Abstract
Background
Traditional neoadjuvant therapy for esophageal cancer has used chemoradiation doses greater than 45 Gy. This study aimed to examine the dose of preoperative radiation in relation to the pathologic complete response (pCR) rate and overall survival (OS) for patients with resectable esophageal cancer.
Methods
The National Cancer Database was queried for all patients with esophageal or gastroesophageal junction cancer who received neoadjuvant chemoradiation (CRT) followed by esophagectomy between 2006 and 2015. The radiation doses were divided into four ranges based on Grays (Gy) received: less than 39.6 Gy, 39.60–44.99 Gy, 45–49.99 Gy, and 50 Gy or more.
Results
The inclusion criteria were met by 10,293 patients. All patients received neoadjuvant CRT, with 689 patients (6.7%) receiving less than 39.6 Gy, 973 patients (9.5%) receiving 39.6–44.9 Gy, 3837 patients (37.3%) receiving 45–49.9 Gy, and 4794 patients (46.6%) receiving 50 Gy or more. The overall pCR rate was 17.2% (1769/10,293) and was significantly lower for those who received less than 39.6 Gy of radiation than for those who received 39.6 Gy or more (13.9% [96/689] vs. 17.4% [1673/9604]; p = 0.017). The median OS of 37.2 months was significantly better for those who received 39.6 Gy or more than for those who received less than 39.6 Gy (38 vs. 29.6 months (p < 0.0001). The pCR and OS did not differ between the three higher radiation doses (39.6–44.9 vs. 45–49.9 Gy vs. ≥ 50 Gy; pCR [p = 0.1] vs. OS [p = 0.097]). The patients who received 39.6–44.9 Gy were propensity matched with those who received 45 Gy or more of radiation. There remained no difference in pCR (p = 0.375) or OS (p = 0.957).
Conclusions
In the United States, the heterogeneity in neoadjuvant CRT dosing is significant, with 84% of patients receiving more than 45 Gy. The benefit of neoadjuvant CRT in terms of pCR and overall survival is seen with doses of 39.6 Gy or more, but not with doses higher than 45 Gy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The optimal treatment for locally advanced, resectable esophageal cancer has been difficult to define due to the wide variation in treatment regimens. It is difficult to delineate which factor has the strongest impact on outcomes; surgical approach, chemotherapy regimen, or radiation dose. Multiple randomized clinical trials have shown a benefit of neoadjuvant therapy before esophagectomy for locally advanced disease,1,2,3,–4 whereas some trials have failed to show a benefit.5,6 Much of the variation in these trials is in radiation dosing, with positive trials using doses ranging from 40 to 50.4 Gy and negative trials using doses ranging from 35 to 45 Gy.
To complicate medical decision making further, the recommended dose of radiation for nonoperative patients is 50.4 Gy.7,8 To date, no prospective trials have compared outcomes based on different neoadjuvant radiation doses.
The most recent large-scale prospective randomized trial evaluating CRT before surgery was the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial.1 The radiation dose used in this trial was 41.4 Gy given in 23 fractions. The dose was lower than the radiation doses used in many prior studies,2,3,–4 but the regimen still showed a significant survival benefit compared with surgery alone.
We sought to evaluate, in the context of the National Cancer Database (NCDB), whether the dose of preoperative radiation for resectable esophageal cancer affects the pathologic complete response (pCR) rate and overall survival (OS). We hypothesized that higher radiation doses would be associated with improved rates of pCR and therefore improved OS.
Methods
National Cancer Database (NCDB)
The NCDB is a hospital-based tumor registry jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB captures data from hospitals accredited by the American College of Surgeons Commission on Cancer and includes more than 70% of all cases with newly diagnosed cancer nationwide.9
Clinical staging in the NCDB is based on the American Joint Committee of Cancer (AJCC) edition corresponding with the year of diagnosis. We used clinical and pathologic T stages because these were consistent between the sixth and seventh editions, which spanned our study period. To keep the data consistent between patients treated at the time of the sixth- and seventh-edition AJCC staging, we also examined clinical and pathologic N stage by node-positive versus node-negative status and did not evaluate based on the number of positive lymph nodes.
The NCDB is a joint project of the Commission on Cancer (CoC) between the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB were the source of the de-identified data used in this study. They have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Study Population and Variables
The NCDB was queried for all patients with esophageal or gastroesophageal junction cancer who received neoadjuvant chemotherapy and radiation followed by esophagectomy from 2006 to 2015. The definition of study variables are available from the NCDB Participant Use Data File data dictionary (http://ncdbpuf.facs.org). Neoadjuvant chemotherapy and radiation were defined as reception of both multi-agent chemotherapy and radiation, all administered before surgical resection.
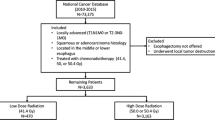
Patients were excluded from the study if they had metastatic disease, an unknown clinical stage, non-beam radiation, a radiation dose greater than 7740 Gy, or surgery more than 90 days after completion of radiation treatment.
The patients were divided into four different cohorts based on the radiation dose received. These cohorts were determined based on the frequency of radiation doses among the patients in the study and on clinically relevant values (Fig. 1), with cohort 1 receiving less than 39.6 Gy, cohort 2 receiving 39.6–44.9 Gy, cohort 3 receiving 45–49.9 Gy, and cohort 4 receiving 50 Gy or more. The interval to surgery was defined as the time from the end of radiation treatment to the date of surgery. The outcomes of interest were pCR and OS, with pCR defined as pathologic T0 and N0 (pT0N0) and OS defined from date of diagnosis to the last follow-up visit or death.
Statistical Analysis
Statistical comparison between the groups was performed using the Wilcoxon rank sum or Kruskal–Wallis for continuous variables and Chi square for categorical data. Demographic and clinical characteristics are described using mean ± SD for continuous variables and frequency and percentages for categorical variables.
Propensity score-matched logistic regression modeling was performed using the known covariates (age, gender, type of institution, payor, Charlson Comorbidity Score, histology, T stage and N stage) to control for confounding effects. Patients with any missing data for the propensity-matched variables were excluded from the analysis.
Nearest-neighbor 1:3 matching was used, with the caliper set at 0.1. A new set of matched low- and high-dose pairs was generated, from which covariates were assessed using the independent sample t test or the Pearson Chi square test to assess the success of the matching algorithm.
The primary outcomes were survival in months and pCR. Survival estimates were calculated by the Kaplan–Meier method, and groups were compared using the log-rank test. Pathologic complete response was compared using Chi square test. Propensity score matching was performed using R, whereas comparative or survival analysis was performed using SAS Enterprise Guide, version 7.1 (SAS Institute, Cary, NC, USA).
The clinical and pathologic features associated with a pCR in the univariate analysis were entered into a multivariable analysis. Cofactors for the multivariable regression were identified using backward selection from the univariate analysis (α < 0.01). Statistical analysis was performed using STATA version 14.2 (StataCorp; College Station, TX, USA). Statistical significance was set at a p value lower than 0.05. The study was determined to be exempt from Institution Review Board review because all the data were de-identified.
Results
The inclusion criteria were met by 10,293 patients who underwent neoadjuvant chemotherapy and radiation followed by esophagectomy. The study enrolled 1593 women (14.9%) and 8754 men (85.1%) with a median age of 62 years (interquartile range [IQR], 56–68 years). The patient characteristics are listed in Table 1.
Neoadjuvant chemotherapy was multi-agent for all the patients. All the patients received radiation preoperatively, with 689 patients (6.7%) receiving less than 39.6 Gy, 973 patients (9.5%) receiving 39.6 to 44.9 Gy, 3837 patients (37.3%) receiving 45–49.9 Gy, and 4794 patients (46.6%) receiving 50 Gy or more.
The patient characteristics were compared between the four radiation-dose cohorts of (Table 2). The four cohorts differed in proportion of male sex, histology, clinical nodal status, and interval from completion of radiation to surgery. Higher radiation doses were used more frequently for the men, those with adenocarcinoma histology, and clinically lymph node-positive patients. Those who received higher radiation doses had a significantly longer interval from completion of radiation to surgery. All patients underwent an esophagectomy after completion of chemotherapy and radiation.
The pCR rate for all the patients was 17.2% (1769/10,293) and was significantly lower for the patients who received less than 39.6 Gy of radiation than for those who received 39.6 Gy or more (13.9% [96/689] vs. 17.4% [1673/9604]; p = 0.017). The pCR rate did not differ among the patients who received 39.6 Gy or more (18.6% [181/973] vs. 16.4% [630/3837] vs. 18% [862/4794]; p = 0.097). Additional factors associated with pCR in the univariate analysis were age, sex, histology, clinical T stage, and interval to surgery (Table 3).
Given the differences in the radiation-dose cohorts and the potential confounding of these variables in the association between radiation dose and pCR, we performed a multivariable logistic regression. This analysis identified radiation dose lower than 39.6 Gy, male sex, adenocarcinoma histology, and higher clinical T stage as factors associated with not achieving pCR (Table 4). A prolonged interval from completion of radiation to surgery was associated with a higher pCR rate.
Among all the patients, the median OS was 37.2 months, and was better for those who received 39.6 Gy of radiation or more than for those who receive less than 39.6 Gy (38 vs. 29.6 months (p < 0.0001). There was no difference in survival between the three higher radiation cohorts (39.6–44.9 vs. 45–49.9 Gy vs. ≥ 50 Gy; 39.5 vs. 36.2 vs. 37.9 months; p = 0.1; Fig. 2).
Overall survival of patients compared by dose of preoperative radiation. The Kaplan–Meier survival estimate of all patients is shown (p < 0.0001). Comparison of cohorts 2–4 (39.6–44.9 vs. 45–49.9 vs. ≥ 50 Gy) showed no significant difference in survival (p = 0.18). The groups were compared using the log-rank test
To address other factors potentially confounding survival, we performed a Cox proportional hazard analysis. Among all the patients, improved survival was observed for those with pCR (hazard ratio [HR], 0.616; p < 0.0001) and decreased survival for those with a radiation dose lower than 39.6 Gy (HR 1.24; p < 0.0001), older age (HR 1.01; p < 0.0001), male sex (HR 1.29; p < 0.0001), higher clinical T stage (HR 1.11; p = 0.004), clinically positive lymph nodes (HR 1.249; p < 0.0001), and prolonged interval from radiation to surgery (HR 1.003, p < 0.0001).
We recognize that an unmatched comparison of patients has significant confounders and therefore decided to perform a propensity match for the patients who received 39.6 Gy of radiation or more. The patients who received 39.6–44.9 Gy were matched with the patients who received 45 Gy radiation or more for age, gender, type of institution, insurance status, Charlson Comorbidity Score, histology, T stage, and N stage in a 3:1 propensity match. This resulted in 669 patients in the 39.6- to 44.9-Gy radiation group and 2006 patients in the group that had 45 Gy of radiation or more. The pCR did not differ significantly between the unmatched group (19.6% [131/669] vs. 17.9% [1198/6707]; p = 0.27) and the matched group (19.6% [131/669] vs. 18.1% [362/2006]; p = 0.375). Additionally, survival did not differ between the two matched groups (p = 0.957; Fig. 3). After propensity score matching, the median survival was 40.7 months and similar between those who received 4500 cGy of radiation or more and those who received 3960 to 4499 cGy (40.7 vs. 40.2 months; p = 0.957; Fig. 3).
Discussion
The survival benefit of neoadjuvant CRT for patients with resectable esophageal cancer has been confirmed by multiple studies.1,2,3,–4 Nationally, there is significant heterogeneity in preoperative radiation dosing. In the NCDB database, 84% of patients received doses exceeding 44 Gy. This higher radiation dosing is supported by prior studies, including the Cancer and Leukemia Group B randomized study, which shows a survival benefit of neoadjuvant chemotherapy and radiation followed by surgery versus surgery alone with radiation dosing of 50.4 Gy.3 Current ongoing clinical trials continue to use the radiation dose of 50.4 Gy.10,11
Published in 2012, the CROSS trial examined clinical T1N1, ≥ T2/T3, N0/N1 resectable esophageal adenocarcinoma, squamous cell carcinoma (SCC) or large-cell undifferentiated carcinoma, and randomized patients to surgery alone or CRT followed by surgery.1 The chemotherapy regimen used was cisplatin and paclitaxel with a radiation dose of 41.4 Gy. A pCR was achieved in 29% of the patients, with a 49% pCR rate in SCC and a 23% pCR rate in adenocarcinoma histology. The OS was longer in the neoadjuvant treatment group than in the surgery-alone group (49 vs. 24 months; p = 0.003). This relationship was stronger for SCC (p = 0.011) than for adenocarcinoma (p = 0.049). Since the publication of the CROSS trial, there has been a trend toward decreasing radiation doses. In the NCDB, the percentage of patients receiving between 39.6 and 44.9 Gy of radiation rose from 7.3% between 2006 and 2011 to 11.1% between 2012 and 2015.
Few studies have investigated varying radiation doses and the rate of pCR. In one small retrospective study that included only patients with squamous cell carcinoma, three different radiation doses were compared as follows: 30–30.6 Gy, 39.6–40 Gy, and 44–45 Gy.12 This study examined 111 patients with an overall pCR rate of 51%. In their small cohort, the authors noted a significant difference between the three groups, with the lowest radiation dose showing a 13% pCR rate compared with 38% and 67% in the higher-dose groups. However, the highest radiation group comprised only six patients.12
Our study also showed that those who received less than 39.6 Gy of radiation had lower pCR rate, at 13.8%, compared to higher radiation doses with a pCR rate of 17.4% (p = 0.017). Additionally, we found that those who received less than 39.6 Gy of radiation had a significantly shorter OS than those who received higher radiation doses. There was no difference in pCR rate or OS based on radiation dosing if radiation doses of 39.6 Gy and above were used in both the unmatched and propensity-matched data.
Consistent evidence shows a correlation between pCR and improved survival.13,14,15,–16 Murphy et al.14 studied almost 1000 patients who received neoadjuvant chemotherapy and 50.4 Gy of radiation before esophagectomy. The overall pCR rate was 24%, and the patients with a pCR had a significantly better OS (71 vs. 36 months; p = 0.0021) and 5-year recurrence-free survival (71 vs. 26 months; p = 0.0011) than those without a pCR. Additionally, they found older age, poor differentiation, higher T stage, and signet ring cell pathology to be independently less likely to result in a pCR. Donahue et al.15 also found improved survival with pCR in a retrospective study of 162 patients after neoadjuvant CRT and surgery. The median radiation dose used was 50.4 Gy, but ranged from 12.6 to 54 Gy. Overall survival and disease-free survival were significantly improved for those with a pCR.15 Singla et al.13 studied 226 patients who received neoadjuvant chemotherapy and radiation before surgery. The majority of the patients received 50.4 Gy of radiation. In a multivariable analysis, they also identified pCR as a strong independent predictor of survival. Our study was consistent with these findings, identifying pCR in the Cox analysis as an independent predictor of improved survival, with a hazard ratio of 0.645.
No strong evidence exists to show that increasing the radiation dose increases pCR, and no direct link exists between increasing radiation dose and longer survival. An abundance of literature reports higher radiation doses for patients who receive definitive CRT without surgery. These data show that escalating radiation doses exceeding 50 Gy do not significantly improve survival and are associated with additional toxic side effects.8,17,18 He et al.19 studied almost 200 consecutive patients receiving definitive CRT and found no significant differences between 54 and 66 Gy versus 50.4 Gy or less in terms of pCR (p = 0.975), regional failure rate (p = 0.336), distant metastasis rate (p = 0.390), or 5-year OS (p = 0.617) at the consequence of increased toxicity. The landmark INT0123 study by Minsky et al.8 showed in a randomized trial of 218 patients undergoing definitive treatment that 64.8 Gy was not superior to 50.4 Gy. The higher radiation dose did not increase survival or locoregional control, and there was a 6% rate of treatment-related deaths, with the majority occurring after 50.4 Gy of radiation or more.
Changing the way that radiation is delivered may have some value, and some have suggested that delivering higher doses of radiation may be beneficial and feasible with improved technologies, including intensity-modulated radiation therapy (IMRT) and/or protons. One single-institution study shows a pCR improved from 30 to 56.2% by the addition of dose-painting IMRT to traditional radiation, increasing the dose from 50.4 to 56 Gy.20 This study observed no increase in postoperative complications or mortality based on the radiation treatment used. Additionally, some evidence shows that IMRT may be associated with improved OS compared with three-dimensional conformal radiotherapy. In a study by Lin et al.21 the cancer-specific survival and recurrence were similar between the two treatment methods, and OS was improved with IMRT. Whether this is due to organ toxicity or pathologic response is unknown. Further research is needed to identify the optimal modality regardless of radiation dose chosen.
Higher radiation doses do not come without a cost, and some studies have shown high doses to be associated with increased morbidity and mortality at the time of esophagectomy. These effects may be related in part to radiation dose and may support decreased radiation doses if equivalent outcomes can be achieved. In a randomized trial of 195 early-stage (T1/2, N1-0, T3N0) esophageal cancer patients, CRT with 45 Gy was compared with surgery alone.22There was no difference in overall 5-year survival, with a significant increase in postoperative mortality (11% vs. 3%; p = 0.049) for those who received CRT.
Reynolds et al.23 studied 200 matched patients, comparing those who underwent surgery with those who had neoadjuvant CRT followed by surgery. The radiation dose ranged from 40 to 50 Gy. They found no difference in operative times or blood loss, but did find that those who received preoperative CRT had a significantly higher incidence of postoperative sepsis, respiratory failure, and acute respiratory distress syndrome.
In a large multi-center study of nearly 3000 patients, this complication risk was less apparent.24 The CRT consisted of 5-fluorouracil and platinum salt with 45 Gy of radiation. Those who received neoadjuvant CRT and those who did not showed similar rates of 90-day postoperative mortality (9.3% vs. 7.2%; p = 0.110) and morbidity (33.4% vs. 32.1%; p = 0.564). The pulmonary complication rates did not differ between the groups (24.6% vs. 22.5%; p = 0.291), whereas the neoadjuvant CRT group had higher rates of chylothorax (2.5% vs. 1.2%; p = 0.020), cardiovascular complications (8.6% vs. 0.1%; p = 0.037), and thromboembolic events (8.6% vs. 6.0%; p = 0.037).
After propensity matching, only the incidence of chylothorax remained significant.24 In the CROSS trial, with a lower radiation dose (41.4 Gy), the postoperative outcomes did not differ based on the use of neoadjuvant CRT.1
Future research should focus on optimization of the neoadjuvant radiation dose and regimen, with fixed chemotherapy protocols. This could ideally be done with the use of a prospective randomized studies comparing neoadjuvant chemoradiation at different radiation doses. Additional studies should evaluate the impact of preoperative factors and histology on the effectiveness of various preoperative radiation doses.
The limitations to this study included its retrospective method for analyzing a national database, which limited its ability to compare patient groups. The database does not contain details on the type of chemotherapy used, which have may influenced response to neoadjuvant treatment and/or survival. Inaccurate clinical staging may have influenced the treatment decisions and/or outcomes examined in this study. Furthermore, the radiation-dose groups used in this analysis were not based on CRT regimen dosing, so the study may not have adequately analyzed differences between the accepted CRT regimens. Additionally, this database does not analyze patients who did not receive surgery.
In conclusion, in this retrospective review of the NCDB database, pCR and OS were inferior for patients who received neoadjuvant radiation doses lower than 39.6 Gy, whereas doses of 45 Gy or more did not show any added benefit. Based on these findings, we believe that adequate radiation dosing would lie between 39.6 and 45 Gy. Neoadjuvant CRT trials randomizing patients to different preoperative radiation doses may further clarify this issue.
References
Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Cao XF, He XT, Ji L, Xiao J, Lv J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:477–81.
Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92.
Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–7.
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13.
Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the esophagus: a randomized controlled phase III trial. Lancet Oncol. 2005;6:659–68.
Tai P, Yu E. Esophageal cancer management controversies: radiation oncology point of view. World J Gastrointest Oncol. 2014;6:263–74.
Minsky BD, Pajak TF, Pisansky TM, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74.
National Cancer Database. American College of Surgeons. Retrieved 11 Jan 2019 at https://www.facs.org/quality-programs/cancer/ncdb.
Radiation Therapy Oncology Group 1010. A phase III trial evaluating the addition of trastuzumab to trimodality treatment of HER2: overexpressing esophageal adenocarcinoma. In: Radiation Therapy Oncology Group. https://clinicaltrials.gov/ct2/show/NCT01196390?term=RTOG+1010&rank=1. Accessed 11 Jan 2019.
Cancer and Leukemia Group B; National Cancer Institute. PET Scan Imaging in Assessing Response in Patients with Esophageal Cancer Receiving Combination Chemotherapy. In: ClinicalTrials.gov. National Library of Medicine (US), Bethesda, MD. http://clinicaltrials.gov/show/NCT01333033. Accessed 11 Jan 2019.
Ordu AD, Nieder C, Geninitz H, et al. Association between radiation dose and pathologic complete response after preoperative radiochemotherapy in esophageal squamous cell cancer. Anticancer Res. 2014;34:7255–61.
Singla S, Gabriel E, Alnaji R, et al. Complete pathologic response is independent of the timing of esophagectomy following neoadjuvant chemoradiation for esophageal cancer. J Gastrointest Oncol. 2018;9:73–9.
Murphy MB, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: the association with baseline variables and survival. The University of Texas MD Anderson Cancer Center experience. Cancer. 2017;123:4106–13.
Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemo-radiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–9.
Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7.
Suh YG, Lee IJ, Koom WS, et al. High-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II–III esophageal cancer. Jpn J Clin Oncol. 2014;44:534–40.
He L, Allen PK, Potter A, et al. Reevaluating the optimal radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol. 2014;9:1398–405.
He L, Allen PK, Potter A, et al. Reevaluating radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol. 2014;9:1398–405.
Venkat PS, Shridhar R, Naghavi AO, et al. Dose-escalated neoadjuvant chemoradiotherapy with dose-painting intensity-modulated radiation therapy and improved pathologic complete response in locally advanced esophageal cancer. Dis Esophagus. 2017;30:1–9.
Lin S H, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2012;84:1078–85.
Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–22.
Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006;132:549–55.
FREGAT Working Group. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg. 2014;260:764–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Christopher W. Towe, MD, reports that he is a consultant for Zimmer Biomet, SigMedical, Atricure, and Medtronic, but that these relationships did affect this study or the accuracy of the data analysis. The other authors have no disclosures, sources of funding, or financial relationships to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Worrell, S.G., Towe, C.W., A. Dorth, J. et al. Higher Doses of Neoadjuvant Radiation for Esophageal Cancer Do Not Affect the Pathologic Complete Response Rate or Survival: A Propensity-Matched Analysis. Ann Surg Oncol 27, 500–508 (2020). https://doi.org/10.1245/s10434-019-07849-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07849-z