Abstract
Background
For pancreatic tumors located in the body or tail of the pancreas, distal pancreatectomy (DP) remains the surgical procedure of choice to achieve radical tumor removal. Purpose of this study was to evaluate outcome and overall survival of patients who underwent DP combined with multivisceral resection (MVR).
Methods
Retrospective single-center case-matched analysis. Between January 1994 and June 2014, 494 consecutive patients were entered into a prospective database, and 126 patients undergoing DP + MVR (cases) were matched with 126 patients undergoing DP (controls) for gender, age, and underlying final diagnosis.
Results
There were no significant differences in patient demographics. Rates of postoperative pancreatic fistula (POPF) (36 (28.6%) vs. 29 (23.0%); p = 0.388) and postpancreatectomy hemorrhage (PPH) (7 (5.5%) vs. 5 (3.9%); p = 0.769) did not reveal any significant differences. Although operative time (237.8 ± 57.9 vs. 203.5 ± 34.5; p < 0.001) and the necessity for intraoperative transfusions (18 (14.3%) vs. 5 (4.0%); p < 0.001) was significantly higher, the number of patients with major complications (the Clavien-Dindo ≥ 3) was not increased (27 (19.8%) vs. 20 (15.9%); p = 0.332) in the DP + MVR group. Midterm survival analysis indicated no significant difference for adenocarcinoma and neuroendocrine tumors for either group.
Conclusion
DP + MVR is a feasible and safe surgical procedure to achieve radical tumor removal and can offer beneficial survival outcomes. Although operative time and intraoperative transfusions are enhanced, POPF, PPH, or major complications (the Clavien-Dindo ≥ 3) are not significantly increased after DP + MVR. DP + MVR can therefore be recommended in selected patients for resection of extended tumors within the concept of interdisciplinary strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For pancreatic tumors that are located in the body or tail of the pancreas, distal pancreatectomy (DP) remains the surgical procedure of choice in order to achieve radical tumor removal and long-term survival.1,2,–3 Due to delayed symptoms, tumors are frequently diagnosed at more advanced stages and often appear with an involvement of adjacent structures. Positive resection margins have been identified as negative prognostic factor for local tumor recurrence and therefore radical tumor removal is requested to achieve the aim of a curative intended therapy.4,5 With increasing institutional expertise, more aggressive surgical techniques have been progressively improved over time and multivisceral resections (MVR) appear as feasible and reliable procedures with decreasing complication rates for selected patients.6 According to this continuous progress, resections of the superior mesenteric vein (SMV), portal vein (PV), or coeliac axis are nowadays considered as a safe and reliable technique when performed at high-volume centers.7,8 While previous studies indicated increased perioperative morbidity and mortality for patients undergoing multivisceral and extended resections during pancreatoduodenectomy, outcomes of tumors that are located within the body/tail of the pancreas requiring DP with additional MVR are less well studied and data are still limited.7,9,10,–11
The objective of this study was to investigate and report outcomes of patients who required DP combined with MVR and have completed at least 36 months of follow-up. In order to control and eliminate confounding factors (age, gender, histology, and indication for surgery), a case-matched study design was chosen to reduce selection bias, improve internal validity, and enable suitable comparisons and conclusions being made.
Methods
Patients’ Inclusion Criteria
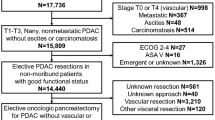
This was a retrospective single-center analysis conducted in a tertiary referral center for pancreatic surgery. All patients undergoing pancreatic resections between January 1994 and June 2014 were entered into a prospective database. Approval was granted by an independent ethics committee. Standard preoperative clinical diagnostics included physical examination and routine laboratory testing, including the tumor marker CA 19-9. Computed tomography (CT) and/or magnet resonance imaging (MRI) were routinely used as radiological diagnostic tools. All included operations were performed by experienced surgeons at the study site. All indications for pancreatic resections underlying malignant diseases were endorsed in an interdisciplinary consensus meeting. Due to the lack of evidence to perform multivisceral resections for pancreatic tumors using minimally invasive techniques, all distal pancreatectomies combined with multivisceral resections were performed by laparotomy. Accordingly, the matching process did not include minimally invasive distal pancreatectomies. We identified and reviewed an overall of 494 consecutive patients undergoing DP in a retrospective manner. A total of 126 patients underwent DP combined with MVR. All 126 patients (cases) have completed at least 3 years of follow-up and were eligible of matching with 126 DPs without MVR (controls) on a one-to-one basis for gender, age, and histology/indication for the surgical procedure. Indications for simple distal pancreatectomies are equivalent to the indications for distal pancreatectomies combined with MVR and are therefore stated singular in Table 4. Definition of DP extended to MVR was determined by the definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS).6
Preconditioning and Surgical Technique
When preoperative CT or MRI showed tumor involvement of the celiac axis and/or the common hepatic artery (CHA) but no affiliation to the superior mesenteric artery (SMA) or the gastroduodenal artery (GDA), patients were further evaluated for distal celiacopancreatectomy. If examinations revealed eligible conditions for resection, preoperative embolization of the celiac axis and the CHA was performed. Hereby, the arterial blood supply to the liver and stomach was enhanced through collateral pathways from the SMA over the pancreatoduodenal arcades to the GDA, the proper hepatic artery (PHA), the gastroepiploic artery, and the right gastric artery as previously described.12 At the beginning of the operation, peritoneal metastases were initially excluded by complete exploration of the abdominal cavity. Access to the omental bursa was established by dissection of the gastrocolic ligament. After retraction of the stomach and inspection of the pancreas, the local resectability of the lesion and the extent of the resection (especially the need for MVR) were determined based on local findings such as vascular and/or another organ infiltration. In cases of underlying malignant disease or precancerous lesions, a splenectomy and standard lymphadenectomy was performed. Patients with benign lesions received a spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Dissection of the pancreas was either done by electrocautery, or a stapling device. In cases of pancreatic resections performed by electrocautery, a subsequent closure of the main pancreatic duct of the pancreatic remnant was achieved by a stitch ligation using 4–0 polypropylene sutures, followed by single U-shaped 4–0 polypropylene sutures (Prolene, Johnson & Johnson Medical GmbH, Norderstedt, Germany). In case of a pancreatoenteral anastomosis, either a pancreatojejunostomy or a pancreatogastrostomy was performed using mattress sutures placed in a “U” shape combined with two corner sutures. Distal closure of the pancreas remnant by stapler was performed using linear stapling devices armed with a 60-mm cartridge (EndoGIA™, Auto-Suture, Covidien) reinforced by a bioabsorbable mesh (SEAMGUARD®, W.L. Gore, Flagstaff, AZ). Every patient received at least one intra-abdominal drain (Degania Silicone Europe GmbH, Regensburg, Germany) to measure postoperative amylase levels and drain output in the postoperative course.
Standard Postoperative Care
Postoperative care was standardized in both groups. All patients were monitored for at least 1 day at a specialized surgical intensive care unit. Amylase levels were monitored in the serum and in the intraoperatively placed abdominal drains on the second and fourth postoperative day. Routine perioperative antibiotics [cefuroxime 1 g intravenously (i.v.) and metronidazole 500 mg i.v.] were given. In the absence of signs of a pancreatic fistula, oral food intake was started depending on the clinical presentation and tolerance. The concept of enhanced recovery after surgery (ERAS) has not been applied within the study period.
Data Collection
The following data were collected for each patient: demographics (age, gender); body mass index; comorbidities (diabetes mellitus, history of pancreatitis); preoperative laboratory findings such as serum levels of CA 19-9; associated resections within MVR (spleen, stomach, liver metastases, left colon, vascular structures such as celiac axis, PV, and/or SMV, left adrenal gland); operative details such as operation time and intraoperative transfusion; results of the final histopathological examination (ductal adenocarcinoma, neuroendocrine tumors, mucinous cystic neoplasm, malignant intraductal papillary mucinous neoplasm (IPMN), metastasis); details of the postoperative course such as postoperative morbidity and frequency of interventional measures to treat severe complications (according to the Clavien-Dindo classification) in terms of postoperative pancreatic fistula (POPF), postpancreatectomy hemorrhage (PPH), and abdominal collection which were all classified according to International Study Group of Pancreatic Fistula (ISGPF) definitions13,14; length of hospital stay (LOS) which was calculated from the day of surgery including the day of discharge; in-hospital mortality after surgery as well as 30- and 90-day mortality and an admission to any hospital for more than 24 h within 30 days after surgery which was defined as readmission; long-term follow-up which was assessed by our oncological outpatient clinic, and the review of medical records as well as direct communication with the general practitioner tracking patient survival or the documented day of death.
Statistics
The analyses were based on a case-matched study design. Statistics were presented as the mean (standard deviation) or numbers (%). The quantitative variables intensive care unit (ICU) stay and length of hospital stay are expressed as median with interquartile range. Paired Student’s t test or the Wilcoxon signed-rank test was used to compare means of quantitative variables as appropriate. Categorical data were compared using chi-square tests or Fisher’s exact tests as appropriate. All 126 patients completed at least 3 years of follow-up and long-term data was available for all cases and controls. Therefore, survival analysis indicates observed survival and does not include any censored data. Significance tests were two-sided, and p < 0.05 was considered to be statistically significant. All statistical analyses, as well as initial matching of cases and controls, were performed using SPSS version 24.0 (SPSS, Chicago, IL, USA).
Results
Baseline Characteristics
Between January 1994 and June 2014, a total of 494 consecutive patients underwent DP at our tertiary referral center for pancreatic surgery. A total of 368 patients (74.4%) underwent DP exclusively, while 126 patients (25.5%) required additional MVR in order to achieve radical tumor removal. In accordance with the matched-pair study design, 126 patients were included in our study and were eligible for matching on a one-to-one basis for sex, age, and histology/indication for surgery. Minimally invasive distal pancreatectomies were not included within the matching process.
Both groups had similar clinical characteristics at baseline, with a mean patient age of 60 years and the majority of patients were male. The BMI (kg/m2) prior to surgery (23.6 ± 3.9 vs. 24.7 ± 3.4; p = 0.351), as well as pre-existing diabetes mellitus (24 (19.0%) vs. 20 (15.8); p = 0.421) and a history of pancreatitis (16 (12.7%) vs. 14 (11.1%); p = 0.599), was similar in both groups. A comparison of preoperative CA 19-9 (kU/l) demonstrated a significant difference between cases and controls (90.5 (3–1050) vs. 75.7 (3–1217); p < 0.001) (Table 1).
Operative Details and Postoperative Course
Within the MVR group, all additional resections were performed during DP. Additional resections included spleen n = 107 (84.9%), stomach n = 77 (61.1%), liver metastasis n = 47 (37.3%), and left colon n = 29 (23.0%). Vascular structures (celiac axis/PV and/or SMV) had to be resected in 27 cases (21.4%), including 11 Appleby procedures and 16 wedge resections to the axis of the SMV/PV. Resection of the left adrenal gland was performed in 19 cases (15.1%) (Table 2).
Operative time was significantly longer in the case group (237.8 ± 57.9 vs. 203.5 ± 34.5; p < 0.001). Furthermore, an increased necessity for intraoperative transfusions was observed in the MVR group (18 (14.3%) vs. 5 (4.0%); p < 0.001). Closure of pancreas remnant was mainly achieved by suture (106 (84.1%) vs. 101 (80.5%)) or a stapling device (19 (15.1) vs. 24 (19.0)). There was only one patient with a pancreatogastrostomy and pancreatojejunostomy in each group. Rates of postoperative pancreatic fistula (36 (28.6%) vs. 29 (23.0%); p = 0.388) and postpancreatectomy hemorrhage (7 (5.5%) vs. 5 (3.9%); p = 0.769) did not reveal any differences. Postoperative abdominal collections were identified in 26 patients (20.6%) in the DP + MVR group and in 18 patients (14.3%) in the DP group with no significant difference in the statistical assessment (p = 0.245). The number of patients with major complications according to the Clavien-Dindo classification (the Clavien-Dindo ≥ 3) was not significantly higher for either group (27 (19.8%) vs. 20 (15.9%); p = 0.332). Patients in the DP + MVR group had a significantly longer ICU and overall hospital stay (3 [1–5] vs. 1 [1–4]; p < 0.001; 12 [6–41] vs. 9 [6–23]; p < 0.001, respectively). Reoperation within 30 days (5 (3.9%) vs. 2 (1.6%); p = 0.447) and readmission within 30 days (10 (7.9%) vs. 8 (6.3%); p = 0.808) were comparable in both groups. Additionally, 30-day mortality rates demonstrated no significant differences between the two groups (3 (2.4%) vs. 1 (0.8%); p = 0.622) (Table 3).
Histology/Indication for Surgery
Indication for surgery included benign and malignant neoplasms. All 126 patients (cases) were matched with 126 DPs without MVR (controls) on a one-to-one basis for gender, age, and histology/indication for the surgical procedure. Indications for surgery for the simple distal pancreatectomies are equivalent to the indications for distal pancreatectomies combined with MVR and are therefore stated singular in Table 4. Sixty-five patients (51.6%) suffered from ductal adenocarcinoma. In 43 patients (34.1%), a neuroendocrine tumor was the cause for the surgical intervention. Mucinous cystic neoplasm was observed in nine cases (7.1%). Histopathological examination revealed metastasis, mainly due to renal cell carcinoma in five patients (4.0%). IPMN with inherent malignant transformation was discovered in four patients (3.2%) (Table 4).
Survival Analysis
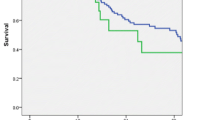
Sixty-one cases of ductal adenocarcinoma and 43 cases of neuroendocrine tumors were matched in this analysis. All 126 patients completed at least 3 years of follow-up and long-term data was available for all subjects. Survival analysis indicates observed survival and does not include any censored data. A median survival of 29 months was observed in patients with adenocarcinoma who received DP + MVR, which was comparable to the survival after standard resection (34 months). Neuroendocrine tumors revealed a higher median survival (36 months in both groups). Again, there was no significant survival difference when comparing cases to controls (Fig. 1).
Discussion
Tumors that are located in the body or tail of the pancreas may in some cases require DP combined with additional MVR in order to achieve radical tumor removal during DP.1,2,–3 In this study, we demonstrated that DP combined with MVR is a safe and feasible procedure with no increased rates of POPF, PPH, or major complications (the Clavien-Dindo ≥ 3) compared to standard procedures. While abdominal collections, reoperations, readmissions, and in-hospital mortality as well as 30- and 90-day mortality following DP + MVR is comparable to DR alone, DP + MVR additionally permitted similar midterm overall survival rates in comparison to patients undergoing standard DP when suffering from adenocarcinoma or neuroendocrine tumors. In consequence of these extended surgical procedures, operative time, need for intraoperative transfusions, and ICU and length of hospital stay are modestly extended.
Numerous studies have evaluated outcomes in patients undergoing multivisceral and extended resections during pancreatoduodenectomy and revealed increased perioperative morbidity and mortality rates.15,16 In contrast, outcomes of patients with tumors located within the body/tail of the pancreas requiring DP with additional MVR are less well studied and guiding data is still limited. Panzeri et al. presented results from a single-center experience and depicted acceptable surgical complication rates and favorable long-term survival for distal pancreatectomies associated with multivisceral resections.11 In another study, Irani et al. described a wide variety of indications for distal pancreatectomy and demonstrated comparable morbidity and mortality rates even for extended or multivisceral resections, recommending extended procedures as clear conclusion.17 Both publications come from high-volume centers and findings are in accordance with our results underlining the potential benefit of DP + MVR for individually selected patients with advanced tumors. In this context, hospital volume has already been identified to be a significant independent variable of morbidity and mortality following pancreatic surgery in a variety of observational studies.18,19 Krautz et al. even demonstrated a clear relation between hospital volume and mortality in case of major complications and therefore strictly recommended a centralization of pancreatic surgery to immediately reduce morbidity and mortality rates of patients undergoing pancreatic resections.20 Other authors added, beyond in-hospital mortality, long-term survival is also improved at high-volume centers.21 Currently, the only chance of cure for a pancreatic neoplasm is the complete margin free radical surgical resection. Based on the present evidence, referral to high-volume pancreatic surgery centers may decrease the risk of mortality and assures optimal interdisciplinary management of pancreatic neoplasms.
From a surgical point of view, POPF remains the most common and clinically relevant complication following DP and may lead to pancreatic fluid collection, intra-abdominal abscesses, wound infection, and sepsis.22 Various technical factors including the method of stump closure, associated organ resection, necessity of intraoperative transfusion, and prolonged operation time were shown to correlate with an increased risk for POPF.23 Although DP + MVR revealed a significant increase in terms of operative time and need for intraoperative transfusions in our study, we did not observe a remarkable difference regarding POPF.
Currently, there is no consensus for the ideal operative technique of stump closure in order to reduce the occurrence of POPF. Numerous studies observed a significant increased risk of POPF when using stapling devices, whereas other reports revealed increased POPF rates with sutured closure.24 The randomized DISPACT study could however not reveal any differences between both methods in postoperative outcomes, keeping a controversial debate ongoing.25 In this cohort, subsequent stump closure of the main pancreatic duct of the pancreatic remnant was mainly achieved by a stitch ligation using 4–0 polypropylene sutures, followed by single U-shaped 4–0 polypropylene sutures and in selected cases, we additionally administered bovine serum albumin-glutaraldehyde (BioGlue, Cryolife Inc., Kennesaw, GA, USA) into the fish-mouth cavity for a reinforced closure.26 However, an increased usage of stapling devices could be observed over the last 10 years with a still ascending trend. Due to the discordant literature, pancreatic surgeons should have the ability to perform different established methods and decide which one to use based on the intraoperative findings targeting the best suitable solution.
Despite all advancements in surgical techniques and institutional expertise, the prognosis of patients adversely affected by adenocarcinoma remains relatively poor.27 A promising development in recent years has been the successful introduction of neoadjuvant treatment concepts for patients with borderline resectable disease.28 Different regimes have been investigated, targeting micrometastatic disease and a traceable reduction of tumor volume to potentially increase the likelihood of a complete resection.29 Again, the focus of attention is mainly directed towards neoplasms that are located in the head of the pancreas, while tumors that are located within the body/tail of the pancreas requiring DP with additional MVR are less well studied. Additional studies need to be determined to further investigate the optimal interaction between neoadjuvant treatment and surgical procedure, which could beneficially impact patients’ outcome.
The strengths of our study include (i) the case-matched study design, (ii) the homogeneity within the groups, and (iii) the possibility to report 3-year survival rates for adenocarcinomas as well as neuroendocrine tumors. The present study is limited by common biases that are mainly due to the retrospective character of this analysis. Precision and completeness of data acquisition are very difficult to control especially over such an extended study period. To encounter this condition, we used a carefully matched control group. In order to accurately access survival, patients with less than 3 years of follow-up have been excluded. This methodical process could possibly penalize the ability to detect differences in perioperative events.
At the present time, procedures tailored to the individual needs of our patients are to be seen as the current advancement of choice. In conclusion, DP + MVR is a feasible and safe surgical procedure to achieve radical tumor removal and can offer beneficial survival outcomes in patients with advanced tumor stages when performed at high-volume centers. The length of hospital stay and morbidity rates are comparable to those of patients that required distal pancreatectomy alone. Prospective multicenter studies are however needed to validate these results.
Conclusion
Our results demonstrate that DP + MVR is a feasible and safe approach that enables survival rates after resection of adenocarcinoma or neuroendocrine tumors comparable to those following standard procedures. Operative time, need for intraoperative transfusions as well as ICU and length of hospital stay are quite modestly increased due to the extended surgical procedures. However, POPF, PPH, or major complication (the Clavien-Dindo ≥ 3) rates are not significantly increased after DP + MVR and therefore, this procedure can be recommended for the treatment of extended tumors in selected patients within the concept of interdisciplinary strategies.
References
Paye F, Micelli Lupinacci R, Bachellier P, Boher JM, Delpero JR, French Surgical A. Distal pancreatectomy for pancreatic carcinoma in the era of multimodal treatment. Br J Surg. 2015;102(3):229–36.
de Rooij T, Tol JA, van Eijck CH, Boerma D, Bonsing BA, Bosscha K, van Dam RM, Dijkgraaf MG, Gerhards MF, van Goor H, van der Harst E, de Hingh IH, Kazemier G, Klaase JM, Molenaar IQ, Patijn GA, van Santvoort HC, Scheepers JJ, van der Schelling GP, Sieders E, Busch OR, Besselink MG, Dutch Pancreatic Cancer G. Outcomes of Distal Pancreatectomy for Pancreatic Ductal Adenocarcinoma in the Netherlands: A Nationwide Retrospective Analysis. Ann Surg Oncol. 2016;23(2):585–91.
Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229(5):693–8; discussion 8-700.
Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–5.
Ruess DA, Makowiec F, Chikhladze S, Sick O, Riediger H, Hopt UT, Wittel UA. The prognostic influence of intrapancreatic tumor location on survival after resection of pancreatic ductal adenocarcinoma. BMC Surg. 2015;15:123.
Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M, Andren-Sandberg A, Asbun HJ, Bassi C, Bockhorn M, Charnley R, Conlon KC, Dervenis C, Fernandez-Cruz L, Friess H, Gouma DJ, Imrie CW, Lillemoe KD, Milicevic MN, Montorsi M, Shrikhande SV, Vashist YK, Izbicki JR, Buchler MW, International Study Group on Pancreatic S. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156(1):1–14.
Andren-Sandberg A. Tumors of the body and tail of the pancreas. N Am J Med Sci. 2011;3(11):489–94.
Hirono S, Kawai M, Tani M, Okada K, Miyazawa M, Shimizu A, Kitahata Y, Yamaue H. Indication for the use of an interposed graft during portal vein and/or superior mesenteric vein reconstruction in pancreatic resection based on perioperative outcomes. Langenbecks Arch Surg. 2014;399(4):461–71.
Kulemann B, Hoeppner J, Wittel U, Glatz T, Keck T, Wellner UF, Bronsert P, Sick O, Hopt UT, Makowiec F, Riediger H. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg. 2015;19(3):438–44.
Bhayani NH, Enomoto LM, James BC, Ortenzi G, Kaifi JT, Kimchi ET, Staveley-O’Carroll KF, Gusani NJ. Multivisceral and extended resections during pancreatoduodenectomy increase morbidity and mortality. Surgery. 2014;155(3):567–74.
Panzeri F, Marchegiani G, Malleo G, Malpaga A, Maggino L, Marchese T, Salvia R, Bassi C, Butturini G. Distal pancreatectomy associated with multivisceral resection: results from a single centre experience. Langenbecks Arch Surg. 2017;402(3):457–64.
Denecke T, Andreou A, Podrabsky P, Grieser C, Warnick P, Bahra M, Klein F, Hamm B, Neuhaus P, Glanemann M. Distal pancreatectomy with en bloc resection of the celiac trunk for extended pancreatic tumor disease: an interdisciplinary approach. Cardiovasc Intervent Radiol. 2011;34(5):1058–64.
Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Buchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–5.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula D. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13.
McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246(2):246–53.
Sun J, Yang Y, Wang X, Yu Z, Zhang T, Song J, Zhao H, Wen J, Du Y, Lau WY, Zhang Y. Meta-analysis of the efficacies of extended and standard pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas. World J Surg. 2014;38(10):2708–15.
Irani JL, Ashley SW, Brooks DC, Osteen RT, Raut CP, Russell S, Swanson RS, Whang EE, Zinner MJ, Clancy TE. Distal pancreatectomy is not associated with increased perioperative morbidity when performed as part of a multivisceral resection. J Gastrointest Surg. 2008;12(12):2177–82.
Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260(2):244–51.
Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37.
Krautz C, Nimptsch U, Weber GF, Mansky T, Grutzmann R. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann Surg. 2017.
Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-Term Survival Is Superior After Resection for Cancer in High-Volume Centers. Transactions of the Meeting of the American Surgical Association. 2005;123(&NA;):234–41.
Fahy BN, Frey CF, Ho HS, Beckett L, Bold RJ. Morbidity, mortality, and technical factors of distal pancreatectomy. Am J Surg. 2002;183(3):237–41.
Kleeff J, Diener MK, Z’Graggen K, Hinz U, Wagner M, Bachmann J, Zehetner J, Muller MW, Friess H, Buchler MW. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245(4):573–82.
Zhang H, Zhu F, Shen M, Tian R, Shi CJ, Wang X, Jiang JX, Hu J, Wang M, Qin RY. Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br J Surg. 2015;102(1):4–15.
Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, Tomazic A, Bruns CJ, Busch ORC, Farkas S, Belyaev O, Neoptolemos JP, Halloran C, Keck T, Niedergethmann M, Gellert K, Witzigmann H, Kollmar O, Langer P, Steger U, Neudecker J, Berrevoet F, Ganzera S, Heiss MM, Luntz SP, Bruckner T, Kieser M, Büchler MW. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. The Lancet. 2011;377(9776):1514–22.
Klein F, Sauer IM, Pratschke J, Bahra M. Bovine Serum Albumin-Glutaraldehyde Sealed Fish-Mouth Closure of the Pancreatic Remnant during Distal Pancreatectomy. HPB Surg. 2017;2017:9747421.
GLOBOCAN. European age-standardised rates calculated by the statistical information team at cancer research UK. 2011 using data from GLOBOCAN 2008 v1.2, IARC, version 1 2. Available at: http://globocan.iarc.fr.
Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, Asbun HJ, Bassi C, Buchler M, Charnley RM, Conlon K, Cruz LF, Dervenis C, Fingerhutt A, Friess H, Gouma DJ, Hartwig W, Lillemoe KD, Montorsi M, Neoptolemos JP, Shrikhande SV, Takaori K, Traverso W, Vashist YK, Vollmer C, Yeo CJ, Izbicki JR, International Study Group of Pancreatic S. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2014;155(6):977–88.
Schorn S, Demir IE, Reyes CM, Saricaoglu C, Samm N, Schirren R, Tieftrunk E, Hartmann D, Friess H, Ceyhan GO. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma—A systematic review and meta-analysis. Cancer Treat Rev. 2017;55:96–106.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception or design of the work: TM, FK, AA, JP, MB. Acquisition, analysis, or interpretation of data for the work: TM, FK, AA, JP, MB. Drafting the work or revising it critically for important intellectual content: TM, FK, AA, JP, MB. Final approval of the version to be published: TM, FK, AA, JP, MB.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
This study was presented as oral presentation at Viszeralmedizin 2017, Dresden, September 13–16, 2017.
Rights and permissions
About this article
Cite this article
Malinka, T., Klein, F., Andreou, A. et al. Distal Pancreatectomy Combined with Multivisceral Resection Is Associated with Postoperative Complication Rates and Survival Comparable to Those After Standard Procedures. J Gastrointest Surg 22, 1549–1556 (2018). https://doi.org/10.1007/s11605-018-3804-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3804-z