Abstract
Background
While several trials have compared laparoscopic to open surgery for colon cancer showing similar oncological results, oncological quality of laparoscopic versus open rectal resection is not well investigated.
Methods
A systematic literature search for randomized controlled trials was conducted in MEDLINE, the Cochrane Library, and Embase. Qualitative and quantitative meta-analyses of short-term (rate of complete resections, number of harvested lymph nodes, circumferential resection margin positivity) and long-term (recurrence, disease-free and overall survival) oncologic results were conducted.
Results
Fourteen randomized controlled trials were identified including 3528 patients. Patients in the open resection group had significantly more complete resections (OR 0.70; 95% CI 0.51–0.97; p = 0.03) and a higher number of resected lymph nodes (mean difference − 0.92; 95% CI − 1.08 to 0.75; p < 0.001). No differences were detected in the frequency of positive circumferential resection margins (OR 0.82; 95% CI 0.62–1.10; p = 0.18). Furthermore, no significant differences of long-term oncologic outcome parameters after 5 years including locoregional recurrence (OR 0.95; 95% CI 0.44–2.05; p = 0.89), disease-free survival (OR 1.16; 95% CI 0.84–1.58; p = 0.36), and overall survival (OR 1.04; 95% CI 0.76–1.41; p = 0.82) were found. Most trials exhibited a relevant risk of bias and several studies provided no information on the surgical expertise of the participating surgeons.
Conclusion
Differences in oncologic outcome between laparoscopic and open rectal surgery for rectal cancer were detected for the complete resection rate and the number of resected lymph nodes in favor of the open approach. No statistically significant differences were found in oncologic long-term outcome parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Colorectal cancer is one of the most frequently diagnosed cancers worldwide and accounts for over 750,000 cancer-related deaths annually. Nearly 30% of all colorectal adenocarcinomas are located in the rectum. Surgical resection is the only curative treatment of the disease in most cases. Over the last decades, survival rates have significantly improved from a 5-year survival rate of 48.1% in the 1970s to 67.7% in 2009.1 According to epidemiological studies in a microsimulation model, modern screening via colonoscopy and fecal occult blood tests may account for more than 50% of this effect.2 Furthermore, locoregional recurrence as another important oncological outcome was significantly reduced by changes in treatment strategies. The most important improvement in the surgical therapy was the widespread implementation of the total mesorectal excision as described by Heald et al. in 19823 which reduced the rate of locoregional recurrence from 25% in the 1980s to rates of under 4% today.
In 1991, Jacobs et al. reported the first laparoscopic colectomy for colon cancer.4 Since then, oncological quality of laparoscopic colorectal surgery has been discussed controversially. In the last 20 years, several studies have been conducted to compare laparoscopic and open surgery in colorectal cancer. The majority of these studies included patients with colon and rectal cancer and found no differences in oncological outcomes between an open and a laparoscopic approach but provided data of lower intraoperative blood loss and shorter length of hospital stay after laparoscopic surgery.5,6,7 These results were confirmed for colon cancer in subsequent meta-analyses, and laparoscopic colonic resection has since become the standard of care in many countries.8,9,10,11
Due to the inhomogeneous population with both colon and rectal cancer patients in most studies, little is known about the oncological quality of laparoscopic resections compared to open surgery in the rectal cancer subgroup. Rectal cancer surgery differs from colon resections in multiple ways and is technically more challenging. A systematic review by Vennix et al.12 in 2014 found no significant differences in survival or recurrence rates between laparoscopic and open total mesorectal excision for rectal cancer. Compared to patients undergoing open surgery, however, patients had less blood loss and a shorter hospital stay following laparoscopic surgery,12 which is well documented.5,6,7,13 Since then, the results of several trials have been published, necessitating an updated systematic review and meta-analysis including further oncologic outcome parameters. Based on these new data, this systematic review and meta-analysis was performed to determine differences of short- and long-term oncological outcome between open and laparoscopic resections for rectal cancer.
Material and Methods
This systematic review was conducted according to the recommendations of the current Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 The study was performed according to a prespecified protocol and all stages of study selection, data extraction, and assessment were carried out by two reviewers (PH and HN). The PRISMA checklist is provided in supplement Figure 2. The study was registered in the PROSPERO database (CRD42017060727).
Literature Search
The electronic databases MEDLINE (via PubMed), Embase, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched systematically from their initiation until February 28, 2016 for relevant randomized controlled trials (RCTs). The patient-intervention-comparison-outcome (PICO) scheme was used to build the search strategy using search terms describing the patient (rectal cancer), intervention (laparoscopic resection), comparison (open resection), and outcome (see outcome measures). The search of the MEDLINE database via PubMed was combined with the Cochrane Highly Sensitive Search Strategy for identifying RCTs in MEDLINE, sensitivity-maximizing version, PubMed format (2008 revision).
The final search strategy for MEDLINE is provided in supplement Figure 1, and for the other databases, search strategies were adapted to the specific vocabulary of the database. Furthermore, references of relevant articles were hand-searched for additional relevant studies. No restrictions were applied regarding to language or publication date.
Eligibility Criteria
Since the aim of this systematic review was to analyze the oncological outcome parameters in rectal cancer only, studies that did not provide subgroup data for rectal cancer patients were excluded from further analyses. Only RCTs performed in humans were included.
Study Selection
Titles and abstracts of all results were screened by two reviewers (PH and HN) independently for relevant studies. In case of disagreement, a third reviewer (TS) was consulted and decisions were made unanimously. For all included studies, full-text articles were retrieved for further evaluation of eligibility. Again, in the event of disagreement, a third reviewer (TS) was consulted and the decision was made after discussion of the article.
Data Extraction
Two authors (PH and HN) independently extracted all relevant data according to the prespecified protocol using a data extraction form (see supplement Figure 1). The extracted data included title, author, year of publication, journal, language, trial duration, trial design, number of treatment groups, total number of patients randomized, evaluable patients, withdrawals, number of patients lost to follow-up, and funding source. Furthermore, data on patient’s baseline characteristics, including age, gender, comorbidities, functional status, and preoperative staging, were extracted. The following primary outcome parameters were collected: complete resection rates (defined as complete total mesorectal excision (TME)), the number of dissected lymph nodes, and the rate of circumferential resection margin (CRM) positivity as surrogate parameters of short-term oncological outcome. CRM positivity was analyzed based on the provided definition in the respective studies. As long-term oncological outcome parameters, the rate of local tumor recurrence, disease-free survival, and overall survival was collected. As a non-oncological outcome parameter, conversion rates and the reasons for failure of laparoscopy were collected. In addition, the perioperative outcomes like operation time, blood loss, length of hospital stay, rates of anastomotic leakage, postoperative morbidity, and mortality were extracted. Since several studies showed no differences between the different approaches in these parameters, we did not include them into our analyses.
If two or more articles reported on the same patient cohort, all relevant data were extracted and the most current and most comprehensive data for each outcome were used. Since the included studies described a various number of survival and disease-free periods reaching from a 1- to a 10-year follow-up, we decided to compare the rate of local recurrence, disease-free survival, and overall survival at a 3- and 5-year follow-up.
Statistical Analyses
Statistical analyses and meta-analyses of the individual outcomes were conducted using Review Manager (version 5.3; the Nordic Cochrane Centre, Copenhagen; the Cochrane Collaboration, 2014). For continuous outcomes, the mean differences (MDs) pooled by the inverse-variance method were measured and 95% confidence intervals (CIs) were provided. For dichotomous outcomes, the odds ratios (ORs) and 95% CI were calculated by the Mantel-Haenszel model. Due to the clinical homogeneity of the patient groups, the exclusion of non-RCTs, and the number of studies included for the single outcomes of patients included in the analyses, a fixed effects model was used for all statistical analyses. Statistical heterogeneity was assessed by using I2 statistics, and results of over 60% were considered as substantial heterogeneity.
Analyses were performed in the intention-to-treat population (i.e., as randomized) which most of the original articles were reported. To adjust the analyses for relevant risk of bias and surgical expertise between groups, sensitivity analyses were performed, excluding trials with a high risk of selection bias, attrition bias, or the ones that did not report any matching of surgical experience between both groups.
Risk of Bias
Bias was judged using the Cochrane Collaboration tool for assessing the quality and risk of bias.15 Trials were defined as having an overall high risk of bias, if they had assessed high risk in any of the following domains: random sequence generation and/or attrition bias with incomplete reporting of the outcome data. Publication bias was assessed using a funnel plot for each outcome.
Results
Study Selection
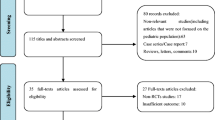
A total number of 3763 potentially relevant results were identified. After exclusion of 600 duplicates, 3163 abstracts were screened. Three thousand one hundred twenty-five were excluded as not relevant to the topic of this systematic review. Full-text articles were retrieved from the remaining 38 references. Out of these, 14 trials were included in this systematic review and meta-analysis. The remaining 24 studies were excluded for the following reasons: 12 of them were due to a lack of primary data, six were double publications, and six reported follow-up data of prior publications. Data from the latter six studies were extracted as necessary. Details of study selection are listed in Fig. 1. In these cases, data of each cohort was summarized using the most current outcome parameters. The articles published by van der Pas et al.5 and Bonjer et al.16 were summarized as Colorectal cancer Laparoscopic or Open Resection (COLOR) II. The references Guillou et al.,7 Jayne et al.,17,18 and Green et al.19 were reported together as Conventional versus Laparoscopic-Assisted Surgery In Colorectal Cancer (CLASICC). The two manuscripts of Kang et al.20 and Jeong et al.21 were reported as Comparision of Open versus laparoscopic surgery for mid and low REctal cancer After Neoadjuvant chemoradiotherapy (COREAN). The references of Leung et al.22 and Ng et al.23 reported the results of an identical patient group and were summarized in Ng et al.23 All other references were described similarly in the forest plots.
Study Characteristics
A detailed overview of the included studies and the patient characteristics is provided in Table 1. Almost half of the studies were multicenter trials (6 of 14) and were performed across various countries, most of them in Europe and Asia. Some trials were performed exclusively in patients undergoing rectal resection for cancer while others had mixed populations with colon and rectal cancer patients. Furthermore, primary outcomes varied between studies. Most trials were powered to detect short-term perioperative outcomes7,24,25,26,27,28,29,30,31 while others were powered to detect differences in long-term oncologic outcome parameters5,16,17,18,19,20,21,22,23,32 (Table 2).
Risk of Bias
Except one study,32 all authors reported random sequence allocation. Since blinding of patients and investigators is impracticable in the investigated setting, none of the studies reported attempts of blinding. For assessment of performance bias, standardization of the different procedures and the postoperative care was analyzed. Two studies failed to report any specific information on the postoperative care of the patients.26,29 Four studies22,25,28,33 were at high risk for attrition bias with incomplete reporting of the outcome data. Three studies26,29,32 were at high risk for selective reporting with missing information on follow-up data. The experience of the operating surgeons was classified as other bias. Two studies7,33 were at high risk for a bias in this domain, and four studies failed to report any information on this topic.16,25,26,30 A detailed summary of the risk of bias is given in Fig. 2a, b. A detailed overview of the experience of the surgeons is provided in Table 3.
Most of the included studies used the same follow-up regime for both groups, but only few clearly described how oncologic follow-up was performed which might account for differences in recurrence rate or disease-free survival.
Short-Term Oncological Outcomes
The number of harvested lymph nodes was reported in eight studies. Meta-analysis showed a statistically significantly higher number of resected lymph nodes in the open resection group compared to laparoscopy (MD − 0.92; 95% CI − 1.08 to − 0.75; p < 0.001; Fig. 3a). The positivity of the circumferential resection margin was described in ten studies with no significant differences between open and laparoscopic surgery (OR 0.82; 95% CI 0.62 to 1.10; p = 0.18; Fig. 3b). Definition of a positive circumferential margin was provided in seven of the ten studies. Six of these seven studies used a distance less than 1 mm to define a margin as positive while the COLOR II study used less than 2 mm (Table 4).
Information on the frequency of complete resections was provided in seven studies with significantly more complete resections in the open approach (OR 0.71; 95% CI 0.54 to 0.93; p = 0.01; Fig. 3c). A composite endpoint including completeness of the mesorectum plus negative distal and circumferential margins was reported in two studies (see Table 5) and showed significantly more complete resections according to this definition in the open group (OR 0.62; 95% CI 0.45–0.86; p = 0.003; Fig. 3d).
Subgroup analysis for tumor localization (high/low rectal cancer) was not possible as only one study5 reported CRM rates in relation to the tumor height. Additionally, the definition of low tumors was different among studies.
Long-Term Oncological Outcomes
Local recurrence rate after 3 years was reported in four studies, and no significant difference was found between laparoscopic and open approach after 3 years (OR 1.03; 95% CI 0.66 to 1.58; p = 0.91; Fig. 4a) and ranged from 3.6% (lap)/4.7% (open)24 to 9.9% (lap)/10.2% (open).7
Five-year local recurrence rates were provided in three studies and ranged from 2.8% (lap)/8.3% (open)28 to 9.3% (lap)/8.6% (open) with no significant difference between the two groups (OR 0.94; 95% CI 0.44 to 2.05; p = 0.89; Fig. 4b).
Regarding disease-free survival (DFS), there was no difference in the 3- or 5-year data between laparoscopic and open surgery in three and four included trials, respectively (OR 1.17; 95% CI 0.89 to 1.52; p = 0.26; OR 1.16; 95% CI 0.85 to 1.58; p = 0.36; Fig. 5a, b).
Overall survival after 3 years was reported in five studies and did not differ significantly between laparoscopic and open rectal resections (OR 1.12; 95% CI 0.92 to 1.37; p = 0.25; Fig. 6a). Comparable results were found in overall survival after 5 years. Four studies showed no difference between laparoscopic and open resections (OR 1.04; 95% CI 0.76 to 1.41; p = 0.82; Fig. 6b).
Conversion Rates and Reasons for Failure of Laparoscopy
This was reported in six studies, and data was available for a total number of 220 conversions. The overall conversion rate ranged from 1.2% (COREAN) to 34% (CLASICC). The most frequent reasons for conversion were large tumors or uncertain tumor clearance (17.7%) followed by extensive adhesions (14.5%). The detailed reasons for conversion are provided in Table 6.
Sensitivity Analyses
To adjust meta-analyses for relevant risk of bias and surgical experience in the included studies, sensitivity analyses have been performed excluding trials with a high risk of selection bias, attrition bias, or the ones that did not report any matching of surgical experience between both groups. The results for each of the outcomes were comparable with the overall results without any significant changes in the additional analyses.
Discussion
In this systematic review, the effect of the surgical approach on the oncological outcome in rectal cancer surgery was investigated. The analyses showed that patients who underwent laparoscopic resection had significantly less complete resections and a reduced number of resected lymph nodes. This had no significant influence on local recurrence rate and the 5-year overall or disease-free survival rates. Furthermore, other short-term oncologic outcomes such as the rate or CRM-positive resections did not differ between the two groups.
Out of the 14 included trials, there were five large trials that directly investigated the oncologic difference (primary endpoint) between the two different approaches in the last 20 years. The first trial that only included patients with rectal cancer was the COLOR II study which was conducted between 2004 and 2010 in eight different countries in Europe and included 1103 patients.5,16 The second large trial on this topic was the COREAN trial conducted in South Korea between 2006 and 2009 and included 340 patients with rectal cancer.20 The third trial was the Australian Laparoscopic Cancer of the Rectum Trial (ALaCaRT) trial which compared laparoscopic and open rectal resections in 475 patients between 2010 and 2014. The fourth study was the American College Of Surgeons Oncology Group (ACOSOG) trial which was conducted in the USA between 2008 and 2013 and included 486 patients. In addition to these rectal cancer-specific trials, the MRC CLASICC trial compared laparoscopic and open resections in colorectal cancer patients. This study was conducted in the UK between 1996 and 20027 and included a total number of 794 patients. Out of the CLASICC study population, only patients undergoing rectal resections were included into this analysis.
Beside these large trials, a high number of other studies compared the short-term perioperative outcome parameters of the two approaches.
A major challenge in surgical studies is to control for surgical experience between the study groups in order to rule out comparing different surgical expertise rather than the effect of surgical interventions.34 To be able to reliably compare the outcome of laparoscopic versus open rectal surgery, both procedures should be performed by surgeons with the same level of experience in both groups. In the smaller single-center studies, the operations in both arms were usually performed by the same surgical team that was experienced in both procedures.29,31 In the larger multicenter trials, laparoscopic surgeons were required to proof experience by a certain number of operations. In contrast to that, there was no minimum requirement for operations in the open group. Kennedy et al.33 reported the number of operations performed by a trainee, and there was a significant difference between the laparoscopic and the open group. While laparoscopic rectal resection was only performed by a trainee in 7% of the cases, less-experienced surgeons were responsible for 33% of the resections in the open group. Analogous in the design of the CLASICC trial, the investigators required at least 20 laparoscopic resections for participating surgeons; however, specific requirements for open resections were not reported. Even though, the requirement for 20 laparoscopic resections might still not be enough which is documented by the high conversion rate of 34% in the CLASICC trial7,19 in contrast to the low conversion rate of 1.2% in the COREAN trial. Since the most frequent reason for conversion was large tumors or uncertainty of tumor clearance, it is unclear how experience in the open group influences the outcome (Table 3). Analyses of learning curves in laparoscopic rectal surgery have shown that at least 50 laparoscopic procedures are required to achieve equal morbidity and oncological outcomes.35,36,37 As a learning curve exists also for open surgery (control group), it ultimately remains unclear which proportion of the results are due to training rather than technical effects.
Investigating short-term oncological outcome parameters, we found a higher rate of complete resections and a higher number of resected lymph nodes in the open group but no difference regarding the frequency of CRM positivity. The number of complete resections was reported in five studies with a total number of 2545 patients. It should be mentioned that an additional number of three studies24,26,27 reported a complete resection rate of 100% in both groups without providing specific information on the definition of completeness. In the studies by Braga et al.24 and Lujan et al.,27 patients having tumor infiltration into adjacent organs were excluded from the study while Liu et al.26 do not provide any information on this point. Four multicenter studies reported the frequency of complete resections as defined as complete TME. In all of them, the frequency of complete resection was higher in the patients operated via the open approach compared to the laparoscopic group. This difference did not show any statistical significance in the single studies, but pooled data showed a significantly higher rate of complete resections in patients with an open operation. It should be mentioned that the ALaCaRT and the Z6051 trial did not only provided the statistically validated, isolated endpoint CRM positivity or completeness of the TME but also compared a composite endpoint defining a negative circumferential margin, a negative distal margin, and the completeness of the TME as a complete resection. By comparing the patients from these two studies, we found significantly more complete resections according to this definition in the patient operated in an open approach.
Regarding the statistically significant difference in harvested lymph nodes, it should be mentioned that the mean difference in resected lymph nodes in our analyses is 0.9 lymph nodes. The COLOR II and the COREAN trial together account for over half of the patients included in this analysis and showed an absolute difference between the approaches of one lymph node in favor of the open approach. However, one study that showed no difference in the minimum number of 12 harvested lymph nodes was found. Additionally, neoadjuvant chemoradiotherapy is negatively affecting the number of harvested lymph nodes,38 and therefore, from a clinical point of view, a difference of one single lymph node might be regarded as irrelevant, if a thorough total mesorectal excision including the mesorectal lymph nodes is performed.
In contrast to these findings, no difference was found in the frequency of CRM positivity, which was reported in ten included studies. The first results from the CLASICC trial suggested that patients who underwent laparoscopic anterior rectal resection have higher rates of CRM positivity compared to patient in an open procedure. Guillou et al.7 reported a CRM positivity rate of 12% for patient undergoing laparoscopic anterior resection and a rate of 6% in the control group without any statistical significant difference. Since patients with anterior resection were only a subgroup and the results in this trial were reported combined with patients who underwent laparoscopic abdominoperineal resection, no difference was seen in the overall rate of CRM positivity (Fig. 3b).
On the other hand, results from the COLOR II trial showed a higher rate of CRM positivity in patients undergoing open rectal resections for low tumors without any statistical difference as well. For this trial, it must be mentioned that in contrast to the other studies, a different definition of a CRM-positive margin was used (see Table 4). This different definition may account for the different results. It is well known that the frequency of complete resections and negative CRM depends highly on the experience of the operating surgeon.39 In the CLASICC trial, participating surgeons were required to have performed at least 20 laparoscopic resections while no requirements for experience in open operations were reported. The high number of CRM-positive tumors especially in low rectal tumors could therefore be caused by a lack of experience.
While the frequency of CRM-positive resections did not show any difference between the two groups, the number of incomplete resections was significantly higher in patients who underwent laparoscopic surgery. This result is even more remarkable as in the studies which reported a higher number of incomplete resections in the laparoscopic group, in which the requirements for participating surgeons were quite high. Stevenson et al.30 reported that only surgeons with more than 130 colorectal resections could include patients without reporting any requirements for open resections. Nevertheless, the number of incomplete resections was higher in the laparoscopic group. The authors of the COLOR trial reported that 86% of the surgeons were experienced in both techniques without providing any details on the specific number of performed operations. Kim et al.39 describe a learning curve of at least 80 performed operations for laparoscopic rectal resections to achieve adequate local recurrence rates and a CRM quality. Since specific numbers of performed operations were not provided in most of the reported trials, an estimation of the experience of the participating surgeons is difficult.
In summary, we found no significant difference in the frequency of the CRM positivity but in the number of harvested lymph nodes, and furthermore, significantly more patients in the laparoscopic group underwent incomplete resection. While the requirements for surgeons in the laparoscopic group were reported in detail, no information was provided on surgeons operating the patients in the open group. This makes the higher number of incomplete resections in laparoscopic resections even more remarkable and indicates that laparoscopic rectal resections should only be performed after sufficient training.
For evaluation of the long-term quality of oncological resections, the disease-free and the overall survival after 3 and 5 years was compared. Most studies reported data for these two time points with follow-up periods ranging from 1 to 10 years. Local recurrence rates did not differ significantly after 3 and 5 years. As short-term outcomes showed significant difference in the number of harvested lymph nodes and complete resections, this does not seem to translate into differences in the long-term outcome.
Survival rates did not differ between the separate groups neither at 3 years nor at 5 years. These results are similar to Vennix et al.12 in 2014 who did not find any statistical significant difference at 3, 5 or 10 years between the two different groups. Long-term follow-up data of the large trials (COREAN and COLOR II) did not influence this finding, and even if survival data from the ACOSOG Z6051 and the ALaCaRT study25,30 is not available yet, it has to be supposed that that there will be no difference in short- or long-term survival between the two groups.
Therefore, in summary, the higher number of incomplete resections does not seem to influence long-term outcome. The phenomenon that a reduced local recurrence rate does not transform into an increased overall survival in rectal cancer is well documented from neoadjuvant chemotherapy trials40 as survival seems to be influenced by distant metastases more than by locoregional recurrence. Furthermore, the more pronounced the beneficial effect of neoadjuvant treatment on local recurrence, the higher the risk/probability of local recurrence. Therefore, one might speculate that neoadjuvant treatment balances out the risk difference of local recurrence brought about by the two surgical techniques.
The most important limitation of all the included studies is the problem of the control group. The requirements for the participating surgeons were very different, and this leads to a high susceptibility to bias in all included studies. In our opinion, there is no need for further high-quality studies to evaluate this point with focus on oncological quality. One of the major problems encountered during this study was the lack of standardization between trials. This hampered comparison and meta-analyses of results not only of outcome parameters (CRM) but also of baseline diagnostic data (definition of low rectal cancer), surgical experience, and follow-up of sensitive oncologic outcome parameters like DFS. Therefore, major efforts should be invested in an international standardization/consensus of outcome parameters to facilitate future trials.
Given our results, laparoscopic surgery might have limitations in rectal cancer; therefore, the goal of the future study should be to identify the specific subgroups which have the equal oncologic outcome as when treated by open surgery. This meta-analysis including only high-quality studies did not show any difference in terms of long-term survival, and most likely, the quality of the oncological resection is not dependent on the approach but on the performing surgeon. The advantages (shorter hospital stay, less blood loss, and potentially reduced costs) of laparoscopic surgery have been well described.5,6,7,13 Taken all these aspects together, laparoscopic rectal resection seems to be an excellent option in selected patient groups if it is feasible and performed by an experienced surgeon. However, we still need to correctly identify the limitations and accordingly stratify the patients correctly to open and laparoscopic surgery. This means that surgeons need to be further trained in the future to provide excellent open surgery to be able to optimally treat patients not suitable to laparoscopic surgery. Additionally, patients cannot be advised against open surgery as oncologically as it is at least as good as laparoscopic surgery. Currently, several ongoing trials investigate the difference between laparoscopic and robotic resections and the design of the control group in these trials will be essential to the quality of these studies.
References
Siegel, R., C. Desantis, and A. Jemal, Colorectal cancer statistics, 2014. CA Cancer J Clin, 2014. 64(2): p. 104–17.
Edwards, B.K., et al., Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer, 2010. 116(3): p. 544–73.
Heald, R.J., E.M. Husband, and R.D. Ryall, The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg, 1982. 69(10): p. 613–6.
Jacobs, M., J.C. Verdeja, and H.S. Goldstein, Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc, 1991. 1(3): p. 144–50.
van der Pas, M.H., et al., Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol, 2013. 14(3): p. 210–8.
Stead, M.L., et al., Assessing the relative costs of standard open surgery and laparoscopic surgery in colorectal cancer in a randomised controlled trial in the United Kingdom. Crit Rev Oncol Hematol, 2000. 33(2): p. 99–103.
Guillou, P.J., et al., Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet, 2005. 365(9472): p. 1718–26.
Theophilus, M., C. Platell, and K. Spilsbury, Long-term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomized controlled trials. Colorectal Dis, 2014. 16(3): p. O75–81.
Wang, C.L., G. Qu, and H.W. Xu, The short- and long-term outcomes of laparoscopic versus open surgery for colorectal cancer: a meta-analysis. Int J Colorectal Dis, 2014. 29(3): p. 309–20.
Di, B., et al., Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol, 2013. 22(3): p. e39–43.
Kuhry, E., et al., Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev, 2008. 34(6): p. 498–504.
Vennix, S., et al., Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev, 2014(4): p. Cd005200.
Biondi, A., et al., Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg, 2013. 13 Suppl 2: p. S12.
Panic, N., et al., Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One, 2013. 8(12): p. e83138.
Higgins, J.P., et al., The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj, 2011. 343: p. d5928.
Bonjer, H.J., C.L. Deijen, and E. Haglind, A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N Engl J Med, 2015. 373(2): p. 194.
Jayne, D.G., et al., Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg, 2010. 97(11): p. 1638–45.
Jayne, D.G., et al., Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol, 2007. 25(21): p. 3061–8.
Green, B.L., et al., Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg, 2013. 100(1): p. 75–82.
Kang, S.B., et al., Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol, 2010. 11(7): p. 637–45.
Jeong, S.Y., et al., Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol, 2014. 15(7): p. 767–74.
Leung, K.L., et al., Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet, 2004. 363(9416): p. 1187–92.
Ng, S.S., et al., Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum, 2009. 52(4): p. 558–66.
Braga, M., et al., Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum, 2007. 50(4): p. 464–71.
Fleshman, J., et al., Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA, 2015. 314(13): p. 1346–55.
Liu, F.L., et al., Hand-assisted laparoscopic surgery versus the open approach in curative resection of rectal cancer. J Int Med Res, 2010. 38(3): p. 916–22.
Lujan, J., et al., Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg, 2009. 96(9): p. 982–9.
Ng, S.S., et al., Laparoscopic-assisted versus open total mesorectal excision with anal sphincter preservation for mid and low rectal cancer: a prospective, randomized trial. Surg Endosc, 2014. 28(1): p. 297–306.
Pechlivanides, G., et al., Lymph node clearance after total mesorectal excision for rectal cancer: laparoscopic versus open approach. Dig Dis, 2007. 25(1): p. 94–9.
Stevenson, A.R., et al., Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA, 2015. 314(13): p. 1356–63.
Zhou, Z.G., et al., Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc, 2004. 18(8): p. 1211–5.
Liang, X., et al., Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A, 2011. 21(5): p. 381–5.
Kennedy, R.H., et al., Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol, 2014. 32(17): p. 1804–11.
Strobel, O. and M.W. Buchler, The problem of the poor control arm in surgical randomized controlled trials. Br J Surg, 2013. 100(2): p. 172–3.
Bege, T., et al., The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution’s experience. Ann Surg, 2010. 251(2): p. 249–53.
Kayano, H., et al., Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc, 2011. 25(9): p. 2972–9.
Son, G.M., et al., Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A, 2010. 20(7): p. 609–17.
Leibold, T., et al., Prognostic implications of the distribution of lymph node metastases in rectal cancer after neoadjuvant chemoradiotherapy. J Clin Oncol, 2008. 26(13): p. 2106–11.
Kim, C.H., et al., Learning curve of laparoscopic low anterior resection in terms of local recurrence. J Surg Oncol, 2014. 110(8): p. 989–96.
Sauer, R., et al., Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis, 2003. 5(5): p. 406–15.
Funding
No funding was used to create this review. However, the resources and facilities of the University of Heidelberg were used in conducting this study.
Author information
Authors and Affiliations
Contributions
HN, PH, RS, BPM, ALM, and TS are responsible for the conception and design of the study. HN, PH, RS, ALM, and TS performed the acquisition and analysis of the data and drafted the manuscript. YK, MKD, JK, MS, BPM, AU, and MWB offered substantial contributions to the interpretation of the data and critically revised the manuscript. All authors gave their final approval of this version of the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Nienhüser, H., Heger, P., Schmitz, R. et al. Short- and Long-Term Oncological Outcome After Rectal Cancer Surgery: a Systematic Review and Meta-Analysis Comparing Open Versus Laparoscopic Rectal Cancer Surgery. J Gastrointest Surg 22, 1418–1433 (2018). https://doi.org/10.1007/s11605-018-3738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3738-5