Abstract
Background
The optimal dose of neoadjuvant radiation for locally advanced, resectable esophageal cancer remains controversial in the absence of randomized clinical trials, with conventional practice favoring the use of 50.4 vs. 41.4 Gy.
Methods
Retrospective analysis of adults with non-metastatic esophageal cancer in the National Cancer Database (2004–2015) treated with neoadjuvant chemoradiotherapy. Outcomes were compared between patients undergoing 41.4, 45, or 50.4 Gy. Primary outcome was overall survival. Secondary outcomes included T and N downstaging and perioperative mortality adjusted for demographics, clinicopathologic factors, and facility volume.
Results
Eight thousand eight hundred eighty-one patients were included: 439 (4.9%) received low-dose (41.4 Gy), 2194 (24.7%) received moderate-dose (45 Gy), and 6248 (70.4%) received high-dose (50.4 Gy) neoadjuvant radiation. Compared to high-dose, low-dose radiation was associated with superior median overall survival (52.6 vs. 40.7 months) and 5-year survival (48.3% vs. 40.2%), and lower unadjusted 90-day mortality (2.3% vs. 6.5%, all p ≤ 0.01). Multivariable proportional hazards models confirmed an increased hazard of death associated with high-dose radiation therapy (HR = 1.38, 95% CI 1.10–1.72, p = 0.005). There was no significant difference in T and/or N downstaging between low-dose vs. high-dose therapy (p > 0.1 for both). Patients receiving 45 Gy exhibited the lowest median overall survival (37.2 months) and 5-year survival (38.7%, log-rank p = 0.04).
Conclusions
Compared to 50.4 Gy, 41.4 Gy is associated with reduced perioperative mortality and superior overall survival with similar downstaging in locally advanced esophageal cancer. In the absence of randomized clinical data, our findings support the use of 41.4 Gy in patients with chemoradiation followed by esophagectomy. Prospective trials are warranted to further validate these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2018, there are estimated to be more than 17,000 new diagnoses and 15,000 deaths from esophageal cancer in the USA.1 Thirty-two percent of esophageal cancer patients have locally advanced disease at the time of diagnosis, with 5-year survival in patients undergoing curative treatment remaining poor at 30–47%.2,3,4 While surgical resection has traditionally been a central component of localized esophageal cancer therapy, recent work has demonstrated improved survival and pathologic complete response (pCR) with the incorporation of neoadjuvant chemoradiotherapy (CRT) as part of a trimodality regimen.5 Current guidelines by the National Comprehensive Cancer Network advocate for concurrent chemotherapy and radiation followed by surgery in managing locally advanced, resectable esophageal cancer.6 Recommended radiation dose ranges from 41.4 to 50.4 Gy in daily 1.8–2 Gy fractions for patients undergoing planned trimodality therapy.
Arguments for 50.4 Gy (high-dose, HD) neoadjuvant CRT over 41.4 Gy (low-dose, LD) include improved survival, tumor burden reduction, and R0 resection rates.7 LD, on the other hand, provides the theoretical benefit of reduced perioperative mortality and complication risk, including cardiac and pulmonary toxicity, perforation, mediastinitis, and stricture and fistula formation.8,9,10 Notably, the Chemotherapy for Oesophgeal Cancer followed by Surgery Study (CROSS) trial was the first major LD study to demonstrate survival rates and pCR comparable to those seen in high-dose trials.
There remains an active debate as to whether the optimal neoadjuvant dose of radiation therapy is 41.4 vs. 50.4 Gy. In the absence of randomized trial data available to address this question, recent efforts have attempted to compare outcomes between patients undergoing low vs. high dose radiation therapy.11 However, due to the mostly recent uptake of 41.4 Gy in general clinical practice, these efforts were limited in their power to detect changes in clinical outcomes and staging. In this study, we used an updated cohort within the National Cancer Database (NCDB) to compare long-term and perioperative survival, downstaging, and pCR of three dosing regimens commonly used for trimodality treatment for patients diagnosed with non-metastatic esophageal cancer treated with neoadjuvant chemoradiation followed by surgery between 2004 and 2015.
Materials and Methods
Adult patients with non-metastatic esophageal cancer diagnosed between 2004 and 2015 and treated with neoadjuvant CRT followed by esophagectomy were selected from the NCDB. Radiation dose group was defined as 41.4 Gy (LD), 45.0 Gy (moderate dose, MD), and 50.4 Gy (HD). Patients who underwent radiation doses other than these were excluded due to concern for premature treatment termination. The primary outcome was overall survival (OS), defined as the time from diagnosis to death from any cause. Secondary outcomes included 30- and 90-day post-operative mortality, and pathologic downstaging. A sensitivity analysis in which patients who died within 30 days after surgery were excluded was conducted to ensure any observed differences in overall survival were not purely attributable to differences in short-term outcomes. Patient and treatment characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Differences were tested using chi-square or Fisher’s exact tests for categorical variables, as appropriate, and analysis of variance or t tests, for continuous variables, as appropriate.
Annual esophagectomy volume was calculated as the total number of esophagectomies performed by a given facility divided by the total number of years that facility reported performing at least one esophagectomy in the NCDB. ypN stage was defined based on pathologic N stage following neoadjuvant radiation (pN1–3 = ypN+, pN0 = ypN–), and patients with pNx were excluded from all analyses of ypN stage. pCR was defined as achievement of ypT0N0; however, we also separately reported tumor pathologic complete response (ypT0) and nodal complete pathologic response (ypN0). Per NCDB guidelines, survival information is not included for patients diagnosed during the last reporting year of the database; therefore, patients diagnosed in 2015 were excluded from all survival analyses. Unadjusted median and 5-year OS were estimated using the Kaplan-Meier method, and study groups were compared using the log-rank test. A Cox proportional hazards model was used to estimate the association of radiation dose with OS, after adjustment for patient gender, race, income level, facility type, annual esophagectomy volume, tumor grade, histology, tumor location, radiation modality, surgical approach, and margin status. Time from radiation to surgery was not seen to be different between the groups on a clinically meaningful level and therefore excluded from the multivariable model. Lastly, in order to account for the correlation of patients treated at the same facility (i.e., clustering), a robust sandwich covariance estimator was incorporated into this model.
Although all patients who underwent low-, moderate-, or high-dose CRT were included in this analysis, the focus was on low-dose vs. high-dose, on account of systematic differences in cohort characteristics and outcomes seen in the MD group. All results presented hereafter should be interpreted as low-dose vs. high-dose unless otherwise specified.
In order to examine differences in OS and downstaging by histology, additional unadjusted analyses were performed separately for patients with adenocarcinoma and squamous cell carcinoma. No adjustments were made for multiple comparisons, and a significance level of 0.05 was used for all statistical tests. Only patients with available data were included in each analysis. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Cohort Demographic and Clinicopathologic Characteristics
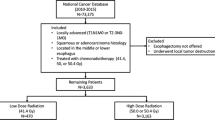
A total of 8881 patients met study criteria: 439 (4.9%) received LD, 2194 (24.7%) received MD, and 6248 (70.4%) received HD radiation (Supplemental Figure A1). Use of LD increased from less than 3% prior to 2010 to 13% by 2015 (Fig. 1). Use of high-dose radiation therapy increased from 50% in 2005 to a steady state of just over 70% by 2009, with a concurrent decrease in MD. Patients receiving LD were more likely to be treated at academic centers (LD 60.6% vs. HD 51.4%, p < 0.001) and at higher volume institutions (median annual esophagectomy volume: LD 6.4 (IQR 2.6–17.6) vs. HD 5.5 (2.5–15.1), p < 0.001), and they were more likely to have squamous cell carcinoma (23.7% vs. 16.6%, p = 0.001) compared to those receiving high dose. Patients receiving MD were least likely to receive care at academic centers (42.1%, p < 0.001) (Supplemental Table A1). There was no clinically meaningful difference between the three groups in terms of time between radiation and surgery [LD 51 (41–62) days vs. MD 48 (38–62) days vs. HD 52 (41–66) days]. The median (IQR) number of lymph nodes examined was 15 (9–21), 11 (5–18), and 12 (7–19) for LD vs. MD vs. HD, respectively (p < 0.001). Median follow-up for survival was 57.3 months (95% CI 55.9–59.0).
Low-Dose Neoadjuvant CRT Is Associated with Superior OS
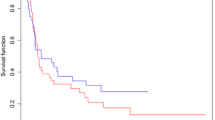
On univariate analysis, LD patients exhibited significantly longer median OS (52.6 vs. 40.7 months) and 5-year survival [48.3% (95% CI 40.1–56.0%) vs. 40.2% (38.7–41.7%), log-rank p = 0.01] compared to high-dose patients (Fig. 2a). A sensitivity analysis in which patients who died within 30 days of surgery were excluded confirmed longer median overall survival in LD patients compared to HD patients (52.8 vs. 42.9 months, p < 0.05). On survival analysis by nodal status, ypN+ patients showed significantly shorter median OS (25.2 vs. 49.1 months) and 5-year survival [21.6% (95% CI 19.5–23.7%) vs. 45.5% (43.6–47.3%), log-rank p < 0.001)] compared to ypN− patients (Fig. 2b). On adjusted analysis, the hazard ratio (HR) was 1.38 for HD vs. LD neoadjuvant CRT (95% CI 1.10–1.72, p = 0.005) (Table 1). After excluding those who died within 30 days of surgery, both MD and HD patients remained at an increased risk of death compared to LD patients [high- vs. low-dose HR = 1.31 (95% CI 1.05–1.65), p = 0.02; moderate- vs. low-dose HR = 1.32 (95% CI 1.05–1.66), p = 0.02]. MD patients had the shortest median OS (37.2 months) and 5-year survival [38.7% (95% CI 36.4–41.0%), log-rank p = 0.04] (Supplemental Figure A2).
Low-Dose CRT Exhibits Comparable T and N Downstaging vs. High-Dose CRT
T and N downstaging rates did not differ significantly for LD vs. HD CRT (T downstaging: LD 48.7% vs. HD 45%, p = 0.13, and N-downstaging: 33.9% vs. 32.9%, p = 0.65) (Table 2). Tumor and nodal pCR (to ypT0 and ypN0, respectively) also did not differ significantly between patients treated with LD vs. HD CRT (T-pCR: LD 21.4% vs. HD 21.1%, p = 0.89, and N-pCR: 31.4% vs. 30.6%, p = 0.73). In order to rule out a more subtle impact of dose on tumor control, we also plotted the distribution of T and N stages by radiation dose to examine whether any evidence of downstaging was apparent (Fig. 3a, b, Supplemental Table A2). MD CRT exhibited a significantly lower rate of downstaging (T downstaging 38.2%, N downstaging 27.6%, p < 0.001) and pCR (T-pCR 16.4%, N-pCR 26.5%, p < 0.001) compared to LD and HD CRT (Table 2).
Low-Dose CRT Is Associated with Reduced Perioperative Mortality
LD patients had a significantly lower rate of 90-day mortality compared to high-dose patients (2.3% vs. 6.5%, p = 0.01). There was no significant association between high- vs. low-dose in length of hospital stay, 30-day readmission, or margin status (all p ≥ 0.10). There was trend towards increased 30-day mortality in the high- vs. low-dose group (2.8% vs. 0.7%; p = 0.05) (Supplemental Table A3).
Subgroup Analysis of Overall Survival and Downstaging by Histology
In planned subgroup analyses, we examined the association of low- vs. high-dose radiation therapy limited to patients with squamous carcinoma vs. adenocarcinoma. We observed a similar trend towards improved median OS with LD radiation therapy; however, in these smaller sample sizes (inherent to subgroup analyses), we did not detect a statistically significant difference for LD vs. HD CRT (adenocarcinoma: LD 52.4 vs. HD 39.2 months, p = 0.14, and squamous cell carcinoma: 144.2 vs. 46.4 months, p = 0.12) (Supplemental Tables A4 and A5). In adenocarcinoma patients, T and N downstaging was not significantly different by dose (T downstaging: LD 45.6% vs. HD 44.3%, p = 0.65, and N downstaging: 31.2% vs. 32.1%, p = 0.73). T- and N-pCR also did not differ by dose (T-pCR: LD 16.5% vs. HD 18.8%, p = 0.3, and N-pCR: 28.1% vs. 29.8%, p = 0.53). In squamous cell carcinoma patients, T and N downstaging was not significantly different by dose (T downstaging: LD 57.7% vs. HD 48.9%, p = 0.09, and N downstaging: 41.3% vs. 36.7%, p = 0.35). T- and N-pCR also did not differ significantly by dose (T-pCR: LD 35.6% vs. HD 32.4%, p = 0.51, and N-pCR: 40.4% vs. 34.9%, p = 0.27) (Supplemental Tables A6 and A7).
Discussion
This is the first study to demonstrate superior outcomes with 41.4 vs. 50.4 Gy in the treatment of patients with non-metastatic esophageal cancer with neoadjuvant chemoradiation. Receipt of low-dose radiation was associated with approximately a year increase in median survival, decreased perioperative mortality, and increased survival at 5 years compared to receipt of high-dose radiation. There was no evidence of increased pathologic downstaging or complete response rates with high-dose radiation. Although impossible to exclude completely within the context of a retrospective study, no evidence of significant selection bias between patients receiving low- vs. high-dose radiation was observed, with only small differences in T stage, N stage, and squamous histology. Our findings are further supported by comparable findings in both unadjusted and adjusted analyses of the primary study outcomes. Finally, previously suggested associations of ypN+ disease with compromised outcomes were confirmed, with these patients exhibiting roughly half the 5-year survival of ypN− patients in unadjusted analysis. In the absence of randomized clinical trials, our data provide evidence to support the use of 41.4 vs. 50.4 Gy in patients undergoing planned trimodality neoadjuvant radiation therapy.
Our values for overall survival and pathologic complete response rates are consistent with several single-dose studies including CROSS and CALGB 9781 (summary of prior studies is shown in Appendix Table 3), which demonstrated survival and downstaging outcomes that were comparable to, or slightly lower, than those of our study (Fig. 2a, Table 2).5,12,13,14,15,16,17 The CROSS trial, which used low-dose neoadjuvant CRT, reported a median survival of 49.4 months and tumor pCR of 29%, while CALGB 9781, which used high-dose, demonstrated a median survival of 53.8 months and tumor pCR of 40%. Distribution of tumor histology in these studies were comparable to that of our cohort, further validating our data. There has been one prior retrospective study of the NCDB, however, that was conducted prior to routine use of 41.4 Gy and was not adequately powered to detect significant differences in median OS or mortality.11
Several factors may explain the improved OS seen in low-dose patients. A reduction in potentially dose-dependent, thoracic radiation-induced toxicities such as pneumonitis, pericarditis, myocardial ischemia, esophageal perforation, and pancytopenia could have contributed to this observed association.5,18,19 Recent studies in advanced non-small cell lung cancer have associated escalated heart dose with increased adverse cardiac events and treatment-related deaths, and shorter OS.20,21,22 A recent randomized controlled trial using 45 Gy demonstrated no survival difference between neoadjuvant CRT + surgery group vs. surgery-only group, which was attributed to the considerably higher post-operative mortality rate in patients receiving radiation.17 Differences in chemotherapeutic regimen could also have contributed to differences in survival.23 Specifically, in the USA, cisplatin and 5-FU are predominantly given with high-dose, while carboplatin and paclitaxel are primarily given with low-dose radiation in the neoadjuvant setting.24,25 A retrospective study by Munch et al. comparing the effect of carboplatin and paclitaxel vs. cisplatin and 5-FU in the context of neoadjuvant chemoradiation in patients with locally advanced squamous cell carcinoma of the esophagus demonstrated that, while no significant difference was seen for overall survival or freedom from recurrence, the cisplatin and 5-FU regimen was associated with significantly more hematologic toxicities compared to its alternative. Further, in another study involving definite chemoradiation for esophageal squamous cell carcinoma using the two chemotherapy regimens, risk of myelotoxicity significantly increased with patient age.26,27 It is therefore possible such differences in outcomes may have been amplified for patients receiving high-dose radiation in our study. Finally, improved survival may be attributed to a non-significant trend towards greater T and N downstaging in low-dose vs. high-dose patients, which has previously been associated with improved survival (Table 2).28,29,30
Patients receiving moderate-dose (45 Gy) neoadjuvant CRT exhibited the most compromised long- and short-term survival and downstaging outcomes. Although it is unclear why these patients had the worst prognosis, we posit that this trend may be explained by patients with either extensive comorbidities or tumor burden who were initially intended to undergo a full 50.4 Gy chemoradiation regimen but were unable to tolerate the treatment and received 45 Gy instead. Alternatively, it is possible these patients were associated with centers with the lowest median annual esophagectomy volume, which could be a measure of the difference in overall quality of care received by this cohort. Lastly, the particularly low rate of intensity-modulated radiation therapy (IMRT) associated with the 45 Gy group may help explain this phenomenon, given that IMRT is associated with improved targeting of tumor and salvaging of adjacent healthy tissue compared to conformal radiation therapy.
While patients with squamous cell carcinoma expectedly saw a higher overall rate of T and N downstaging and pCR compared to those with adenocarcinoma likely on account of their greater radiosensitivity, long-term survival outcomes were relatively comparable between the two groups.31 This finding is unexpected due to the well-known association between downstaging and survival.30,32 However, the tendency for squamous cell carcinoma patients to have more significant comorbidities, including extensive tobacco and alcohol use, compared to adenocarcinoma patients, may have contributed heavily to their long-term mortality, thus offsetting the survival benefit attributed to greater tumor downstaging.33,34,35
Nodal involvement, regardless of tumor size, is a critical determining factor for consideration of pre-operative CRT in esophageal cancer, and the significantly higher mortality rate observed in node-positive patients warrants particular attention.36 Recent data analyzing patients receiving low-dose neoadjuvant CRT for esophageal cancer have suggested locoregional recurrence rates as low as 14%, with only 1% of patients experiencing isolated in-field recurrence.37 If these patterns hold true in general clinical practice, it seems unlikely that meaningful improvements in local control would be achieved through dose increase.13,15,16,37 Considering this, and our finding that high-dose CRT is not necessarily associated with superior survival or downstaging outcomes, the focus should perhaps shift from increased radiation dose to incorporation of additional modalities in managing node-positive populations, who exhibit markedly compromised survival outcomes compared to their node-negative counterparts. Potential options include concurrent or adjuvant chemotherapeutic agents, more aggressive surgery, or non-standard approaches to targeted radiation therapy, such as integrated boost plans that limit higher dose radiation therapy to the gross tumor volume.
This study has several limitations. Notably, it is limited by its retrospective nature, which cannot exclude the possibility of selective administration of low-dose radiotherapy to healthier patients or to those with less advanced or aggressive tumors. Furthermore, the NCDB does not contain any indicators of patient functional status, though it instead includes the Charlson/Deyo comorbidity score, which demonstrated no clinically meaningful differences between groups on univariate analysis. In order to mitigate the potential for confounding, we compared and adjusted for several patient demographic and clinicopathologic factors between low-dose and high-dose patients, and only saw minimal differences between groups, which demonstrated a robust and stable association of low-dose radiation with superior outcomes. In addition, a key limitation of the NCDB is that it does not specify chemotherapeutic agents, only that a patient received concurrent chemoradiation prior to surgery. Therefore, the impact of differences in chemotherapy on OS and downstaging cannot be directly inferred. However, 41.4 Gy in the USA is thought to be used almost entirely with carboplatin and paclitaxel, given that its adoption was directly influenced by the CROSS trial. The NCDB does not specify treatment intent, and therefore, it is possible that patients who were meant to receive high dose were unable to tolerate the treatment and instead received a lower dose. Finally, toxicity and cancer-specific survival data are not recorded in the NCDB and were unavailable for analysis.
Conclusion
In conclusion, a neoadjuvant dose of 41.4 Gy is associated with decreased perioperative mortality and superior OS with similar downstaging compared to 50.4 Gy in patients with locally advanced, resectable esophageal cancer. Further prospective trials that control for histology, chemotherapy, and radiation modality are warranted to further validate the results of this study.
References
Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2018. CA Cancer J Clin, 2018. 68(1): p. 7–30.
Shapiro, J., et al., Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol, 2015. 16(9): p. 1090–1098.
Cunningham, D., et al., Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 2006. 355(1): p. 11–20.
Ychou, M., et al., Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol, 2011. 29(13): p. 1715–21.
van Hagen, P., et al., Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med, 2012. 366(22): p. 2074–84.
NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. Version 1.2017. 2017 [cited 2018 March 30, 2018]; Available from: http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
Elliott DA, et al., Locally Advanced Esophageal Chemoradiation Therapy Practice Patterns: Results From a National Survey of ASTRO Members (Abstract). IJROBP, 2015. 93: p. S219.
Murro, D. and S. Jakate, Radiation esophagitis. Arch Pathol Lab Med, 2015. 139(6): p. 827–30.
Novak, J.M., et al., Effects of radiation on the human gastrointestinal tract. J Clin Gastroenterol, 1979. 1(1): p. 9–39.
Beukema, J.C., et al., Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer?. Radiother Oncol, 2015. 114(1): p. 85–90.
Haque, W., et al., Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol, 2018. 9(1): p. 80–89.
Walsh, T.N., et al., A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med, 1996. 335(7): p. 462–7.
Urba, S.G., et al., Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol, 2001. 19(2): p. 305–13.
Lee, J.L., et al., A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol, 2004. 15(6): p. 947–54.
Tepper, J., et al., Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol, 2008. 26(7): p. 1086–92.
Burmeister, B.H., et al., Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol, 2005. 6(9): p. 659–68.
Mariette, C., et al., Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol, 2014. 32(23): p. 2416–22.
Kwa, S.L., et al., Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys, 1998. 42(1): p. 1–9.
Vivekanandan, S., et al., The Impact of Cardiac Radiation Dosimetry on Survival After Radiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys, 2017. 99(1): p. 51–60.
Wang, K., et al., Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol, 2017. 35(13): p. 1387–1394.
Speirs, C.K., et al., Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol, 2017. 12(2): p. 293–301.
Bradley, J.D., et al., Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol, 2015. 16(2): p. 187–99.
Xi, M., et al., Comparing docetaxel plus cisplatin versus fluorouracil plus cisplatin in esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Jpn J Clin Oncol, 2017. 47(8): p. 683–689.
Almhanna, K., R. Shridhar, and K.L. Meredith, Neoadjuvant or adjuvant therapy for resectable esophageal cancer: is there a standard of care?. Cancer Control, 2013. 20(2): p. 89–96.
Nabavizadeh, N., et al., Preoperative carboplatin and paclitaxel-based chemoradiotherapy for esophageal carcinoma: results of a modified CROSS regimen utilizing radiation doses greater than 41.4 Gy. Dis Esophagus, 2016. 29(6): p. 614–20.
Munch, S., et al., Comparison of neoadjuvant chemoradiation with carboplatin/ paclitaxel or cisplatin/ 5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat Oncol, 2017. 12(1): p. 182.
Munch, S., et al., Comparison of definite chemoradiation therapy with carboplatin/paclitaxel or cisplatin/5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat Oncol, 2018. 13(1): p. 139.
Donahue, J.M., et al., Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg, 2009. 87(2): p. 392–8; discussion 398-9.
Zanoni, A., et al., ypN0: Does It Matter How You Get There? Nodal Downstaging in Esophageal Cancer. Ann Surg Oncol, 2016. 23(Suppl 5): p. 998–1004.
Davies, A.R., et al., Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol, 2014. 32(27): p. 2983–90.
Kasten-Pisula, U., et al., The extreme radiosensitivity of the squamous cell carcinoma SKX is due to a defect in double-strand break repair. Radiother Oncol, 2009. 90(2): p. 257–64.
Korst, R.J., et al., Downstaging of T or N predicts long-term survival after preoperative chemotherapy and radical resection for esophageal carcinoma. Ann Thorac Surg, 2006. 82(2): p. 480–4; discussion 484-5.
Baan, R., et al., Carcinogenicity of alcoholic beverages. Lancet Oncol, 2007. 8(4): p. 292–3.
Morita, M., et al., Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery, 2002. 131(1 Suppl): p. S1–6.
Humans, I.W.G.o.t.E.o.C.R.t., Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum, 2004. 83: p. 1–1438.
Wagner, T.D., N. Khushalani, and G.Y. Yang, Clinical T2N0M0 carcinoma of thoracic esophagus. J Thorac Dis, 2010. 2(1): p. 36–42.
Oppedijk, V., et al., Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol, 2014. 32(5): p. 385–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Julie A. Sosa, M.D., M.A., is a member of the Data Monitoring Committee for the Medullary Thyroid Cancer Consortium Registry supported by Novo Nordisk, GlaxoSmithKline, Astra Zeneca, and Eli Lilly. The other authors declare that they have no competing interests.
Additional information
This study was an oral presentation at the 12th Annual Academic Surgical Congress in Las Vegas, Nevada, 2017 and since has been updated to include data through 2015.
Electronic Supplementary Material
Supplementary Figure 1
(PDF 10 kb)
Supplementary Figure 2
(PDF 91 kb)
Supplementary Table 1
(DOCX 24 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Table 3
(DOCX 14 kb)
Supplementary Table 4
(DOCX 13 kb)
Supplementary Table 5
(DOCX 13 kb)
Supplementary Table 6
(DOCX 15 kb)
Supplementary Table 7
(DOCX 15 kb)
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Ji, K.S.Y., Thomas, S.M., Roman, S.A. et al. Low- vs. High-Dose Neoadjuvant Radiation in Trimodality Treatment of Locally Advanced Esophageal Cancer. J Gastrointest Surg 23, 885–894 (2019). https://doi.org/10.1007/s11605-018-4007-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-4007-3