Abstract
Objectives
To evaluate gender differences in initial presentation, pathology and outcomes with GC (GC).
Methods
The 1973–2013 Surveillance Epidemiology and End Results (SEER) 17-registry database was analysed for renal tumours from 1973 to 2013 coded as primary site “stomach”. After various exclusions, a final study group of 99,922 cases with complete data was obtained. Demographic variables analysed included age, sex, marital status and race. Tumour variables included size, stage at diagnosis, grade, primary site, treatment and histology. Primary outcome variables included overall survival (OS) and cancer-specific survival (CSS).
Results
Overall, 96,501 gastric cancer patients were identified. Of those, 34,862 (36.2%) were women. For woman, log-rank test showed that OS and CSS were significantly longer in man (p < 0.0001). In Cox regression analysis, woman was associated with a significantly improved OS [(HR of death in 1973 to 2003 = 0.87, 95% CI = 0.85–0.89, P < 0.001) (HR of death in 2004 to 2013 = 0.94, 95% CI = 0.91–0.97, P < 0.001)] and cancer-specific survival [(HR of death in 1973 to 2003 = 0.90, 95% CI = 0.87–0.92, P < 0.001) (HR of death in 2004 to 2013 = 0.90, 95% CI = 0.87–0.93, P < 0.001)]. When performing a Kaplan-Meier curve analysis after propensity score matching, gender persisted to be a significant survival of woman for OS and CSS.
Conclusions
Men present with larger, higher stage, higher grade GC than women. OS and CSS are better in women, which is significantly different.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the third most common cause of cancer-specific death around the world, although the incidence has decreased in the last few decades. An estimated 951,600 GC cases were newly diagnosed, and 723,100 deaths attributable to GC occurred in 2012 throughout the world.1 The 5-year relative survival rate of stomach cancer varies widely, ranging from 10 to 30% in western countries and from 50 to 70% in East Asia.2
Multimodality therapy including surgery, chemotherapy and radiation is widely accepted for the treatment of stomach cancer. However, recurrence and metastasis are common, and the long-term prognosis of gastric cancer remains poor.3 Chronic infection with Helicobacter pylori, Epstein–Barr virus infection, dietary patterns, and some environmental factors (smoking, alcohol and obesity) have been identified as risk factors for stomach cancer.4 Additionally, GC rates are approximately twice as high in men as those in women and vary widely across countries. The estimated mortality rates for GC are 9.2 per 100,000 for males and 4.2 per 100,000 for females in developed countries.5
Gender differences in incidence and mortality have been observed in several types of cancer. Leoncini et al. demonstrated that in head and neck cancer, male gender was positively associated with tumour recurrence.6 Uhlig et al. suggested that female gender was an independent predictor for poor cancer-specific survival (CSS) and overall survival (OS) in bladder cancer.7 However, the role of gender in stomach cancer remains unclear. This study investigated the effect of gender on CSS and OS in gastric cancer patients using the Surveillance, Epidemiologic and End Results (SEER) database.
Materials and Methods
We conducted the primary cohort analyses according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting observational studies in epidemiology.8
Study Population
This study used SEER*Stat software to identify cancer patients in the SEER database who were coded with the primary site “stomach” from 1973 to 2013. The SEER database is managed by the National Cancer Institute, which provides access to data on 18 population-based cancer centres located throughout the USA, accounting for approximately 26% of the US population. A total of 150,265 cases were coded with the primary site stomach.
Participants
Patients were excluded if (1) their information was incomplete, (2) a non-pathological diagnosis was provided, (3) race was not recorded and (4) tumour size was not recorded. The flow diagram of patient selection is shown in Fig. 1. Finally, we included 96,501 patients. Because the TMN database appeared in the SEER database in 2004, to ensure the integrity of the data, we compared the data from 1973 to 2003 and from 2004 to 2013 separately.
Variables
The demographic variables of the patients in 1973–2003 included age, gender, ethnicity and marital status. The tumour variables included diagnostic stage, SEER grade, histology, tumour location and treatments, including surgery and radiotherapy. The demographic variables and treatment variables of the patients in 2004–2013 were the same as those of the patients in 1973–2003. The tumour variables included diagnostic stage, TMN classification, histology, tumour location and tumour size. Histological results were classified based on the International Classification of Oncology (Third Edition (ICD-O-3)) system. The primary outcome variables were OS and CSS. SEER staging includes the following categories: localized, regional, distant and unstaged. TMN stages are classified according to 2004 AJCC standards. A description of SEER staging can be found on the official SEER website (http://seer.cancer.gov/manuals/CD2.SEERDic.pdf).
Data Sources/Measurement
This study is based on data from the SEER database (http://seer.cancer.gov/), which does not contain any identifiers and is publicly available. Due to the retrospective nature of the study, informed consent is not required. The analysis does not involve interactions with human subjects or the use of personally identifiable information. Prior to the analysis, patient records and data were anonymized and de-identified, and the methods were implemented in accordance with approved guidelines.
Bias
Selection bias due to gender differences is unavoidable. Social factors related to gender may lead to different follow-up outcomes. Given that this study is a retrospective investigation, data collection was not strictly randomized, and the presence of confounding factors is unavoidable.
Quantitative Variables and Statistical Methods
Continuous variables are expressed as the mean, and categorical variables are expressed as percentages. For differential statistical analyses, two-category variables were analysed by the chi-squared test, and continuous variables were assessed using t tests. Univariate analysis of continuous and binary categorical dependent variables was performed using simple linear analysis and logistic regression analysis. OS and CSS were calculated using the Kaplan-Meier method. Log-rank tests were used only for comparisons between groups, and the results are presented as survival curves. Univariate analysis was performed using the Cox proportional hazard model, and variables with statistical significance were introduced into a multivariate analysis. We included all factors with an impact on the survival prognosis for propensity score matching (PSM) (Fig. S1 and S2). Variables with meaningful univariate analysis results were subjected to PSM at a 1:1 ratio to achieve rigorous matching (calliper value 0.0000001), and total survival time and tumour-specific survival time were calculated again. The test level was α = 0.05. P < 0.05 was considered statistically significant. The above data were statistically analysed using SPSS 22.0 statistical software.
Results
Demographic Data
From 1973 to 2013, a total of 96,501 GC patients were documented in the database, including 61,639 (63.8%) male patients and 34,862 (36.2%) female patients (Tables 1 and 2). A total of 58,627 (60.8%) patients were diagnosed from 1973 to 2003, while 37,874 (39.2%) GC patients were diagnosed from 2004 to 2013. Compared with males, females were more often older and divorced and had more advanced tumours and higher tumour stages (P < 0.001). A low tumour grade, fewer white men, a lower incidence of adenocarcinoma and a lower incidence of distant metastases. Tables 1 and 2 show the gender differences in the demographic data of the GC patients. Male and female GC patients in the 1973–2003 cohort were matched via PSM. Variables with evident effects on prognosis and imbalanced basic data were included as covariates in the logistic regression model. After 1:1 matching, 13,630 male and female patients were analysed according to subgroups. No statistically significant differences were found between the two groups, which were comparable. Using the same method, 4325 female patients and 4325 male patients in the 2004–2013 cohort were matched. No statistically significant differences were found between the two groups, which were comparable.
Survival Analysis
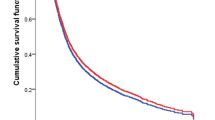
Figures 2a, b, 3a, b show Kaplan-Meier curves of OS and CSS for male and female patients. Women had better OS and CSS compared with men. Figure 4 displays the trends for the median OS and CSS values for men and women (P < 0.0001). The median OS and CSS significantly improved over time in both subsets, but the OS and CSS rates of women were higher than those of men. In an unadjusted Cox proportional hazard regression analysis, female gender was identified as a protective factor for OS [(HR of death in 1973 to 2003 = 0.94, 95% CI = 0.93–0.96, P < 0.001) (HR of death in 2004 to 2013 = 0.91, 95% CI = 0.89–0.93, P < 0.001)] and CSS [(HR of death in 1973 to 2003 = 0.93, 95% CI = 0.91–0.95, P < 0.001) (HR of death in 2004 to 2013 = 0.90, 95% CI = 0.87–0.92, P < 0.001)] (Fig. S1A, B, C, D). After multivariable risk adjustment in the Cox proportional hazard regression analysis, female gender had a significant protective effect on OS [(HR of death in 1973 to 2003 = 0.87, 95% CI = 0.85–0.89, P < 0.001) (HR of death in 2004 to 2013 = 0.94, 95% CI = 0.91–0.97, P < 0.001)] and CSS [(HR of death in 1973 to 2003 = 0.90, 95% CI = 0.87–0.92, P < 0.001) (HR of death in 2004 to 2013 = 0.90, 95% CI = 0.87–0.93, P < 0.001)] (Fig. S2A, B, C, D).
Kaplan-Meier CSS after the diagnosis of GC. The mean survival time for female patients was significantly prolonged compared with that for male patients (P < 0.001). a, b Kaplan-Meier curves for OS in female patients and male patients in 1973–2003. b, d Kaplan-Meier curves for CSS in female patients and male patients in 1973–2003. a and b reflect unadjusted results, while c and d reflect adjusted PSM
Kaplan-Meier CSS after the diagnosis of GC. The mean survival time for female patients was significantly prolonged compared with that for male patients (P < 0.01). a, c Kaplan-Meier curves for OS in female patients and male patients in 2004–2013. b, d Kaplan-Meier curves for CSS in female patients and male patients in 2004–2013. a and b reflect unadjusted results, while c and d reflect adjusted PSM
Adjustments for Patient Characteristics with Propensity Score Matching
Female patients were more often older and divorced and had more advanced tumours and higher tumour stages (Tables 1 and 2). To further corroborate the findings from the univariate and multivariable Cox proportional hazard regression analyses, a propensity score with a stratification factor was calculated to optimally adjust for the aforementioned bias between the two groups. In the Kaplan-Meier curve analysis after PSM, female gender remained a significant contributor to prolonged OS and CSS (Figs. 2c, d, 3c, d).
Discussion
Summary of the Findings
In this study, we analysed 150,265 GC patients in the SEER database, with males accounting for 60.9% of the sample and a male-to-female ratio of 1.56:1. This ratio is similar to those in several prior studies, indicating that the incidence of GC in men is almost twice as high as that in women, and women with GC have smaller tumours and lower tumour grades. The incidence of regional or metastatic spread is higher in male patients, and this difference in tumour characteristics may be the most important reason why male GC patients have lower survival rates than females. In this study, we found that males had relatively poor survival after PSM. According to the SEER data, female GC patients appear to exhibit greater tumour differentiation and lower tumour stages. Interestingly, the female GC patients were older than their male counterparts, and age has an impact on prognosis, but why do women generally have a favourable outcome? The reason is not clear but may be associated with the lower grades and smaller sizes of tumours. Few studies are available on the tumour characteristics of GC in different genders, but Aron et al.9 studied patients with renal cell carcinoma in the SEER database and found that the tumour sizes and tumour stages of females are lower than those of males, which is similar to the results for the GC groups that we studied. Mungan et al.10 studied the effects of gender on bladder cancer and found that women with bladder cancer have higher tumour stages than male patients, which is different from the results of our GC study and those of the study by Aron et al.9 This finding may be explained by the diversity of different tumours.
Comparison with Previous Work
Few studies have examined the prognosis of GC in different genders. Song et al.11 studied 80–90% of cancer cases in more than 150 large hospitals in Korea from 1975 to 1999, including 189,853 male and 90,902 female GC patients. Prognostic analysis revealed that the prognosis of female patients with GC was better than that of male patients. The results of the study are similar to our findings, which unfortunately did not match the results for differences in the variables. Another large sample of GC patients was recruited in two hospitals in Severance and Gangnam Severance, South Korea. Kim et al.12 analysed 4722 patients undergoing radical gastrectomy, including 3136 males and 1586 females. The study found that younger female patients had a higher incidence of signet ring cell carcinoma than men, and the prognosis of women with GC was worse than that of men, but his study did not balance the variables of the two groups, and the conclusions may have some bias. Aron et al.9 found that OS among male patients with renal cell carcinoma was longer than that among women, but no significant difference in CSS was found. The results of Mungan et al.10 revealed that the prognosis of male bladder cancer patients is better than that of female patients. Yang et al.13 studied the survival prognosis of patients with colorectal cancer by meta-analysis. The prognosis of female colorectal cancer patients was significantly better than that of males. We also found that the OS and CSS of female patients with GC were higher than those of male GC patients.
Possible Explanations for the Findings
Recent studies have found that androgen receptors play an important role in GC. Endogenous factors, such as sex hormones, have been suggested to play a key role in providing protection to women or increasing the GC risk for men. The expression of the AR is closely related to the prognosis of patients with GC.14 Some in vivo or in vitro studies have found that AR not only accepts the action of androgens but also interacts with other molecules to play the role of oncoprotein, including regulation of cell proliferation and tumour growth.15 Tumour AR receptors activate cyclin-dependent kinases (CDKs), which allow cells to proliferate in different directions, resist apoptosis and promote angiogenesis.16 AR activates the vascular endothelial growth factor (VEGF) gene, promotes massive synthesis of tumour blood vessels and provides adequate blood supply.17 In 60% of patients with GC, the expression of the tumour suppressor gene p53 is inhibited.18 Abnormal expression of p53 is a major cause of tumorigenesis. In addition to inhibiting cell growth, p53 also regulates chromatin. Binding harnesses the specificity of the AR to control its activity.19 p53 can control the activity of the AR, but the AR can interfere with p53-controlled DNA damage sensing and repair systems, leading to tumour proliferation and differentiation.20 p27 can inhibit cells from entering the cell cycle, while more than half of GC cells lack p27 expression, and loss of p27 is a major cause of a poor prognosis in GC. The AR can inhibit the expression of p27, leading to differentiation and proliferation of GC cells.15 Carcinogenic β-catenin is composed of the cadherin complex subunit and is considered an important activator of the Wnt signalling pathway, which plays an important role in the development and poor prognosis of GC.21 Upon activation, β-catenin is transferred to the nucleus to bind to transcription factors of the T cell factor (TCF)/LEF family, regulating target gene expression and cell proliferation. This process can be activated by the AR, thus resulting in greater effects.22 Compared with female patients, a series of reactions caused by androgen through the AR may be an important cause of the poor prognosis in male patients. GC has also been confirmed to be a male-dominant tumour, especially in liver cancer, bladder cancer and pancreatic cancer.
Strengths and Limitations
Some limitations exist to our study. First, the SEER database does not clarify some important biochemical test indicators. Chemotherapy is a key factor affecting the survival of tumours, but the SEER database did not provide detailed information on postoperative adjuvant chemotherapy and neoadjuvant therapy. Second, these data are observational, and observed associations are susceptible to confounding by unmeasured clinical factors. In the experimental research, in addition to the processing factors that have an impact on the experimental results, some non-processing factors, also known as confounding factors, remain and will also have an impact on the research results. According to this study, age, race, marital status, the primary tumour site, the tumour differentiation extent, treatment mode and tumour staging are all important factors affecting the survival and prognosis of patients. The impacts of these confounding factors between different genders may affect our results. PSM can effectively reduce the confounding effect, balance the difference between the treatment group and the control group, use non-randomized group data to estimate the relationship between treatment factors and outcomes and facilitate assessment of the treatment effect.23 We can only perform matching analyses with known confounding factors, and the influence of some unclear confounding factors is still unknown. Therefore, additional randomized controlled studies that will further validate our results are anticipated.
Conclusion
Through a retrospective study of a large sample of GC patients, we found that the effect of gender on GC is significant, and the prognosis of female GC patients is better than that of male GC patients. The results indicated that gastric cancer is characterized by gender differences. Compared with women, the prognosis of GC and the risk of developing GC are significantly worse in men. In our future clinical practice, we should promptly intervene and screen high-risk male patients, which is beneficial to our early intervention. Although confounding factors existed in this study, we cannot easily ignore the impact of gender on GC. Identifying the causes of the differences in male and female GC patients will help us detect and treat GC in the early stage.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. Jan-Feb 2014;64(1):9–29.
Matsuda T, Saika K. The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Japanese journal of clinical oncology. Nov 2013;43(11):1157–1158.
Rawicz-Pruszynski K, van Sandick JW, Mielko J, Cisel B, Polkowski WP. Current challenges in gastric cancer surgery: European perspective. Surgical oncology. Dec 2018;27(4):650–656.
Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. May 2014;23(5):700–713.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European journal of cancer (Oxford, England : 1990). Apr 2013;49(6):1374–1403.
Leoncini E, Vukovic V, Cadoni G, et al. Tumour stage and gender predict recurrence and second primary malignancies in head and neck cancer: a multicentre study within the INHANCE consortium. European journal of epidemiology. Dec 2018;33(12):1205–1218.
Uhlig A, Seif Amir Hosseini A, Simon J, et al. Gender specific differences in disease-free, cancer specific and overall survival after radical cystectomy for bladder cancer: a systematic review and meta-analysis The Journal of urology. Jul 2018;200(1):48–60.
Fitchett EJA, Seale AC, Vergnano S, et al. Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. The Lancet. Infectious diseases. Oct 2016;16(10):e202-e213.
Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. European urology. Jul 2008;54(1):133–140.
Mungan NA, Aben KK, Schoenberg MP, et al. Gender differences in stage-adjusted bladder cancer survival. Urology. Jun 2000;55(6):876–880.
Song M, Kang D, Yang JJ, et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. Jul 2015;18(3):580–589.
Kim HW, Kim JH, Lim BJ, et al. Sex disparity in gastric cancer: female sex is a poor prognostic factor for advanced gastric cancer. Annals of surgical oncology. Dec 2016;23(13):4344–4351.
Yang Y, Wang G, He J, et al. Gender differences in colorectal cancer survival: a meta-analysis. International journal of cancer. Nov 15 2017;141(10):1942–1949.
Gan L, He J, Zhang X, et al. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC cancer. Dec 2 2012;12:566.
Fang Z, Zhang T, Dizeyi N, et al. Androgen receptor enhances p27 degradation in prostate cancer cells through rapid and selective TORC2 activation. The Journal of biological chemistry.Jan 13 2012;287(3):2090–2098.
Liu X, Gao Y, Ye H, et al. Positive feedback loop mediated by protein phosphatase 1alpha mobilization of P-TEFb and basal CDK1 drives androgen receptor in prostate cancer. Nucleic acids research. Apr 20 2017;45(7):3738–3751.
Yoshida S, Aihara K, Ikeda Y, et al. Androgen receptor promotes sex-independent angiogenesis in response to ischemia and is required for activation of vascular endothelial growth factor receptor signaling. Circulation. Jul 2 2013;128(1):60–71.
Ozguroglu M, Demir G. Expression of p53 protein and resistance to preoperative chemotherapy in locally advanced gastric carcinoma. Cancer. Aug 1 1999;86(3):547–549.
Koivisto PA, Rantala I. Amplification of the androgen receptor gene is associated with P53 mutation in hormone-refractory recurrent prostate cancer. The Journal of pathology. Jan 1999;187(2):237–241.
Wu W, Karelia D, Pramanik K, et al. Phenylbutyl isoselenocyanate induces reactive oxygen species to inhibit androgen receptor and to initiate p53-mediated apoptosis in LNCaP prostate cancer cells. Molecular carcinogenesis. Aug 2018;57(8):1055–1066.
Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. The Journal of clinical investigation. Aug 2011;121(8):3159–3175.
Grumolato L, Liu G, Haremaki T, et al. beta-Catenin-independent activation of TCF1/LEF1 in human hematopoietic tumor cells through interaction with ATF2 transcription factors. PLoS genetics. 2013;9(8):e1003603.
Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Statistics in medicine. Sep 10 2010;29(20):2137–2148.
Author information
Authors and Affiliations
Contributions
CZ and HL conceived and designed the study. ZW, CW, WC and YH performed the statistical analysis. HL and ZW wrote the paper. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huafu Li and Zhewei Wei are joint first authors.
Electronic Supplementary Material
ESM 1
Univariate Cox Analyses of GC Patients According to Various Clinicopathological Variables. A and B, Analyses of OS in 1973–2003 and 2004–2013. C and D, Analyses of CSS in 1973–2003 and 2004–2013. (ZIP 8.89 mb)
ESM 2
Multivariate Cox Analyses of GC Patients According to Various Clinicopathological Variables. A and B, Analyses of OS in 1973–2003 and 2004–2013. C and D, Analyses of CSS in 1973–2003 and 2004–2013. (ZIP 7.33 mb)
Rights and permissions
About this article
Cite this article
Li, H., Wei, Z., Wang, C. et al. Gender Differences in Gastric Cancer Survival: 99,922 Cases Based on the SEER Database. J Gastrointest Surg 24, 1747–1757 (2020). https://doi.org/10.1007/s11605-019-04304-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-019-04304-y