid use were less likely to be a never-smoker (26.4 vs. 34.5 %), more likely to be frail (34.9 vs. 10.6 %), and more likely to have back pain (61.2 vs. 27.6 %) and hip pain (59.7 vs. 49.0 %).
Falls
Over 9.1 (SD 4.0) years of follow-up, 2413 (83.1 %) participants reported at least one fall, and 479 (16.5 %) experienced at least one clinical fracture, including 96 (3.3 %) with a hip fracture. The unadjusted relative risk (RR) of falling was higher for the opioid use group than the non-use group (1.37, 95 % CI 1.23, 1.54; see Table 2). In the unrestricted cohort, including all participants regardless of PS, the adjusted risk of falling was attenuated but remained significantly higher among participants with opioid use (RR 1.14, 95 % CI 1.02, 1.28). In the PS-restricted cohort, the adjusted risk of falling was not statistically different between groups (RR 1.10, 95 % CI 0.99, 1.24). Results of sensitivity analyses did not differ substantially from those of the primary analysis.
Fractures
Fracture results are shown in Table 3. For the primary fracture outcome of any incident clinical fracture, participants with and without opioid use had similar rates of incident clinical fracture in all models (adjusted model in PS-restricted cohort: HR 1.13, 95 % CI 0.94, 1.36). For the secondary outcome of hip fracture, the unadjusted fracture rate was approximately twice as high in the opioid use group (HR 2.14, 95 % CI 1.36, 3.38) as in the non-use group. In the adjusted unrestricted-cohort model, the magnitude of the difference between groups was somewhat attenuated (HR 1.74, 95 % CI 1.06, 2.87), but still statistically significant. In the adjusted PS-restricted cohort analysis, the difference between groups was no longer statistically significantly (HR 1.64, 95 % CI 0.97, 2.79). Results of sensitivity analyses for both fracture outcomes did not differ substantially from those of the primary analyses.
Physical Performance
At baseline, the unadjusted mean physical performance score was 10.4 in the opioid group and 11.6 in the non-use group. This difference between groups was significant in the adjusted PS-restricted model, with the opioid group 0.5 points (95 % CI 0.20, 1.16) worse than the non-use group at baseline. Table 4 shows results for change in physical performance over time. Both groups experienced declines in physical performance over time, but we found no significant between-group difference in the annualized change in physical performance scores (adjusted mean between-group difference in PS-restricted cohort = −0.022, 95 % CI −0.138, 0.093).
Secondary Analyses
Five hundred twenty-eight (18.2 %) participants died during study follow-up. In analyses to examine potential effects of the competing risk of mortality, we found no statistically significant excess hazard associated with opioid use for death (adjusted model in PS-restricted cohort: HR 1.22, 95 % CI 0.94, 1.58), clinical fracture/death composite outcome (HR 1.14, 95 % CI 0.88, 1.48), or hip fracture/death composite outcome (HR 1.22, 95 % CI 0.94, 1.58).
DISCUSSION
In this cohort of older men with persistent musculoskeletal pain, we found that initial between-group differences in falls and fractures were attenuated and not statistically significant after adjustment for likely confounders and exclusion from analysis of participants with an extremely low likelihood of being treated with opioids. We also found that participants who reported opioid use had worse physical performance at baseline, but did not differ from those without opioid use in the rate of decline in physical performance over time.
A major advantage of our analysis of MrOS data is the detailed prospectively collected information about functional status and other important potential confounders. Confounding by indication and unmeasured patient characteristics is particularly relevant for research on the benefits and harms of opioids. Chronic pain is itself associated with fall and fracture risk factors, such as physical deconditioning and mobility impairment,15–19,34 and with increased falling.19,35 Even among patients with chronic pain, opioid users systematically differ from non-users; in general, they are more functionally impaired and psychosocially distressed, more likely to smoke, and less likely to be physically active.36–38 Most prior studies of opioid-related harms have used data from administrative sources, which often have high-quality prescription dispensing and health care utilization data but lack important information about chronic pain, functional impairment, and psychological distress. As a result, these studies likely overestimate the associations of opioid use with adverse clinical outcomes.
We found no significant association between opioid use and our primary fracture outcome in any model; however, for both fall and hip fracture outcomes, we found significant associations present in unadjusted models, attenuated but still statistically significant in adjusted unrestricted-cohort models, and not significant in the PS-restricted analysis after exclusion of participants at the extreme low end of the propensity score distribution. For the fall outcome in particular, this pattern demonstrates an advantage of propensity score analysis. In practice, some patients have contraindications that make them extremely unlikely to receive opioid therapy, resulting in non-overlapping exposure groups and residual confounding despite adjustment. Propensity score methods allow recognition and management of this potential source of residual confounding.39 In the case of the secondary hip fracture analyses, given the relatively small number of incident hip fracture cases, an alternate or additional reason for this pattern may be that cohort restriction led to lower power to detect an association.
MrOS included rigorous prospective ascertainment of falls, which is important because most falls do not result in clinical encounters and are absent from administrative records. Falling was common in this study population and was not significantly associated with opioid use in the primary analysis. Our findings are consistent with those of a meta-analysis of medication use and falling in adults over the age of 60.13
Opioids are thought to increase the risk of fracture either by increasing the likelihood of falling or by increasing the risk of fracture when falling occurs, most likely through adverse central nervous system (CNS) effects such as sedation, dizziness, or impaired reaction time. Because tolerance may develop to some of these CNS effects, the risk may be highest shortly after initiation of therapy. Our finding of no significant association between opioid use and clinical fracture contrasts with findings of most prior studies, including a meta-analysis that reported a pooled RR of 1.38 (1.15, 1.66) for the association of opioids with fracture.12
Prior studies that examined risk immediately after new opioid initiation have generally found stronger associations with fracture than those that examined opioid use of longer or unspecified duration. For example, a study of older adults that used data from Medicare and state pharmacy benefit programs found that patients with new opioid prescriptions had a fivefold higher risk of hip or upper extremity fracture than those with new NSAID prescriptions.10 Fracture incidence was highest in the initial 2 weeks of opioid therapy. Similarly, a recent registry-based study evaluating fracture and other fall-related injuries in Swedish adults found the strongest association in the first week after initiating opioid therapy (OR = 5.14), with risk decreasing in each 7-day period thereafter (OR = 1.23 in the fourth week).14 Interestingly, this relationship was strongest in the youngest age group (18–29 years), which could suggest confounding by high-risk behavior. MrOS did not collect information about the timing of medication initiation, but most medication use in the study was likely prevalent use; thus we cannot assess potential associations between opioids and fractures in the initial weeks or months of opioid therapy.
The major limitation of this study is that our exposure data were limited to repeated cross-sectional assessments of current medication use. Because we did not have data on duration and dose, we were not able to evaluate gradations of risk associated with duration or dose of opioid therapy. As discussed above, our findings likely apply to ongoing opioid use rather than recent onset of use. Furthermore, given that the most commonly reported opioids in this study were “weak” opioids and dose-limited combination products, our findings likely apply to relatively low-dose opioid use. This study has several other limitations. Treatment was not randomized, so we cannot rule out bias due to unmeasured confounding. We observed a relatively small number of fractures, and may not have had adequate power to detect clinically important group differences for the secondary outcome of hip fracture. Given this possibly insufficient power and the observed wide confidence intervals in adjusted models, a clinically meaningful effect of opioid use on hip fractures is not excluded. Finally, this study was conducted in a cohort of community-dwelling older men, so conclusions may not be generalizable to women, younger adults, or other patient populations.
In summary, we found no significant association of opioid use with falls or incident clinical fractures among older men with persistent musculoskeletal pain. Participants with opioid use had significantly more impaired physical performance, but did not have a greater rate of performance decline. Our findings suggest that additional research is needed to determine whether opioid use is a marker of risk or a cause of risk for falls, fractures, and progressive physical impairment.
References
Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35.
Parsells KJ, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138:507–13.
Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105:1776–82.
Edlund MJ, Martin BC, Fan MY, DeVries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112:90–8.
Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–76.
Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297:77–86.
Masud T, Morris RO. Epidemiology of falls. Age Ageing. 2001;30(Suppl 4):3–7.
Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51:364–70.
Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–93.
Miller M, Sturmer T, Azrael D, Levin R, Solomon DH. Opioid analgesics and the risk of fractures in older adults with arthritis. J Am Geriatr Soc. 2011;59:430–8.
Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310–5.
Takkouche B, Montes-Martinez A, Gill SS, Etminan M. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30:171–84.
Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–60.
Soderberg KC, Laflamme L, Moller J. Newly initiated opioid treatment and the risk of fall-related injuries. A nationwide, register-based, case-crossover study in Sweden. CNS Drugs. 2013;27:155–61.
Eggermont LH, Leveille SG, Shi L, et al. Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc. 2014;62:1007–16.
Bryant LL, Grigsby J, Swenson C, Scarbro S, Baxter J. Chronic pain increases the risk of decreasing physical performance in older adults: the San Luis Valley Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2007;62:989–96.
Reid MC, Williams CS, Gill TM. Back pain and decline in lower extremity physical function among community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:793–7.
Shega JW, Weiner DK, Paice JA, et al. The association between noncancer pain, cognitive impairment, and functional disability: an analysis of the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2010;65:880–6.
Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302:2214–21.
Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–68.
Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85.
Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11.
Everson-Rose SA, Paudel M, Taylor BC, et al. Metabolic syndrome and physical performance in elderly men: the osteoporotic fractures in men study. J Am Geriatr Soc. 2011;59:1376–84.
Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8.
Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–14.
Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33.
Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62.
Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8.
Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58:550–9.
Weitzen S, Lapane KL, Toledano AY, Hume AL, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13:841–53.
Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63.
Varadhan R, Weiss CO, Segal JB, Wu AW, Scharfstein D, Boyd C. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care. 2010;48:S96–105.
Ekholm O, Kurita GP, Hojsted J, Juel K, Sjogren P. Chronic pain, opioid prescriptions, and mortality in Denmark: A population-based cohort study. Pain. 2014;155:2486–90.
Eggermont LH, Shmerling RH, Leveille SG. Tender point count, pain, and mobility in the older population: the mobilize Boston study. J Pain. 2010;11:62–70.
Leveille SG, Bean J, Bandeen-Roche K, Jones R, Hochberg M, Guralnik JM. Musculoskeletal pain and risk for falls in older disabled women living in the community. J Am Geriatr Soc. 2002;50:671–8.
Hudson TJ, Edlund MJ, Steffick DE, Tripathi SP, Sullivan MD. Epidemiology of Regular Prescribed Opioid Use: Results from a National, Population-Based Survey. J Pain Symptom Manag. 2008;36:280–8.
Krebs EE, Lurie JD, Fanciullo G, et al. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. 2010;11:44–52.
Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use. Arch Intern Med. 2006;166:2087–93.
Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–9.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no potential conflicts of interest.
Funding
This material is based on work supported by the National Institutes of Health (NIH) National Institute on Aging (NIA #R03 AG042980) and with resources and facilities of the Minneapolis VA Health Care System. The Osteoporotic Fractures in Men (MrOS) Study is supported by NIH funding from the following institutes: the NIA, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and the NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Rights and permissions
About this article
Cite this article
Krebs, E.E., Paudel, M., Taylor, B.C. et al. Association of Opioids with Falls, Fractures, and Physical Performance among Older Men with Persistent Musculoskeletal Pain. J GEN INTERN MED 31, 463–469 (2016). https://doi.org/10.1007/s11606-015-3579-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-015-3579-9