Abstract
Background
The purpose of the present study was to evaluate the efficacy of extended pancreatoduodenectomy (EPD) and standard pancreatoduodenectomy (SPD) for ductal adenocarcinoma of the head of the pancreas via meta-analysis.
Methods
Relevant articles (published between 1995 and 2012) were compiled from online data sources. A total of nine studies satisfied the selection criteria, including a total of 973 patients (478 in the SPD group and 495 in the EPD group). Evaluation parameters included 1-, 3-, and 5-year survival, as well as mortality, morbidity, and specific morbidity outcomes.
Results
Meta-analysis revealed (1) differences in morbidity (Odds ratio [OR] = 1.740; 95 % confidence interval [CI], 0.840–3.600; P = 0.140), mortality (OR = 0.890; 95 % CI, 0.560–1.400; P = 0.620), 1-year overall survival (OS) rate (OR = 1.20; 95 % CI, 0.490–2.930; P = 0.69), 3-year OS rate (OR = 0.770; 95 % CI, 0.460–1.280; P = 0.190), and 5-year OS rate (OR = 1.12; 95 % CI, 0.690–1.810; P = 0.560) were not significant between EPD and SPD. (2) For bile leak (OR = 2.640; 95 % CI, 1.040–6.700; P = 0.040), pancreatic leak (OR = 1.740; 95 % CI, 1.040–2.91; P = 0.030), delayed gastric emptying (OR = 2.090; 95 % CI, 1.240–3.520; P = 0.006), and lymphatic fistula (OR = 6.120; 95 % CI, 1.06–35.320; P = 0.040) differences between EPD and SPD were significant, whereas other specific morbidities were not significantly different.
Conclusions
Extended pancreatoduodenectomy does not improve 1-, 3-, 5-year OS rates compared to SPD and there is a trend toward increased bile leak, pancreatic leak, delayed gastric emptying, and lymphatic fistula after EPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic adenocarcinoma is an aggressive malignant disease of the pancreas with a 5-year survival rate of less than 5 %. In the United States, it is the fourth leading cause of cancer-related deaths, with an estimated 43,920 new cases and 37,390 deaths in 2012. The majority of patients initially present with advanced and metastatic disease, with only 10 % to 15 % of patients being candidates for surgical resection [1]. Surgery remains the mainstay of treatment, with extended pancreatoduodenectomy (EPD) on patients with metastasis to the lymph nodes first performed by Fortner [2] in the mid-1970s. However, there has been controversy over whether EPD could reduce recurrence or prolong survival. The first prospective randomized controlled trial (RCT) to compare the results of SPD versus EPD in radical pancreatoduodenectomy for carcinoma of the head of the pancreas was conducted by Pedrazzoli et al. [3]. Their study objectively evaluated the value of SPD and EPD in radical pancreatoduodenectomy for treating ductal adenocarcinoma of the head of the pancreas, with the aim of providing a better reference-point for improved clinical decision making.
Materials and methods
Study objectives
Published prospective randomized studies and prospective non-randomized studies comparing SPD and EPD in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas over the past 20 years were reviewed. All studies included experimental details and complete follow-up data.
Data sources
PubMed and EMBASE were searched for articles published in the English language using the terms: pancreatic cancer; pancreatic neoplasm; extended; radical; standard.
Inclusion criteria
The following were inclusion criteria for the present study: (1) patients were diagnosed with pancreatic cancer (data sources published in English); (2) source materials included comparison between pancreatoduodenectomy with SPD (the head of the pancreas, a portion of the bile duct, the gallbladder, and the duodenum are removed, the distal 2/3 to 3/4 of the stomach, 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, 12a, 12b1, 12c, 13a, 13b, 14a, 14b, 17a, 17b lymph nodes) and EPD (the head of the pancreas, a portion of the bile duct, the gallbladder, and the duodenum are removed, the distal 2/3 to 3/4 of the stomach, 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9, 11p, 12a, 12b1, 12c, 13a, 13b, 14a, 14b, 17a, 17b, all8, 9, all12, all14, 16a2, 16b1 lymph nodes) [4]. (3) the article included survival, mortality, and morbidity data, as well as the number of resected lymph nodes and detailed morbidity.
Exclusion criteria
The following were the exclusion criteria for the present study: (1) patients with ampullary, distal bile duct, or duodenal carcinoma; (2) retrospective studies; (3) studies lacking follow-up data and control groups.
Data extraction
Two authors searched the literature and selected documents independently of each other; they extracted data according to the same standards. Data extracted included the first author, date of publication, standard for selected patients, EPD and SPD study groups, operative method, endpoints, withdrawal cases, statistical methods, mortality, complications, and 1-, 3-, and 5-year survival rates. All articles included were assessed for quality using the Jada score (Table 1) [5].
Statistical analysis
All data were analyzed with RevMan 4.2 software. Endpoints, including mortality, complications, and 1-, 3-, and 5-year survival rates, and specific complication heterogeneity were tested with the χ 2 test (α = 0.01). Data showing heterogeneity were subjected to the random effects model. In cases of non-heterogeneity, data were subjected to the fixed-effects model. The odds ratio (OR) and confidence intervals (CI) were calculated. If P < 0.05, values were deemed statistically significantly different between the two groups.
Results
Overview of included studies
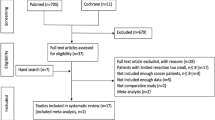
Nine studies, including four prospective randomized and four prospective non-randomized studies, were included, with a combined total of 973 cases (478 cases in the SPD group and 495 in the EPD group) (Table 2 and Fig. 1).
Comparison of postoperative outcomes
Eight of the nine studies evaluated morbidity for surgery. The homogeneity test result was not significant (χ 2 = 28.57; df = 7; P = 0.002; I 2 = 75.5 %). Results of the analysis showed that total morbidity in the EPD group was not significantly lower than that in the SPD group (OR = 1.740; 95 % CI, 0.840–3.600, P = 0.140) (Fig. 2).
Eight of the nine studies compared mortality after surgery between the EPD and SPD groups. The homogeneity test revealed the presence of heterogeneity (χ 2 = 2.56; df = 7; P = 0.92; I 2 = 0 %) and therefore we adopted the fixed-effects model for further analysis. Here, the mortality of the EPD group was not significantly different compared with the SPD group (OR = 0.890; 95 % CI, 0.560–1.400; P = 0.620) (Fig. 3).
Six of the nine studies reported 1-year survival rates after surgery. Analysis of the pooled data showed that the 1-year survival rate in the EPD group was not significantly different from that in the SPD group (χ 2 = 28.72; df = 6; P < 0.0001; I 2 = 82.6 %), (OR = 1.20; 95 % CI, 0.490–2.930; P = 0.690) (Fig. 4).
Seven of the nine studies compared the 3-year survival rates after surgery between the EPD and SPD groups. The results of the homogeneity test revealed heterogeneity in the data (χ 2 = 8.84; df = 6; P = 0.18; I 2 = 32.1 %) and therefore the fixed-effects model was adopted. Here, the 3-year survival rate of the EPD group was not significantly different to the SPD group (OR = 0.770, 95 % CI, 0.460–1.280, P = 0.190) (Fig. 5).
Seven of nine studies tested the 5-year survival rates after surgery. The 5-year survival rate of the EPD group was not significantly different to the SPD group (OR = 1.12; 95 % CI, 0.690–1.810; P = 0.560) (Fig. 6).
The difference was significant between EPD and SPD for bile leak (OR = 2.640; 95 % CI, 1.040–6.700; P = 0.040) (Fig. 7), pancreatic leak (OR = 1.740; 95 % CI, 1.040–2.91; P = 0.030) (Fig. 8), delayed gastric emptying (OR = 2.090; 95 % CI, 1.240–3.520; P = 0.006) (Fig. 9), and lymphatic fistula (OR = 6.120; 95 % CI, 1.06–35.320; P = 0.040) (Fig. 10).
Differences between the two groups in intra-abdominal hemorrhage (OR = 1.42; 95 % CI, 0.620–3.250; P = 0.410); intra-abdominal abscess (OR = 0.770; 95 % CI 0.460–1.280; P = 0.190), wound infection (OR = 2.050; 95 % CI, 1.010–4.180; P = 0.050), gastroenteric leak (OR = 0.330; 95 % CI, 0.050–2.060; P = 0.230), pneumonia (OR = 0.570; 95 % CI, 0.150–2.270; P = 0.430), stump pancreatitis (OR = 2.470; 95 % CI, 0.350–17.150; P = 0.360), obstruction (OR = 1.870; 95 % CI, 0.230–15.540; P = 0.560), thrombosis (OR = 1.020; 95 % CI, 0.180–5.590; P = 0.980), cholangitis (OR = 1.29; 0,95 % CI, 0.340–4.910; P = 0.710), reoperation (OR = 1.130; 95 % CI, 0.570–2.220; P = 0.730), and diarrhea (OR = 5.950; 95 % CI, 0.680–51.80; P = 0.110) were not statistically significant.
Discussion
Pancreatic lymph node metastasis at the head of the pancreas occurs during the progression of ductal adenocarcinoma of the head of the pancreas, extended or standard lymphadenectomy in pancreatoduodenectomy for pancreatic head adenocarcinoma remains controversial. Extended pancreatography was first performed in Japan in the mid-1970 s, and was widely used in the late 1980s and 1990s. Two studies reported significantly better survival rates following EPD compared with SPD [18, 19], while in the 1980s and 1990s, the 5-year survival rate in EPD from seven retrospective studies J. F. Sun, Y. X. Yang, X. Lu, and J. Song contributed equally to this work. All authors contributed to the design and interpretation of the study and to further drafts. Y. W. Zhang is the guarantor [20–26]. was found to increase by as much as 29.7 %.
No differences in the 1-, 3-, and 5-year overall survival (OS) rates and mortality between EPD and SPD were found in the current analysis. In subgroups of carcinoma of the head of the pancreas with node-negative patients, however, some increases in 5-year survival rates have previously been reported. In two prospective, non-randomized studies [27], the rates of diarrhea were higher in the EPD groups than in the SPD groups, but overall morbidity and mortality did not differ [6, 28]. In the article by Farnell et al. [15], 42 % of the 19 patients in the EPD group surveyed experienced “very much” diarrhea, compared with 8 % of the 24 patients surveyed in the SPD group (P = 0.01) at 4 months. Eight months later, the incidence of diarrhea was not different between the SPD and EPD groups. In our meta-analysis, overall morbidity and mortality were not statistically significantly different.
Some prospective randomized controlled studies investigated the long-term advantages and disadvantages of EPD. None of the randomized controlled studies, except in a subgroup of carcinoma of the head of the pancreas revealed improvement of survival rates for EPD, but delayed gastric emptying, diarrhea, and overall morbidity tended to occur more frequently in EPD [3]. Except for bile leak, pancreatic leak, lymphatic fistula, and delayed gastric emptying, no other specific morbidity difference in EPD was found in our analysis.
This meta-analysis is limited by several factors: (1) differences in ranges of lymphadenectomy from several studies; (2) the different proportion of patients with different adjuvant therapy; (3) differences in the diagnostic criteria of complications and the overall mortality; (4) the small number of cases in all studies.
Conclusions
From this meta-analysis, EPD is not generally recommended, This conclusion has limitations, however, and further large, multicenter, randomized studies are required to confirm this finding.
References
Feig C, Gopinathan A, Neesse A et al (2012) The pancreas cancer microenvironment. Clin Cancer Res 18(16):4266–4276
Fortner JG (1973) Regional resection and pancreatic carcinoma. Surgery 73(5):799–800
Pedrazzoli S, DiCarlo V, Dionigi R et al (1998) Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 228(4):508–517
Pedrazzoli S, Beger HG, Obertop H et al (1999) A surgical and pathological based classification of resective treatment of pancreatic cancer. Summary of an international workshop on surgical procedures in pancreatic cancer. Dig Surg 16(4):337–345
Huang ZY, Ma J, Pei F, Yang J et al (2013) Meta-analysis of temporary versus no clamping in TKA. Orthopedics 36(7):543–550
Henne-Bruns D, Kremer B, Meyer-Pannwitt U et al (1993) Partial duodenopancreatectomy with radical lymphadenectomy in patients with pancreatic and periampullary carcinomas: initial results. Hepatogastroenterology 40(2):145–149
Henne-Bruns D, Vogel I, Luttges J et al (1998) Ductal adenocarcinoma of the pancreas head: survival after regional versus extended lymphadenectomy. Hepatogastroenterology 45(21):855–866
Henne-Bruns D, Vogel I, Luttges J et al (2000) Surgery for ductal adenocarcinoma of the pancreatic head: staging, complications and survival after regional versus extended lymphadenectomy. World J Surg 24(5):595–601 Discussion 601–602
Gazzaniga GM, Cappato S, Papadia F et al (2001) D1 versus D2 pancreatoduodenectomy in surgical therapy of pancreatic head cancer. Hepatogastroenterology 48(41):1471–1478
Yeo CJ, Cameron JL, Sohn TA et al (1999) Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 229(5):613–622 Discussion 622–614
Yeo CJ, Yeo CJ, Cameron JL, Lillemoe KD et al (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphade nectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236(3):355–366 discussion 366–368
Iacono C, Accordini S, Bortolasi L et al (2002) Results of pancreaticoduodenectomy for pancreatic cancer: extended versus standard procedure. World J Surg 26(11):1309–1314
Popiela T, Kedra B, Sierzega M (2002) Does extended lymphadenectomy improve survival of pancreatic cancer patients? Acta Chir Belg 102(2):78–82
Capussotti L, Massucco P, Ribero D et al (2003) Extended lymphadenectomy and vein resection for pancreatic head cancer: outcomes and implications for therapy. Arch Surg 138(12):1316–1322
Farnell MB, Pearson RK, Sarr MG et al (2005) A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery 138(4):618–628 Discussion 628–630
Nimura Y, Nagino M, Takao S et al (2012) Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci 19(3):230–241
Nimura Y, Nagino M, Kato H, et al (2004) Regional versus extended lymph node dissection in radical pancreaticoduodenectomy for pancreatic cancer: a multicenter, randomized controlled trial. HPB 6 (Suppl 1):2 (abstract)
Ishikawa O, Ohhigashi H, Sasaki Y et al (1988) Practical usefulness of lymphatic and connective tissue clearance for carcinoma of the pancreas head. Ann Surg 208(2):215–220
Manabe T, Ohshio G, Baba N et al (1989) Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer 64(5):1132–1137
Nagakawa T, Kurachi M, Konishi K et al (1982) Translateral retroperitoneal approach in radical surgery for pancreatic carcinoma. Jpn J Surg 12(3):229–233
Ishikawa O, Ohhigashi H, Sasaki Y et al (1998) Practical usefulness of lymphatic and connective tissue clearance for carcinoma of the pancreas head. Ann Surg 208(2):215–220
Manabe T, Ohshio G, Baba N et al (1989) Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer 64(5):1132–1137
Nagakawa T, Konishi I, Ueno K et al (1991) Surgical treatment of pancreatic cancer. The Japanese experience. Int J Pancreatol 9(1):135–143
Nagakawa T, Kobayashi H, Ueno K et al (1994) Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer 73(4):1155–1162
Nakao A, Harada A, Nonami T et al (1995) Lymph node metastases in carcinoma of the head of the pancreas region. Br J Surg 82(3):399–402
Kayahara M, Nagakawa T, Ueno K et al (1995) Surgical strategy for carcinoma of the pancreas head area based on clinicopathologic analysis of nodal involvement and plexus invasion. Surgery 117(6):616–623
Michalski CW, Kleeff J, Wente MN et al (2007) Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 94(3):265–273
Gazzaniga GM, Cappato S, Papadia F et al (2001) D1 versus D2 in surgical therapy of pancreatic head cancer. Hepatogastroenterology 48(41):1471–1478
Acknowledgments
No benefits in any form have been received or will be received by any of the authors from a commercial party related directly or indirectly to the subject of this article. This work was supported by the Special Funds of the National Natural Science Foundation of China (61371066), Natural Science Foundation of Jiangsu Province (SBK2011856), Key Medical Talents of Jiangsu Province (RC2011090), and 333 program for high level talents of Jiangsu Province (grant no. 2011III-2640).
Author information
Authors and Affiliations
Corresponding author
Additional information
J. F. Sun, Y. X. Yang, X. Wang, Z. Yu, and T. Zhang contributed equally to this work. All authors contributed to the design and interpretation of the study and to further drafts. Y. W. Zhang is the guarantor.
Rights and permissions
About this article
Cite this article
Sun, J., Yang, Y., Wang, X. et al. Meta-analysis of the Efficacies of Extended and Standard Pancreatoduodenectomy for Ductal Adenocarcinoma of the Head of the Pancreas. World J Surg 38, 2708–2715 (2014). https://doi.org/10.1007/s00268-014-2633-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-014-2633-9