Abstract
Background
A gender difference in survival has been documented in colorectal cancer (CRC) patients, although the underlying mechanism remains undefined. This study aimed to gain improved insight into this difference, with a special focus on improved cancer-specific survival.
Methods
The study population consisted of 82,402 patients with invasive CRC who had undergone surgery in Japan between 1985 and 2004. To estimate improved survival, multivariate adjustment using patient demographics and tumor characteristics was performed.
Results
Patient characteristics changed over time. The 5-year survival rates increased from 66.5 to 76.3 % during the study period. Higher survival rates persisted in women over time (multivariate-adjustment model—hazard ratio [HR] 0.87, 95 % confidence interval [CI] 0.85–0.90). Patients who received surgery during the period 2000–2004 had significantly longer survival than those during the period 1985–1989 (men: HR 0.70, 95 % CI 0.67–0.74; women: HR 0.72, 95 % CI 0.67–0.76). However, there was no gender difference regarding improved survival.
Conclusions
A reduced risk of cancer-specific death for women relative to men persisted over time; however, enhancement of survival was equally observed in both genders. Identification of factors associated with gender differences and changes over time in CRC survival may serve as targets for further improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) remains a serious health problem. It is the second most common cancer in women and the third in men, accounting for ≥1.3 million newly diagnosed cases annually worldwide [1]. Current estimates indicate that the incidence of CRC could increase to >2 million in the near future [1]. In contrast to such a steeply increasing incidence, a downward trend in CRC mortality has been observed in some countries, suggesting a potential improvement in CRC survival in recent years [2, 3]. According to the CONCORD-2 study that analyzed survival regarding 11 common cancers in >25 million cases collected from 67 countries, the CRC survival rate increased over the period from 1995−2009, and the 5-year relative survival rates >60 % in 22 countries [4]. A report from the Epidemiology and End Results (SEER) program in the United States indicated an indisputable improvement in CRC survival between 1970 and 1973 and between 2004 and 2010 [5]. In particular, the 5-year relative survival rates of patients with colon and rectal cancer increased by 14.8 and 19.7 %, respectively, during these periods [5]. In Japan, small but significant improvements in the 5-year relative survival rate of 1.2 % for colon cancer and 2.9 % for rectal cancer, between 1993 and 1996 and between 2003 and 2005 were reported in a study involving a nationwide population-based cancer registry [6].
Such improvements in CRC survival are probably attributable to substantial advances in early detection and treatment of this disease over the past few decades. For instance, the survival benefit of adjuvant chemotherapy for stage III disease has been augmented by progress in medical therapy [6]. For metastatic disease, improvements in survival have been shown to be associated with the increasing use of metastasectomy and active chemotherapeutic regimens such as FOLFOX and FOLFIRI involving the use of molecular-targeting agents [7].
In Japan, male to female ratios for both age-adjusted CRC incidence and mortality rates have been reported to exceeded 1.7 in 2011 [8, 9]. In general, the incidence of CRC and mortality for women is lower than that for men worldwide, although there is some geographical variation [1]. However, mortality is not a direct indicator of survival, because it is affected by both incidence and individual fatality. Recently, some studies have evaluated gender differences in CRC survival and have indicated that women tend to have a higher CRC survival rate than men. However, few studies have addressed the time trend in gender differences with respect to CRC survival. To gain a better insight into gender differences regarding CRC survival with a special focus on the time trend in Japan, we analyzed the large-scale multi-institutional database of the Japanese Society for Cancer of the Colon and Rectum (JSCCR). Given that the impact of advances in oncology concerning CRC differs with gender, we should be vigilant regarding the causative factors of the disparities in providing appropriate health care for all patients.
Patients and methods
Data sources and study population
We used the database of the JSCCR. The JSCCR has a hospital-based CRC registration system that was initiated in 1980 [10]. The member hospitals of the JSCCR located throughout Japan voluntarily register information on CRC patients who are treated at each hospital. Data are presented regarding initial surgical treatment following CRC diagnosis and post-operative surveillance. The database currently contains information on approximately 170,000 CRC patients treated between 1974 and 2005. Because the registry data were mainly collected from the surgical department of each member hospital, the subject of this study was limited to patients who had undergone surgical resection for primary CRC. The study population consisted of 82,402 patients with invasive adenocarcinoma of the colon and rectum who underwent surgical resection between 1985 and 2004. Demographic (gender and age) and tumor characteristics were obtained from the database including tumor subsite, tumor histological types, pathological depth of tumor invasion (pT), pathological lymph node status (pN), and pathological stage (pStage). We excluded patients with unspecified gender and age, cancers that originated from the appendix and anal canal, unknown pathological stage, and unknown follow-up status.
The present study was reviewed and determined to be exempt from evaluation by the institutional review board at Tochigi Cancer Center, because it used pre-existing data with no personal identifiers.
Study variables and data analysis
The primary independent variable in this study was gender (male or female). CRC was divided into three anatomic site groups—the right colon, left colon and rectum. The right colon included the cecum, ascending colon and transverse colon. The left colon included the descending colon and sigmoid colon. The rectum included the rectosigmoid, upper rectum and lower rectum. The histological type of tumor was divided into three categories—well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and the combined category of poorly-differentiated adenocarcinoma, mucinous carcinoma and signet-ring cell carcinoma. We used the TNM staging system of the UICC/AJCC classification with the exception of the N category; this was because the pN category was described in accordance with the Japanese classification, and it was not possible to translate it into the UICC/AJCC classification. The time of surgery was described according to four periods with 5-year increments (1984–1989, 1990–1994, 1995–1999 and 2000–2004). The summary statistics for each variable were compiled using frequency and proportion, and the P values for trend were calculated using the chi-squared test for linearity.
The primary outcome of interest was the measurement of cancer-specific survival (CSS), which was calculated in months relative to the date of surgery, and patients who died of CRC only were defined as an event. Five-year CSS probabilities for each period of surgery according to the selected variables were calculated using the Kaplan–Meier method; the statistical significance of the differences was tested using the log-rank test, with the length of follow-up being truncated at 60 months. Cox-proportional models were fitted to determine the association between the investigated variables and cancer-specific death by calculating hazard ratios (HR) and 95 % confidence intervals (CI). The multivariate-adjusted HRs with P values of <0.05 were considered as being potential confounders. Subsequently, multivariate-adjusted HRs for cancer-specific death associated with gender by age strata were evaluated. Finally, to assess gender differences regarding improvement in CRC survival over time, multivariate-adjusted HRs for cancer-specific death associated with the gender and age for patients who had surgery during 1990–1994, 1995–1999 and 2000–2004, as compared with those in 1985–1989 were calculated. In this analysis, all models were adjusted for gender, age, tumor subsite, histological type and pT and pN status, if applicable. Possible interaction of each variable was assessed using the likelihood ratio test in the Cox models. All statistical analyses were performed using SPSS Statistics version 22 (IBM Corporation, Somers, NY, USA). All statistical tests were two-sided, and statistical significance was defined when the P value was <0.05.
Results
Table 1 details the changes in the demographic and tumor characteristics stratified by gender. Of these, 59.0 % occurred in men and 41.0 % occurred in women. During this period, the distribution of all of the investigated variables in both genders significantly changed over the 20-year study period. In general, the percentage of patients aged ≥70 years, right colon cancer, and moderately differentiated cancer increased, whereas that of patients aged ≤59 years, rectal cancer, and well-differentiated adenocarcinoma decreased. The percentage of patients with pStage I disease increased from 16.2 to 21.1 %, whereas the percentage of patients with pStage III plus IV was consistently nearly half of the entire patient population in each period. In every pStage, pT shifted towards a lower grade, and this was also true for pN in pStage III (Table 1). In a given pStage, the range of cancer extent expressed in terms of pT and/or pN diminished over time, although the rate of increase differed slightly between the genders. The changes in the male to female ratio according to tumor subsite are illustrated in Fig. 1. Although the ratio as a whole did not essentially change, it increased significantly for rectal cancer over time.
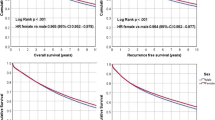
The 5-year overall survival and CSS rates for the entire cohort in this study increased from 61.6 and 66.5 %, respectively (1985–1989) to 71.5 and 76.3 %, respectively (2000–2004), and both ultimately >70 % (Fig. 2). Changes in the 5-year CSS rates by age, tumor subsite, histological type and pStage stratified by gender are detailed in Table 2. The 5-year CSS rates of all the investigated variables increased significantly between 1985–1989 and 2000–2004. The higher 5-year CSS rates in women than in men persisted in every subset of the age group, tumor subsite, histological type and pStage, with the exception of patients aged ≤59 years and patients with right colon cancer. The 5-year CSS declined with increasing age, with the lowest rates observed among patients aged >80 years. As for tumor subsite, the CSS rate was highest in patients with left colon cancer.
The results of univariate analysis regarding the identification of factors affecting the CSS are detailed in Table 3. All of the investigated variables, namely gender, age, tumor subsite, histological type and pStage, were associated with CSS and were added in a forward stepwise (likelihood ratio) fashion in the multivariate regression model. The final step, with associated HRs, is shown in Table 3. In this model, differences in CSS between both genders were statistically significant (HR 0.92, 95 % CI 0.90–0.95). As expected, the pStage was the most robust factor affecting CSS followed by histological type. Tumor subsite also significantly affected CSS. Regarding age, the HR for patients aged ≥60 years significantly increased as compared with their younger counterparts. In the light of the confounding effects of these variables on CSS, the subsequent analyses of them were adjusted when applicable. Furthermore, pT and pN status were used as covariates instead of pStage, because the proportion of patients with pT and pN status at a given pStage changed over time (Table 2).
Adjustment of these variables resulted in a significant survival advantage for women with an HR of 0.87 (0.85–0.90). This was true for women in every age group except for patients aged <50 years, but the degree of reduction differed by age group (Fig. 3). Gender stratified multivariate-adjusted HRs and the 95 % CI for cancer-specific death, associated with year of surgery according to age group, are presented in Fig. 4 (data are given in Online Resource). Significant improvements shown by a decrease in the adjusted HR over time were observed for every age group for both genders, with the exception of female patients aged >80 years. The degrees of improvements in CSS differed among the age groups (the test for the interaction indicated significant differences), whereas there was no apparent relationship between gender and age gradient. Moreover, the degree of improvement did not differ between men and women (for interaction P = 0.94).
Additionally, the proportion of male and female patients with pStage III colon cancer who received adjuvant chemotherapy was compared (Table 4). Although the type of regimen and duration of adjuvant chemotherapy was not investigated in this database, there was no significant difference with regard to whether or not any adjuvant chemotherapy was administrated. Although it is noteworthy that there was a decreasing trend in administration of chemotherapy for pStage III colon cancer, factors affecting this trend were not investigated in this study.
Discussion
The present study demonstrated that the distribution of the baseline characteristics of CRC apparently differ between men and women, and change over time. The most pronounced difference was a rightward shift of tumor site, i.e., an increase in right colon cancer in women. The right colon has become the top subsite for women and the third for men. The rightward shift has already been documented, not only in Western countries but also in Asian countries [11, 12]. The present study also revealed a widening gap in the incidence of rectal cancer between men and women, which is in accordance with findings already reported in Western countries. Together with a rightward shift, this may suggest that the Japanese population shares etiologic and clinical characteristics with Western countries. Differences in the distribution of histological type and pStage between both genders were statistically significant. However, the clinical implication of these differences may be limited because of their very narrow margins. The trend towards patients presenting with earlier stage disease at surgery was observed in both genders in accordance with our previous study [10]. Furthermore, it is noteworthy that a shift towards lower pT and pN categories was evident in a given pStage.
In the current study cohort, overall CSS steadily increased over time in both genders, and the 5-year CSS rate increased by 9.8 % between 1985 and 1989 and between 2000 and 2004. Of these, women had a slight but significantly higher overall CSS rate than men; this was true for every stratified subset of the age groups, tumor subsite, histological type and pStage. In the multivariate adjustment model, a significantly better prognosis was demonstrated in women, with a 13 % reduction in HR for cancer-specific death as compared with men, and the difference widened with increasing age. This higher 5-year CSS rate in women was similar to those reported in recent studies [13–15]. McArdle et al. reported that both overall survival and CSS were significantly higher in women, among patients who underwent elective surgery and among those who underwent curative resection; this was true even if the results were adjusted by several covariates including age, tumor subsite and stage [14]. Paulson et al. reported that women in the United States had a significantly lower multivariate-adjusted HR for cancer-specific death than men, using data from the SEER-Medicare linked database that consisted of 30,795 CRC patients who had undergone surgery during the period from 1996−2006 [15].
As for the time trend regarding the survival of CRC patients, Siegel et al. reported that the age-adjusted survival rate for colon cancer increased from 50.6 to 65.4 % between 1975 and 1977 and between 2003 and 2009, and the corresponding rates of rectal cancer were 48.1 and 67.7 %, respectively using the SEER program database [16]. Zeng et al. also showed that the multivariate adjusted HRs for cancer-specific death declined significantly during the 1990–2009 period using the SEER program database [5]. However, there was no significant difference in improvement between men and women. This observation is entirely in accordance with the results of the present study (Fig. 4). In our study, improvements in the survival of women (HR 0.72, 95 % CI 0.67–0.76) and of men (HR 0.70; 95 % CI 0.67–0.74) were virtually equivalent, and there were no distinct gender differences among the age groups, with the exception of women aged >80 years. Thus, we demonstrated that gender differences in CRC survival existed even after adjustment of the available covariates. However, there was no discrepancy regarding improvement in their survival.
The underlying mechanism driving gender differences regarding CRC survival remains to be investigated. Given that local recurrence rates after surgery for rectal cancer were reportedly higher in men, difficulty concerning the surgical approach as a result of the narrow male pelvis could be a causative factor regarding the gender differences in CSS [17, 18]. However, anatomy is unlikely to contribute to the gender differences in colon cancer CSS, because of a limited sex-related difference regarding surgical difficulty. Other studies have speculated that differences in host-related factors such as circulating hormones [19, 20] and immunological response to tumor cells contribute to the gender-related differences in survival [13, 21]. Furthermore, recent studies have indicated that considerably more female than male patients with CRC had microsatellite instability and the CpG island methylation phenotype [22–24]. These genomic backgrounds could affect gender differences regarding survival. Martling et al. investigated gender differences in the treatment of rectal cancer in Swedish patients, and found that the percentage of women who had preoperative radiation therapy was significantly smaller than was the case for men [25]. Similarly, some studies have reported differences in the administration of adjuvant chemotherapy between men and women [26–29]. We did not include adjuvant therapy as a covariate in our survival analysis, because the non-controlled and retrospective nature of this study could have been biased by patient selection. In addition, we could not find gender differences concerning the administration of adjuvant chemotherapy for pStage III colon cancer in our study. However, the impact on adjuvant therapy on gender differences in survival warrants further investigation.
The strength of this study was to the ability to access a large-scale database consisting of data from JSCCR member hospitals that basically consisted of a single ethnic group. However, the database covered only 6–9 % of CRC patients in Japan, and was exclusively sampled from member hospitals of the JSCCR; this may have affected the generalizability of our findings regarding the general population. Other limitations included those inherent in retrospective database analysis. The study may have been confounded by other important contributing factors such as comorbidities, frailty, operative morbidity, individual socioeconomic status, lifestyle factors and other factors. Therefore, the potential confounding effects of these factors cannot be excluded. Additionally, because patients with no follow-up information were excluded from the analyses, survival probabilities in this study could have been overestimated or underestimated.
In conclusions, in the present study, significant differences in CRC survival between Japanese men and women were demonstrated. Even after adjustment for available confounders, gender differences regarding survival persisted; gender was found to be an independent prognostic factor for CRC. The degree of improvement in survival during the study period was virtually equivalent in both genders, suggesting that men and women similarly experienced the benefits of advancements in oncology treatment. To develop more individualized healthcare, an increased understanding of the biochemical and molecular backgrounds behind this phenomenon will be required. Gender differences in CRC survival merit further investigation.
References
Ferlay J, Soerjomataram I, Ervik M et al (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. http://globocan.iarc.fr. Accessed on 27 Apr 2015
Gao RN, Neutel CI, Wai E (2008) Gender differences in colorectal cancer incidence, mortality, hospitalizations and surgical procedures in Canada. J Public Health (Oxf) 30(2):194–201
Cheng X, Chen VW, Steele B et al (2001) Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992–1997. Cancer 92(10):2547–2554
Allemani C, Weir HK, Carreira H, CONCORD Working Group et al (2015) Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385(9972):977–1010
Zeng C, Wen W, Morgans A et al (2015) Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol 1(1):88–96
Matsuda T, Ajiki W, Marugame T et al, Research Group of Population-Based Cancer Registries of Japan: monitoring of Cancer Incidence in Japan—Survival 2003–2005 Report (Center for Cancer Control and Information Services, National Cancer Center, 2013) (2011) Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 41:40–51
André T, Boni C, Mounedji-Boudiaf L et al (2004) Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators: oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351
Kopetz S, Chang GJ, Overman MJ et al (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 27(22):3677–3683
Matsuda A, Matsuda T, Shibata A et al (2013) Cancer Incidence and Incidence Rates in Japan in 2008: a Study of 25 Population-based Cancer Registries for the Monitoring of Cancer Incidence in Japan (MCIJ) Project. Jpn J Clin Oncol 44(4):388–396
Ministry of Health, Labour and Welfare (2015) Vital Statistics Japan. http://www.mhlw.go.jp/english/database/db-hw/index.html. Accessed on 27 Apr 2015
Kotake K, Honjo S, Sugihara K et al (2003) Changes in colorectal cancer during a 20-year period: an extended report from the multi-institutional registry of large bowel cancer, Japan. Dis Colon Rectum 46(10 Suppl):S32–S43
Rhodes JB, Holmes FF, Clark GM (1997) Changing distribution of primary cancers in the large bowel. JAMA 238(15):1641–1643
Ji BT, Devesa SS, Chow WH et al (1998) Colorectal cancer incidence trends by subsite in urban Shanghai, 1972–1994. Cancer Epidemiol Biomark Prev 7(8):661–666
Wichmann MW, Müller C, Hornung HM et al (2001) Gender differences in long-term survival of patients with colorectal cancer. Br J Surg 88(8):1092–1098
McArdle CS, McMillan DC, Hole DJ (2003) Male gender adversely affects survival following surgery for colorectal cancer. Br J Surg 90(6):711–715
Paulson EC, Wirtalla C, Armstrong K et al (2009) Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum 52(12):1982–1991
Siegel R, DeSantis C (2014) Colorectal cancer statistics, 2014. CA Cancer J Clin 64(2):104–117
Verschueren RC, Mulder NH, Van Loon AJ et al (1997) The anatomical substrate for a differences in surgical approach to rectal cancer in male and female patients. Anticancer Res 17(1B):637–641
Fietkau R, Rödel C, Hohenberger W et al (2007) Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control: results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys 67(4):1008–1019
Slattery ML, Friedman GD, Potter JD et al (1996) A description of age, sex, and site distributions of colon carcinoma in three geographic areas. Cancer 78(8):1666–1670
Hendifar A, Yang D, Lenz F et al (2009) Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res 15(20):6391–6397
McMillan DC, Wotherspoon HA, Fearon KC et al (1995) A prospective study of tumor recurrence and the acute-phase response after apparently cur active colorectal cancer surgery. Am J Surg 170(4):319–322
Ward R, Meagher A, Tomlinson I et al (2001) Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 48(6):821–829
Hawkins N, Norrie M, Cheong K et al (2002) CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology 122(5):1376–1387
Ogino S, Cantor M, Kawasaki T et al (2006) CpG island methylator phenotype (CIMP) of colorectal cancer is best characterised by quantitative DNA methylation analysis and prospective cohort studies. Gut 55(7):1000–1006
Martling A, Granath F, Cedermark B et al (2009) Gender differences in the treatment of rectal cancer: a population based study. Eur J Surg Oncol 35(4):427–433
Rayson D, Urquhart R, Cox M et al (2012) Adherence to clinical practice guidelines for adjuvant chemotherapy for colorectal cancer in a Canadian province: a population-based analysis. J Oncol Pract 8(4):253–259
Jessup JM, Stewart A, Greene FL et al (2005) Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 294(21):2703–2711
Verschueren RC, Mulder NH, Van Loon AJ et al (1997) The anatomical substrate for a differences in surgical approach to rectal cancer in male and female patients. Anticancer Res 17(1B):637–641
Acknowledgments
This work was supported in part by the National Cancer Center Research and Development Fund (26-A-32).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kotake, K., Asano, M., Ozawa, H. et al. Gender differences in colorectal cancer survival in Japan. Int J Clin Oncol 21, 194–203 (2016). https://doi.org/10.1007/s10147-015-0868-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0868-6