Abstract
Background
Although the benefits of identifying and treating asymptomatic HIV-infected individuals are firmly established, health care providers often miss opportunities to offer HIV-testing.
Objective
To evaluate whether a multi-component intervention increases the rate of HIV diagnostic testing.
Design
Pre- to post-quasi-experiment in 5 Veterans Health Administration facilities. Two facilities received the intervention; the other three facilities were controls. The intervention included a real-time electronic clinical reminder that encourages HIV testing, and feedback reports and a provider activation program.
Patients
Persons receiving health care between August 2004 and September 2006 who were at risk but had not been previously tested for HIV infection
Measurements
Pre- to post-changes in the rates of HIV testing at the intervention and control facilities
Results
At the two intervention sites, the adjusted rate of testing increased from 4.8% to 10.8% and from 5.5% to 12.8% (both comparisons, p < .001). In addition, there were 15 new diagnoses of HIV in the pre-intervention year (0.46% of all tests) versus 30 new diagnoses in the post-intervention year (0.45% of all tests). No changes were observed at the control facilities.
Conclusions
Use of clinical reminders and provider feedback, activation, and social marketing increased the frequency of HIV testing and the number of new HIV diagnoses. These findings support a multimodal approach toward achieving the Centers for Disease Control and Prevention’s goal of having every American know their HIV status as a matter of routine clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The benefits of identifying and treating asymptomatic HIV-infected individuals are firmly established.1–3 Providing timely antiretroviral medications, immunizations, and prophylactic antimicrobials to HIV-infected individuals vastly reduces mortality and prevents hospitalizations.1,4–6. Testing for HIV infection is highly cost-effective; when HIV prevalence is 1%, the cost of one-time testing is approximately $15,000 per quality-adjusted life-year gained for tested individuals, lower than many accepted clinical preventive services.2,3 Furthermore, knowledge of HIV-infection and treatment-induced reductions in viral replication are both associated with decreases in further HIV transmission caused by behavioral changes and decreased infectivity.7–10
However, while the U.S. Preventive Services Task Force, Centers for Disease Control and Prevention (CDC), Veterans Health Administration (VHA), and other governmental and professional groups have strongly recommended targeted testing of adults with risk factors for HIV infection,11–18 25% of the 1.2 million HIV-infected persons in the United States remain undiagnosed.12 In a similar way, only 30 to 50% of VHA patients with known, documented risk factors for HIV infection have been tested.11,19 Consequently, despite frequent opportunities to establish an early diagnosis of HIV infection during patient visits to outpatient clinics, urgent care clinics, emergency rooms and hospitals, 12% to 43% of newly diagnosed patients are already in the advanced stages of immunodeficiency or have a concurrent acute opportunistic infection.3,20–22
In response to these shortcomings in HIV testing, using a before-and-after study design with concurrent controls, we sought to determine whether a multi-modal intervention that is based on the provision of computerized decision support, provider activation, audit-feedback, and removal of organizational barriers would significantly increase HIV testing rates in at-risk individuals who receive care at VHA medical facilities. This intervention made use of VHA resources, including a universally implemented electronic medical record, an integrated delivery system, and routine performance measurement, which have fostered quality measures that often exceed that of the rest of the U.S. health care system.23,24 We report here the 1-year results of our program.
METHODS
The intervention program was put in place for 1 year in 2 of the 5 administratively independent, geographically separate major regional health care systems (health care systems [HCS] A and B) located in southern Nevada or California, i.e., in Veterans Integrated Service Network 22 (VISN22 5 to 12 facilities in which primary care and specialty services, including mental health and substance abuse treatment programs, were provided by mixtures of academic and non-academic staff physicians, postgraduate medical trainees and mid-level providers. The other control system lacked an inpatient center. At some facilities, care was provided solely by non-academic physicians and mid-level providers. The 2 intervention HCSs had a total of 18 facilities, whereas the control HCSs had a total of 19 facilities. This study was approved by the appropriate Institutional Review Boards.
Components of the Intervention
Decision support
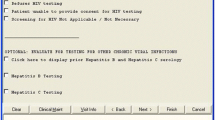
We implemented a real-time, electronic clinical reminder to identify patients at increased risk for HIV infection and to encourage providers to offer HIV testing to such individuals (Fig. 1). Clinical reminders, widely used to implement quality improvement, are well-suited for use in the VHA because of the system-wide computerized patient record. 25,26
Opening screen of HIV testing clinical reminder. The HIV testing clinical reminder appears for all persons with identified HIV risk factors who have not been previously tested for HIV, who have not been otherwise identified as being HIV-infected or for whom there has been no previous indication of HIV testing outside of the VA, refusal of HIV testing, or inability to provide informed consent.
The HIV Testing Clinical Reminder was triggered by any prior evidence of Hepatitis B or C infection, illicit drug use, a sexually transmitted disease (gonorrhea, chlamydia, syphilis, or genital herpes), homelessness, and certain behavioral risk factors (Appendix 1). All data elements were automatically extracted from the VHA electronic medical record. Once triggered, the reminder is resolved by either ordering an HIV test, recording the result of an HIV test performed elsewhere, indicating that the patient is not competent to consent to testing, or specifying that the patient refused HIV testing. Once resolved, the reminder was no longer triggered.
Audit-feedback
We designed an audit-feedback system to inform health care providers of clinic-level performance in regards to HIV evaluation and testing rates in at-risk patients.27 These reports were distributed once per quarter via email to senior and junior managers and clinical leaders responsible for ambulatory care programs and delivery of care at Systems A and B. The contents of the reports were also discussed during academic detailing visits to primary care team meetings and in the social marketing campaign.
Provider activation
The provider activation program included academic detailing, social marketing, and provider and patient educational materials.28–31 The academic detailing component involved regular one-on-one, in-person informal discussions of the basis for and benefits of increased rates of HIV testing by project staff during frequent ad hoc visits to the primary care clinics.32,33 Social marketing involved identifying physician and nursing staff clinical opinion leaders who encouraged HIV testing by primary care health care providers and who also made presentations to substation and clinic leadership.34 Finally, we developed and distributed provider educational hand-outs, pocket cards, and posters to instruct providers on the structure and use of the HIV Testing Clinical Reminder, to promote HIV testing, to make providers aware of HIV risk factors not captured by the reminder (i.e., multiple unprotected sexual contacts), and to further increase provider comfort and abilities to provide pre- and posttest HIV counseling. Whereas providers were encouraged to perform HIV testing, no specific incentive was provided.
Organizational factors
Under Federal Laws specific to the VA, written informed consent and pretest HIV counseling have been, and still are, required for all HIV tests.35 To expedite this process, we encouraged nurse-based rather physician-based pretest counseling36 and the use of a streamlined HIV counseling process that, together with the VHA HIV Consent form, covers all the required elements of HIV pretest counseling and documents consent in 2–3 minutes.37,38 Finally, we reduced the logistical challenges of posttest HIV counseling by encouraging telephone notification and brief posttest counseling after negative HIV test results.12,39–42. To ensure compliance with posttest counseling requirements, we distributed sample scripts for transmitting the results of the test.
Data Sources
We obtained administrative and clinical data from the preexisting VISN22 data warehouse, which included patient demographics, laboratory tests, diagnostic codes, and health factors of inpatient and outpatient encounters from August 2004 to July 2006. Records were linked across the data files by the common patient identifiers. Patients were defined as having been tested for HIV if there was documentation of HIV testing done within the VHA.
Analytic Methods
Our analytic goal was to evaluate the effects of the intervention by comparing pre- to post changes in the rate of HIV testing at the intervention versus the control HCSs. In addition, we were interested in identifying patient, provider, and subfacility-level factors that were associated with HIV testing. To do so, we performed a multilevel logistic regression analysis. We included in the analysis patients who visited VISN22 facilities in the pre (August 2004 to July 2005) and post (August 2005 to July 2006) intervention years. The unit of analysis was a patient who was at risk but had never received HIV testing prior to the year of visit. Patients were considered as at risk for HIV infection if they were diagnosed with hepatitis B or C infections, drug use, sexually transmitted diseases (STD: gonorrhea, chlamydia, syphilis, and genital herpes), had a history of homelessness, or had VHA-defined risk factors for infection by hepatitis C.
The dependent variable in the multi-level logistic regression analysis was the performance of HIV testing. The independent variables included patient, provider, and subfacility-level factors. Patient-level factors included demographic and clinical characteristics such as age, race and ethnicity, marital status, lack of housing, co-payment status, being at risk for hepatitis C (see Appendix), hepatitis C infection, hepatitis B infection, drug use, and STD. Provider-level factor referred to HIV testing performance of the “key” provider whom the patient encountered most frequently. We measured providers’ HIV testing experience by the proportion of at-risk patients whom they tested for HIV during the pre-intervention year. Subfacility-level factors referred to annual patient load, and prevalence of at-risk patients at the “key” subfacility where the patient received primary care most frequently. For each “key” subfacility, we estimated its annual patient load by the number of unique patients who were seen at that subfacility in a year, and its proportion of at-risk patients. The key provider and key subfacility was determined separately for the pre- and post-interventional years. Since patients who visited the same provider tended to receive similar care, we adjusted the covariance matrix of the dependent variable for intra-provider correlations. We addressed 2 questions: whether the intervention significantly improved the rate of HIV testing and whether there was a higher or lower impact of the intervention among patient subgroups. We answered the first question by comparing the pre- to post-adjusted rates of HIV testing between the intervention and control facilities, and the second question by comparing the pre- to post-adjusted odds ratios (OR) of HIV testing among patient subgroups. The data analysis was generated using SAS v9.1 proc genmod (SAS version 9.1. SAS Institute, Cary, NC, USA).
RESULTS
The characteristics of patients with HIV risk factors seen during the intervention period are shown in Table 1. These characteristics were similar in the pre- and post-intervention periods at each HCS (data not shown). When compared to the other HCSs, persons with HIV risk factors at HCS A more often were African Americans, had a history of homelessness, had lower income levels, had a history of sexually transmitted diseases, and had a history of injection drug use. Patients at HCS B were younger and more often had a history of Hepatitis B infection. HCSs C and D had higher proportions of at-risk patients who also had hepatitis C-related risk factors. None of these differences were judged to be meaningful. Provider testing performance before the intervention was low at all facilities; fewer than 2 patients per 100 at-risk patients seen by a provider were tested for HIV. Baseline rates of HIV testing for patients who were at risk but had never been tested varied between 2.2% to 6.6% across the 5 facilities.
Table 2 compares adjusted testing rate from pre-intervention to post-intervention between the intervention and control HCSs. At HCS A, the adjusted HIV testing rate increased from 4.8% to 10.8% (p < .001). Similarly, at HCS B, the adjusted HIV testing rate increased from 5.5% to 12.8% (p < .001). The adjusted magnitude of the increase in the rate of HIV testing was similar across all patient and subfacility strata (Table 3).
As shown in Table 4, the pre-versus-post adjusted OR of HIV testing showed that the intervention worked well in all patient subgroups, and especially so among the patients who were 65 years or older, had HCV risk factors, were seen at subfacilities with annual patient loads <40,000 and prevalence of at-risk patients <15% (pre-versus-post OR > = 2.0). Finally, although the magnitude of the increase in HIV testing was greatest in patients seen in Primary Care clinics, similar increases were seen among patients who received care in mental health and substance abuse clinics (data not shown). In contrast, the adjusted HIV testing rates showed no increases across the 3 control HCSs.
To assess the overall impact of our intervention, we also evaluated the cumulative proportion of at-risk patients receiving care who had ever been tested for HIV infection. This analysis included all at-risk patients receiving care in the time period of interest, including those who had been tested before the implementation of the intervention. As shown in Figure 2, the increases in the proportion of previously untested at-risk patients who were tested for HIV (quarterly rate) were maintained over the first 4 quarters of the intervention, whereas no change was seen at the control HCSs. As a consequence, the proportion of at risk patients who were seen and tested for HIV infection in each quarter or ever previously (i.e., the cumulative rate of HIV testing) steadily increased from 16% in the quarter before the intervention to 25% in the fourth quarter afterward at HCS A and from 22% to 35% at HCS B. Again, no change was observed at the control HCSs. On a yearly basis, among all at-risk patients, the cumulative rate of ever being tested for HIV increased from 20.1% for at-risk persons who received care in the pre-intervention year to 53.7% for such persons in the post- intervention year.
Longitudinal incident and cumulative HIV testing rates at intervention vs control sites. “−1” refers to results in the quarter (90 days) prior to implementation of the intervention, whereas 1–4 refers to results in the 4 subsequent 90-day periods. The quarterly testing rate (striped bars) refers to the proportion of at risk, previously untested patients who received care and were tested for HIV infection during each quarter. The cumulative proportion ever tested (solid bars) is the proportion of at risk patients who received care and who had a previous HIV test. The cumulative rate is sum of the incident and previous testing rates.
Finally, we found that among the 1,906 HIV diagnostic tests done at HCS A during the prior year, there were 12 new diagnoses of HIV infection (0.63%) versus 19 new diagnoses among 3,858 diagnostic tests during the year after the implementation of the intervention (0.49%). Similarly, at HCS B during the prior year there were 3 new diagnoses of HIV infection (0.24% of 1,341 HIV tests) before the intervention versus 11 new diagnoses among 2,793 diagnostic tests afterward (0.39%).
DISCUSSION
We demonstrate that implementation of an integrated package of quality improvement interventions that utilized decision support, academic detailing and audit feedback resulted in a doubling of HIV testing for a population of at-risk individuals who had not previously been tested at 2 large VHA HCSs. No change in HIV testing occurred at the 3 control HCSs. These results were robust with dramatic increases in the likelihood of being tested for HIV being observed across patient-level, provider-level, and subfacility-level factors. Furthermore, the rate of positive HIV tests remained constant despite the doubled rate of testing. In aggregate, the percentage of tests, which resulted in new diagnoses of HIV infection was 0.46% in the pre-intervention year versus 0.45% in the post-intervention year and thus well within the range at which the costs of HIV testing is less than $50,000 per quality-adjusted life year when the societal benefits of testing are considered 2.
Our intervention relied on several components. First, we implemented a real-time computerized clinical reminder to identify patients with risk factors associated with HIV infection. Previous work has shown that the use of clinical reminders in individual patients when combined with audit/feedback and organizational changes improves vaccination, cardiovascular risk reduction, and breast and colorectal cancer screening rates.24–26,43–49 However, the use of clinical reminders alone is generally insufficient to achieve and sustain a transformation of group norms and maximize quality improvement.28–30,43,50–52. Consequently, we also implemented a multi-faceted provider activation program that included academic detailing and social marketing with the aim of increasing the priority with which providers view HIV testing, routinizing the test ordering process, and encouraging providers to routinely test at risk patients HIV.28–30,32–34. We also provided clinic level feedback to health care providers regarding the rate at which HIV screening and testing was performed.31
Several of our interventions were specifically designed to address congressionally mandated legal requirements that VHA patients provide written informed consent for HIV testing and that providers document pre- and posttest counseling.35 As a consequence, many VHA providers have regarded HIV testing to be a time-consuming process that cannot be readily accomplished in the setting of a busy outpatient clinic.53 In response, we developed a streamlined script for HIV pretest counseling that reduces the time required for HIV pretest counseling to 2–3 minutes.38 We also addressed the processes of posttest counseling to specifically allow for telephone notification of negative test results. Studies from other clinical settings including urgent care clinics, emergency departments, and STD clinics have previously shown that HIV testing rates increase after implementation of similar measures.39–41 Our study confirms these results and extends their applicability to a geographically dispersed system of primary care clinics.
The strengths of our study include a quasi-experimental design in which the effect of the intervention was clearly demonstrated in comparison of pre- to post-increases in HIV testing at the intervention HCSs with no pre- to post-changes at the control HCSs. This was very much a real-world effectiveness study that examined the impact of our intervention in an unselected population of at-risk veterans receiving care in a routine clinical setting.
Limitations of our work include the fact that the intervention relied heavily on the quality improvement infrastructure in the VHA, including the electronic medical record, clinical reminder software, and familiarity with performance measurements. This makes it difficult to generalize the intervention to health care systems that do not currently have access to these tools. However, such tools are becoming increasingly common and some components of the intervention, such as provider activation, do not require the infrastructure of an integrated health care system. Another limitation of the design was that we were unable to quantitatively dissect the contributions of the individual elements of our intervention. To address this critical issue, we are undertaking a qualitative process (or formative) evaluation to better understand the influences that impact the success of the intervention; specifically, by identifying contextually relevant factors (i.e., facilitators and barriers) and assessing the degree that behaviors leading to improved testing performance become part of routine practice.54 Areas of particular interest will be to formally evaluate the contribution of nurse-based vs physician-based HIV testing and evaluation as well as the role of intensive provider activation as this is the most costly and time-consuming activity. Finally, since the intervention facilities were selected for convenience and not randomly, this may have biased the results. In this regard, it is relevant that there were little difference in the distribution of patient, provide, subfacility- and facility-level factors between the intervention and control facilities (Table 1).
In summary, we found that the coordinated use of computerized real-time clinical reminders, audit/feedback, provider activation, and removal of systemic barriers significantly increases HIV testing rates and thus allows early diagnosis and treatment for these vulnerable patients. These findings support a multimodal approach toward achieving the CDC’s goal of having every American aged 13–64 regardless of the presence of known risk factors know their HIV status as a matter of routine clinical practice. If sustained, dissemination of this program holds promise of substantial benefit to the VA, the largest single HIV provider in the United States and potentially in other jurisdictions where logistical barriers such as obtaining written informed consent impede implementation of routine opt-out HIV testing as recommended by the CDC.55
References
DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-infected adults and adolescents. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed February 9, 2008.
Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85.
Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95.
Yeni PG, Hammer SM, Hirsch MS, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society—USA Panel. JAMA. 2004;292:251–65.
Goetz MB, Morreale A, Berman S, et al. Effect of highly active anti-retroviral therapy (HAART) on outcomes in Veterans Affairs Medical Centers (VAMC). Clin Infect Dis. 1997;25:396.
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA. Declining morbidity and mortality among patients with advance human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60.
Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9.
Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53.
Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–50.
Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806.
Perlin JB. Need for routine Human Immunodeficiency Virus (HIV) risk assessment and testing. Under Secretary for Health’s Information Letter. IL 10-2005-017. Available at http://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1302. Accessed February 9, 2008.
Anonymous. Screening for HIV: recommendation statement. Ann Intern Med. 2005;143:32–7.
Anonymous. Periodic health examination, 1992 update: 3. HIV antibody screening. Canadian Task Force on the Periodic Health Examination. CMAJ. 1992;147:867–76.
American Academy of Family Physicians. HIV Infection. Available at http://www.aafp.org/online/en/home/clinical/clinicalrecs/hiv.html. Accessed April 4, 2008.
Anonymous. ACOG committee opinion number 304, November 2004. Prenatal and perinatal human immunodeficiency virus testing: expanded recommendations. Obstet Gynecol. 2004;104:1119–24.
Anonymous. Human immunodeficiency virus (HIV) infection. American College of Physicians and Infectious Diseases Society of America. Ann Intern Med. 1994;120:310–9.
Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–29.
Anonymous. Adolescents and human immunodeficiency virus infection: the role of the pediatrician in prevention and intervention. Committee on Pediatric AIDS and Committee on Adolescence. Pediatrics. 2001;107:188–90.
Owens DK, Sundaram V, Lazzeroni LC, et al. HIV testing of at risk patients in a large integrated health care system. J Gen Intern Med. 2007;22:315–20.
Chou R, Huffman LH, Fu R, Smits AK, Korthuis PT. Screening for HIV: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143:55–73.
Cases of HIV infection and AIDS in the United States, 2003. Available at http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2003report/pdf/2003SurveillanceReport.pdf. Accessed February 9, 2008.
Gandhi NR, Skanderson M, Gordon K, Concato J, Justice AC. Delayed Presentation for HIV Care Among Veterans: An Opportunity for Intervention. Conf Retroviruses Opportunistic Infect. 2006;13:Abstract 924
Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348:2218–27.
Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141:938–45.
Patterson ES, Doebbeling BN, Fung CH, Militello L, Anders S, Asch SM. Identifying barriers to the effective use of clinical reminders: bootstrapping multiple methods. J Biomed Inform. 2005;38:189–99.
Patterson ES, Nguyen AD, Halloran JP, Asch SM. Human factors barriers to the effective use of ten HIV clinical reminders. J Am Med Inform Assoc. 2004;11:50–9.
Lomas J, Enkin M, Anderson GM, Hannah WJ, Vayda E, Singer J. Opinion leaders vs. audit and feedback to implement practice guidelines: Delivery after previous cesarean section. JAMA. 1991;265:2202–7.
Thomson O’Brien MA, Oxman AD, Haynes RB, Davis DA, Freemantle N, Harvey EL. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2005;CD000125. DOI 10.1002/14651858.CD000125.
Thomson O’Brien MA, Oxman AD, Davis DA, Haynes RB, Freemantle N, Harvey EL. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2000;CD000409.
Jamtvedt G, Young JM, Kristoffersen DT, Thomson O’Brien MA, Oxman AD. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2003;CD000259.
Jamtvedt G, Young J, Kristoffersen D, O’brien M, Oxman A. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006;19;CD000259.
Collins B, Hawks J, Davis R. From theory to practice: Identifying authentic opinion leaders to improve care. Managed Care. 2000;9:56–62.
Solomon D, Van Houten L, Glynn R, et al. Academic detailing to improve use of broad spectrum antibiotics at an academic medical center. Arch Intern Med. 2001;161:1897–902.
Lefebvre RC, Rochlin L. Social marketing. In: Lewis FM, Rimer BK, eds. Health Behavior and Health Education: Theory, Research, and Practice. 2nd ed. Jossey-Bass; 1988.
VA Policy on Confidential HIV Testing and Counseling. Available at http://www.hiv.va.gov/vahiv?page=prtop02-ov-02#S2X. Accessed February 9, 2008.
Robson J, Boomla K, Fitzpatrick S, et al. Using nurses for preventive activities with computer assisted follow up: a randomised controlled trial. BMJ. 1989;298:433–6.
Pre-Test Counseling and Consent for HIV Testing. VHA-10-0121. Available at http://www.publichealth.va.gov/documents/vha-10-0121-fill.pdf. Accessed February 9, 2008.
Anaya, HD, Golden J, Asch SM. Improving HIV Screening by Nurse Rapid Testing/Streamlined Counseling. Unpublished Work.
Kendrick SR, Kroc KA, Withum D, Rydman RJ, Branson BM, Weinstein RA. Outcomes of offering rapid point-of-care HIV testing in a sexually transmitted disease clinic. J Acquir Immune Defic Syndr. 2005;38:142–6.
Spielberg F, Branson BM, Goldbaum GM, Kurth A, Wood RW. Designing an HIV counseling and testing program for bathhouses: the Seattle experience with strategies to improve acceptability. J Homosex. 2003;44:203–20.
Tsu RC, Burm ML, Gilhooly JA, Sells CW. Telephone vs. face-to-face notification of HIV results in high-risk youth. J Adolesc Health. 2002;30:154–60.
Perry SW, Jacobsberg LB, Fishman B, Weiler PH, Gold JW, Frances AJ. Psychological responses to serological testing for HIV. AIDS. 1990;4:145–52.
Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–51.
Austin SM, Balas EA, Mitchell JA, Ewigman BG. Effect of physician reminders on preventive care: meta-analysis of randomized clinical trials. Proc Annu Symp Comput Appl Med Care. 1994;121–4:121–4.
Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc. 1996;3:399–409.
Hulscher ME, Wensing M, van der Weijden T, Grol R. Interventions to implement prevention in primary care. Cochrane Database Syst Rev. 2001;CD000362.
Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JThMv, Assendelft WJJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2005;CD001481. DOI 10.1002/14651858.CD001481.
Saleem JJ, Patterson ES, Militello L, Render ML, Orshansky G, Asch SM. Exploring barriers and facilitators to the use of computerized clinical reminders. J Am Med Inform Assoc. 2005;12:438–47.
Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765.
Berwick DM. Developing and testing changes in delivery of care. Ann Intern Med. 1998;128:651–66.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–14.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–9.
Centers for Disease Control and Prevention. Advancing HIV Prevention. Interim Technical Guidance for Selected Interventions. Available at http://www.cdc.gov/Hiv/topics/prev_prog/AHP/resources/guidelines/pdf/AHPIntGuidfinal.pdf. Accessed February 9, 2008.
Stetler CB, Legro MW, Wallace CM, et al. The role of formative evaluation in implementation research and the QUERI experience. J Gen Intern Med. 2006;21I(Suppl 2):S1–8.:S1–S8.
Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17.
Acknowledgments
This project was supported by a research grant to Drs. Goetz and Asch by the Veterans Health Administration Health Services Research & Development Service (SDP 06–001).
Conflicts of Interest
Matthew Bidwell Goetz: consultancy with Monogram Biosciences, grant support from Gilead Pharmaceuticals, GlaxoSmithKline; Henry D. Anaya: stock ownership in Trinity Biotechnology, which develops biomarker devices, one of which is a test for the HIV virus, and educational support in the form of unrestricted grants from both Trinity Biotechnology and OraSure Technologies; Allen Gifford: royalties for authorship of Living Well With HIV And AIDS, Ball Publishing Company; Steven Asch: unrestricted travel grant from Trinity Pharmaceuticals. The other authors have no conflicts of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
Triggers for the HIV testing Clinical Reminder
Hepatitis C Infection:
ICD-9 codes
070.41, 070.44, 070.51, 070.54, 070.6, 070.70, 070.71, 070.9, 571.40, 571.41, 571.49, 571.5, 571.8, 571.9, 573.3, 573.8, V02.62
Laboratory tests
Positive HCV antibody test or HCV viral load test
VHA-defined Hepatitis C Risk Factors:
Excessive alcohol use, injection drug use, lack of housing, multiple sexual partners, tattoos, body piercing, receipt of blood/blood products before 1992, unequivocal blood exposure (e.g., in combat), hemodialysis, or unexplained liver disease (including abnormal serum alanine aminotransferase levels). These data are collected through the VHA hepatitis C risk factors dataset.
Hepatitis B Infection:
ICD-9 codes
070.20, 070.21, 070.22, 070.23, 070.3, 070.30, 070.31, 070.32, 070.33, 070.52
Laboratory tests
Positive HBV core antibody test or positive surface antigen
Sexually-transmitted Disease: Includes Gonorrhea, Chlamydia, Syphilis, Herpes
ICD-9 codes
054.10, 054.11, 054.12, 054.13, 054.19, 098.xx, 099.40, 099.41, 099.50, 099.51, 099.52, 099.53, 099.54, 099.55, 099.56, 099.59, 099.8, 099.9, 090.0, 090.1, 090.2, 090.3, 090.40, 090.41, 090.42, 090.49, 099.56, 090.5, 090.6, 090.7, 090.9, 091.0, 091.1, 091.2, 091.3, 091.4, 091.50, 091.51, 091.52, 091.61, 091.61, 091.69, 091.7, 091.81, 091.82, 091.84, 091.89, 091.9, 092.0, 092.9, 093.0, 093.1, 093.20, 093.21, 093.22, 093.23, 093.24, 093.81, 093.82, 093.89, 093.9, 094.0, 094.1, 094.2, 094.3, 094.51, 094.52, 094.7, 094.81, 094.82, 094.83, 094.84, 094.85, 094.86, 094.87, 094.89, 094.9, 095.0, 095.1, 095.2, 095.3, 095.4, 095.5, 095.6, 095.7, 095.8, 095.9, 096.0, 097.0, 097.1, 097.9
Laboratory tests
None
Drug Abuse:
ICD-9 codes
304.00, 304.01, 304.02, 304.03, 304.20, 304.21, 304.22, 304.23, 304.40, 304.41, 304.42, 304.43, 304.60, 304.61, 304.62, 304.63, 304.70, 304.71, 304.72, 304.73, 304.90, 304.91, 304.92, 304.93, 305.50, 305.51, 305.52, 305.53, 305.60, 305.61, 305.62, 305.63, 305.70, 305.71, 305.72, 305.73, 305.90, 305.91, 305.92, 305.93
Laboratory tests
None
HIV Infection
ICD-9 codes
042., 042.1, 042.2, 042.9, 043.0, 043.1, 043.2, 043.3, 043.9, 044.9, 079.53, V08
Laboratory tests
Positive HIV antibody test or viral load
Homelessness
ICD-9 codes
V60.0
Laboratory tests
None
Rights and permissions
About this article
Cite this article
Goetz, M.B., Hoang, T., Bowman, C. et al. A System-wide Intervention to Improve HIV Testing in the Veterans Health Administration. J GEN INTERN MED 23, 1200–1207 (2008). https://doi.org/10.1007/s11606-008-0637-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-008-0637-6