Abstract
Purpose
The aim of this study is to describe a modified treatment strategy with image-guided percutaneous ablation after hepatic resection as a completion method to surgical eradication of liver metastases (“completion ablation [CA]”).
Methods
We conducted a retrospective analyses of patients who underwent CA within 180 days from the liver surgical resection to eradicate liver metastases present on the pre-surgical cross-sectional imaging or identified during intraoperative ultrasound that were not resected due to various reasons. Lesions treated with CA were evaluated for local tumor progression (LTP). Patients were evaluated for hepatic- and overall-recurrence-free survivals (hepatic-RFS and overall-RFS, respectively) and overall survival (OS).
Results
Sixteen patients (10 females; median age 55 years, range 28–69) underwent CA of 21 lesions (median size 8 mm, range 6 to 22). Indications for the use of CA were small future liver remnant in 10 (63%), inability to identify the lesion during surgical exploration in 3 (19%), and technical difficulty of resection in 3 (19%) patients. No liver-related complications were recorded following the surgical resection or the CA procedures. Primary and secondary CA efficacy rates were 95 and 100%, respectively. LTP was 0% at a median clinical follow-up of 27 months (range 4.0–108 months). Five-year hepatic-RFS, overall-RFS, and OS were 36, 16, and 51%, respectively.
Conclusion
The use of CA as a complement to surgical resection is safe and effective. Such approach could potentially expand the surgical candidacy for patients with limited liver functional reserve and reduce postoperative morbidity and mortality in this selected patient population with more advanced disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The management of patients with limited liver metastases is based on the predicate that liver resection is the preferred treatment option if all viable tumors detected prior to chemotherapy can be removed with negative surgical margins, while still preserving an adequate functional liver remnant.1 , 2 To achieve this goal, adjunctive measures such as preoperative systemic chemotherapy,3 , 4 two-stage hepatectomy,5 portal vein embolization (PVE),6 , 7 and percutaneous fiducial marker of liver metastasis at risk of disappearing8 , 9 have been utilized with high degrees of success. Nevertheless, in a small number of patients, surgical eradication of all visible liver metastases is not possible either due to a limited functional liver volume to undergo surgical resection or to technical limitations encountered during the surgical exploration.
Liver ablation has been advocated as an alternative treatment to surgery for patients with limited liver metastases, with both intraoperative and percutaneous approaches routinely utilized.10 – 16 Although intraoperative ablation is traditionally used as the combined approach of choice for patients undergoing liver resection,2 , 17 high rates of local tumor progression (LTP) following intraoperative ablation, postoperative liver failure, and mortality for patients submitted to hemihepatectomy combined with intraoperative ablation have been described.18 , 19 Furthermore, technical limitations encountered during surgical exploration such as surgical adhesions and inability to identify the target tumor(s) with intraoperative ultrasound are also known factors that might preclude the use of intraoperative ablation.20
Recent advances on the understanding of the use of image-guided percutaneous ablation in respect adequate patient selection, use of contrast-enhanced cross-sectional imaging for optimal definition of tumor extent, and close monitoring of optimal ablation margins have reduced associated rates of LTP to rates equivalent to surgical resection.11 , 13 – 16 , 21 – 23 Therefore, the use of planned image-guided percutaneous ablation after hepatic resection as a completion method to surgical eradication of liver metastases (“completion ablation [CA]”) could be advocated in selected patients undergoing resection of liver metastases where complete surgical eradication of all visible liver metastases could not be achieved.
Based on that hypothesis, we proposed a modified treatment strategy where CA was utilized to complement the surgical eradication of existing liver metastases which were present but not resected at the time of surgical resection. The aim of this retrospective cohort study is to describe our experience with such approach in respect indications, safety, and outcomes.
Patients and Methods
Study Population
This single-institution retrospective study was compliant with the Health Insurance Portability and Accountability Act and approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center with a waiver of informed consent (IRB protocol PA15-1036). The prospective institutional surgical oncology liver database was searched between 2006 and 2016 to identify patients who underwent image-guided percutaneous ablation within 180 days from hepatic resection.
Surgical Resection and Ablation Strategy
Multidisciplinary management of patients with liver metastasis was discussed at the Institutional multidisciplinary hepatobiliary meeting. The presence of limited, resectable extrahepatic disease was not necessarily considered an exclusion criterion. Response to chemotherapy was evaluated after every 4 cycles by cross-sectional imaging. Second-line chemotherapy was considered for patients with disease progression or suboptimal tumor response after first-line chemotherapy.24
Liver metastases were deemed treatable when hepatectomy could achieve a negative margin and image-guided percutaneous ablation could achieve complete coverage of the target tumor while preserving more than 20–30% of the total estimated liver volume, sparing two continuous hepatic segments, and maintaining vascular inflow and outflow and biliary drainage.25 In order to expand the number of patients eligible for potentially curative surgical resection, portal vein embolization and two-stage hepatectomy were also utilized by our institutional protocol when indicated.
The use of CA was reserved for liver metastases where surgical resection was not feasible due to intraoperative technical limitations as regards adequate identification and surgical exposure of the liver segment harboring the target metastasis or when the surgeon determined that the resection of such liver metastasis would require encompassing a considerable functional liver volume on patients expecting to have a small future liver remnant.
Completion Ablation Definition
CA was defined by the use of image-guided percutaneous ablation within 180 days from the liver surgical resection to eradicate known liver metastases present on the pre-surgical cross-sectional imaging or identified during intraoperative ultrasound that remained visible in all cross-sectional imaging studies following hepatic resection preceding the percutaneous thermal ablation session (Fig. 1). Finally, in order to be considered a CA, the surgical report should have mentioned the reason why the lesion subsequently treated with image-guided percutaneous ablation was not resected.
Representative case receiving completion ablation. a, b A 42-year-old man with synchronous multiple bilobar colorectal liver metastases (CLM) (red arrowheads). After resection of primary cecal cancer (T3N1), the patient underwent 8 cycles of FOLFOX at outside hospital and was referred to our institution. c After preoperative right portal vein embolization (red arrow), the future liver remnant volume was estimated as 30%. Patient underwent right hemi-hepatectomy and 2 limited resections in the left liver. d Intraoperative ultrasonography incidentally detected small CLM at segment IVA (yellow arrowhead), later identified on subsequent contrast-enhanced computed tomography. Decision was made to ablate percutaneously this lesion given its deep location and high risk of postoperative liver failure due to small liver size. e Percutaneous radiofrequency ablation was performed 30 days after surgery. f A CT scan performed 3 months after ablation showed ablation zone in segment IVA and absence of local tumor progression
Completion Ablation Technique
All percutaneous ablations were performed under computed tomography (CT) or magnetic resonance (MR) by one of four interventional radiologists (B.C.O., 7 years of experience; S.Y.H., 6 years of experience; K.A., 15 years of experience; and S.G., 17 years of experience). General anesthesia with continuous hemodynamic monitoring by an anesthesiologist was utilized for all patients. CA were performed with radiofrequency (Cool-tip ablation system, Covidien, Boulder, CO, USA) or microwave (Certus probe, Certus 140 2.4-GHz ablation system, Neuwave, Madison, WI, USA) and cryoablation (Galil Medical Inc., SeedNet® MRI cryoablation system, Arden Hills, MN, USA) according to the operator’s choice. Patients were discharged home within 24 h of the procedure.
Clinical and Imaging Follow-up
Imaging assessment was performed by two readers (B.C.O., and S.Y., a hepatobiliary research fellow with 7 years of experience). Disagreements in interpretation were resolved by consensus. All available pre- and post-CA cross-sectional studies available in the electronic medical record were reviewed. If a liver lesion was present on the first cross-sectional imaging study available, the date of this study was considered the date of diagnosis of that particular lesion. Baseline cross-sectional imaging was defined as the contrast-enhanced CT or MR available before the first CA session. The initial post-CA cross-sectional imaging assessment of the efficacy of ablation was performed within 4 to 8 weeks. Minimal ablation margin was assessed by comparing the distances of the ablated lesion on the baseline cross-sectional imaging and the ablation zone on the initial post-CA cross-sectional imaging from intrahepatic landmarks on portal venous phase CT images.11 After the initial post-CA imaging assessment, sequential imaging assessments were performed at 2- to 4-month intervals until patient death or loss to follow-up.
The updated standardization of terminology and reporting criteria for image-guided tumor ablation was utilized to assess CA outcomes.26 Residual unablated tumor was defined as irregular peripheral or nodular enhancement <1 cm of the ablated area on the initial post-CA cross-sectional imaging. LTP was defined as the appearance of tumor foci <1 cm of the edge of the ablation zone on cross-sectional imaging after at least one post-ablation contrast-enhanced imaging study had documented adequate ablation and an absence of viable tissue in the target tumor and surrounding ablation margin. Primary efficacy rate was defined as the percentage of ablated tumors successfully eradicated after the initial procedure or course of treatment. Secondary efficacy rate was defined as the percentage of target tumors that have undergone successful repeat ablation after documentation of LTP. Time to LTP was measured in months from the date of the last percutaneous ablation session to the date when LTP was detected on cross-sectional imaging. All adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.
Statistical Analysis
Variables extracted from the database or updated by review of electronic medical records for each patient included date of procedure, sex, age, type of primary malignancy (colorectal versus non-colorectal), use of preoperative chemotherapy, indication for CA, timing of liver metastasis (metachronus versus synchronous), presence of extrahepatic metastasis at the time of surgical resection, presence of bilateral hepatic metastases, type of surgical resection preceding ablation, total number of liver metastases per patient, number of percutaneously ablated lesions per patient, time from lesion to ablation to its discovery, preceding surgery and most recent imaging follow-up, complications associated with ablation, use of post-ablation chemotherapy, local tumor progression, and intra- and extrahepatic disease recurrence. Continuous data (age, lesion size) were expressed as median (range). Hepatic- and overall recurrence-free survival (hepatic-RFS and overall-RFS, respectively) were measured in months from the date of the last image-guided percutaneous ablation session to the date of detection of hepatic and any organ recurrence on cross-sectional imaging or last follow-up, respectively. Overall survival (OS) was measured in months from the date of the last image-guided percutaneous ablation session to the date of death or last follow-up. Survival curves were generated using the Kaplan-Meier method. Statistical analyses were performed with the JMP software (version 12.1.0; SAS Institute Inc, Cary, NC).
Results
A total of 1150 patients with liver metastases underwent surgical resection during the 10-year study period. Of these, 16 patients (10 females; median age 55 years, range 28–69) underwent CA of 21 liver lesions as a complement to surgical resection and comprised the present study population. Patient’s clinicopathologic factors are depicted in Table 1. Colorectal liver metastasis was the most common type of tumor treated with the CA strategy. Over two thirds of the patients underwent preoperative systemic chemotherapy. The factors associated with the inability to resect the lesions who led to the use of CA were small future liver remnant 10 (63%) patients, inability to identify the lesion with intraoperative ultrasound in 3 (19%) patients, and technical difficulty of resection by tight adhesion in 3 (19%) patients.
In terms of operative procedures preceding CA, there were 7 (44%) patients treated with major resection (≥3 Couinaud’s segments). The two-stage hepatectomy was performed in 3 (19%) patients. Preoperative portal vein embolization was performed in 5 (31%) patients (Tables 1 and 2). One third of the patients had multiple liver lesions that were treated with CA. No liver-related complications were recorded in respect either the surgical resection or the CA procedures. As of July 2016, the median imaging follow-up period from the CA procedure was 27 months (range 2.8–88.8 months).
The characteristics of the 21 lesions treated with the CA are listed in Table 3. Primary and secondary efficacy rates were 95% (20 of 21) and 100% (21 of 21), respectively. Radiofrequency ablation was the most common (62%) thermal ablation modality utilized, followed by microwave (33%) and cryoablation (4.8%). Lesions were almost equally located on the right and left hepatic lobes. The median size of the ablated lesions at the time of ablation was 8 mm (range 6 to 22 mm). Nine (43%) of 21 ablated lesions were incidentally found at the time of surgical exploration with intraoperative ultrasound and not detected on pre-surgical cross-sectional imaging. Of those, all progressed to visible lesions on subsequent cross-sectional contrast-enhanced imaging study following the liver resection. Lesion pattern of growth since its identification on cross-sectional imaging demonstrated stable lesion size in 16 (76%) lesions, reducing in size in 3 (14%) lesions, and growing in 2 (9.5%) lesions. Minimal ablation margins >5 mm were achieved in 15 (72%) of the lesions. No cases of LTP at the lesions treated with CA were recorded at a median clinical follow-up of 27 months (range 4.0–108 months).
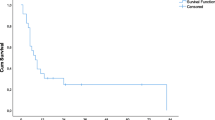
Recurrence-free survival analyses overall-RFS to be 16% for both 3 and 5 years. Three- and 5-year hepatic-RFS were both 36%. Finally, 3- and 5-year OS were 85 and 51%, respectively (Fig. 2).
Discussion
In the present study, we demonstrated for the first time the use of CA as a part of a planned treatment in addition to surgery for eradication of known liver metastases that were intentionally not resected during surgical removal of other sites of liver metastases in a small patient population with widespread liver metastases. The efficacy of such treatment strategy was confirmed by the successful eradication of all 21 small liver metastases treated with ablation in all 16 patients with no cases of LTP at the end of study follow-up. Also, we were able to verify the safety of this approach as demonstrated by the absence of postoperative and post-CA complications in our present series. Finally, despite of the extensive disease burden of our present study population, we were able to achieve satisfactory 3- and 5-year rates of hepatic-RFS and OS comparable to other series in the literature.2
In our casuistic, the use of CA avoided the escalation of the surgical resection to encompass a considerable functional liver volume in approximately two thirds of the patients, potentially reducing the associated risks of liver dysfunction and consequent postoperative morbidity and mortality,19 while still maintaining the candidacy for the intended surgical resection. In the other remaining one third of the patients, surgical resection of those liver metastases was not possible due technical limitations such as adequate lesion identification with intraoperative ultrasound because of the effect of preoperative chemotherapy or difficulty in obtaining an adequate surgical exposure of the affected liver segment(s) due to tight adhesions. Therefore, the use of CA as a complement to surgery for the eradication of small liver metastases could potentially expand the surgical indication for patients with limited functional liver reserve where the resection of the ablated lesion would pose a high risk of liver insufficiency or where resection or intraoperative ablation would not be feasible.
Our findings also provide pertinent information in respect the management of patients undergoing liver resection in which incidental lesions were found during surgical exploration. In our series, 43% of the patients had incidental lesions identified with intraoperative ultrasound that would require a change in the initial surgical plan. This finding is in keeping with existing literature, where 26 to 38% of patients present with incidental liver lesions on intraoperative ultrasound at the time of surgical resection.27 , 28 Although surgical resection should be considered the treatment of choice on those situations, it is not uncommon that such approach could increase the postsurgical morbidity and mortality due to the requirements of an extensive liver resection.19 Performing CA as a complement to surgery in a distinct time point brings the theoretical advantage of splitting the metabolic response associated with both surgery and ablation in two distinct moments, potentially reducing the associated postsurgical morbidity. Finally, the proposed approach suggested in this study could provide a “test of time” strategy for those patients presenting with incidental lesions that were considered undetermined at the time of the surgery. Performing close monitoring of the incidental lesion with contrast-enhanced cross-sectional imaging could be helpful to define the real nature of such incidental lesion(s) before a definitive therapy is applied, while still achieving high rates of local tumor control as demonstrated in our series by absence of LTP at the lesions treated with CA.
The use of percutaneous liver ablation with advanced imaging guidance can also provide advantages over intraoperative ablation. The use of contrast-enhanced cross-sectional imaging is advocated as an important technical factor for reducing rates of LTP.13 , 29 , 30 Our institution routinely utilizes CT or MR imaging for guidance and monitoring of percutaneous liver ablation with acquisition of intravenous contrast-enhanced studies for monitoring ablation results at the time of treatment. Such strategy permits clear depiction of the ablation zone, providing information in respect completeness of the tumor ablation, as well assessment of the minimal ablation margins in all dimensions.26 Therefore, additional ablation of the tumor can be performed at the same ablation session, potentially promoting lower rates of LTP.
The present study has some limitations. Firstly, it encompasses a small patient population with different types of liver metastases. Nevertheless, this is a reflection of the strict selection criteria employed by our group to select patients for the use of CA as a complement to surgery; secondly, no histological confirmation of liver metastases of the incidentally found lesions treated with CA was obtained. Nonetheless, all incidental lesions grew on the subsequent postoperative cross-sectional contrast-enhanced imaging, therefore supporting the understanding that they were, in fact, liver metastasis; thirdly, it is unclear if the liver metastasis not treated at the time of surgical resection could affect overall RFS. However, we were able to achieve acceptable rates of hepatic-RFS and OS with the proposed approach. Moreover, the resection of liver metastases preceding the primary tumor resection has been routinely utilized in many centers, therefore probably making the presence of such small liver metastases of little contribution to the overall patient’s tumor burden. Finally, the minimal follow-up period of the current study might be insufficient to evaluate LTP; however, this is comparable with previous literature dealing with LTP after percutaneous ablation for colorectal liver metastases.10
In conclusion, our present results demonstrate that the use of CA for eradication of liver metastasis identified but not resected at the time of surgical resection is safe and effective in a selected patient population. This approach could potentially expand the surgical candidacy for patients with limited liver functional reserve and reduce postoperative morbidity and mortality in this selected patient population while still achieving acceptable rates of hepatic-RFS and OS. In order to achieve optimal results, we encourage that the use of such modified treatment strategy should be discussed on a multidisciplinary setting, with hepatobiliary surgeons and interventional radiologists familiar with the optimal patient selection for hepatic ablation therapies.
References
Adams RB, Aloia TA, Loyer E, Pawlik TM, Taouli B, Vauthey JN et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91-103.
Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208-15.
Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg 2003;7:109-15.
Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85.
Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7.
Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg 2007;94:1386-94.
Passot G, Odisio BC, Zorzi D, Mahvash A, Gupta S, Wallace MJ et al. Eradication of missing liver metastases after fiducial placement. J Gastrointest Surg 2016;20:1173-8.
Zalinski S, Abdalla EK, Mahvash A, Vauthey JN A marking technique for intraoperative localization of small liver metastases before systemic chemotherapy. Ann Surg Oncol 2009;16:1208-11.
Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265:958-68.
Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM et al. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes-A 10-year Experience at a Single Center. Radiology 2016;278:601-11.
Gillams AR, Lees WR Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13.
Hamada A, Yamakado K, Nakatsuka A, Uraki J, Kashima M, Takaki H et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol 2012;30:567-74.
Abitabile P, Hartl U, Lange J, Maurer CA Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol 2007;33:67-71.
Liang P, Dong B, Yu X, Yang Y, Yu D, Su L et al. Prognostic factors for percutaneous microwave coagulation therapy of hepatic metastases. AJR Am J Roentgenol 2003;181:1319-25.
Yan DB, Clingan P, Morris DL Hepatic cryotherapy and regional chemotherapy with or without resection for liver metastases from colorectal carcinoma: how many are too many? Cancer 2003;98:320-30.
Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol 2003;10:1059-69.
Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6.
Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065-72.
Van Vledder MG, Boctor EM, Assumpcao LR, Rivaz H, Foroughi P, Hager GD et al. Intra-operative ultrasound elasticity imaging for monitoring of hepatic tumour thermal ablation. HPB (Oxford) 2010;12:717-23.
Berber E, Pelley R, Siperstein AE Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005;23:1358-64.
Sotirchos VS, Petrovic LM, Gonen M, Klimstra DS, Do RK, Petre EN et al. Colorectal cancer liver metastases: Biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology 2016;151005.
Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36:166-75.
Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009;302:2338-44.
Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540-8.
Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014;273:241-60.
Arita J, Ono Y, Takahashi M, Inoue Y, Takahashi Y, Matsueda K et al. Routine Preoperative Liver-specific Magnetic Resonance Imaging Does Not Exclude the Necessity of Contrast-enhanced Intraoperative Ultrasound in Hepatic Resection for Colorectal Liver Metastasis. Ann Surg 2015;262:1086-91.
Arita J, Takahashi M, Hata S, Shindoh J, Beck Y, Sugawara Y et al. Usefulness of contrast-enhanced intraoperative ultrasound using Sonazoid in patients with hepatocellular carcinoma. Ann Surg 2011;254:992-9.
Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol 2008;31:948-56.
van Duijnhoven FH, Jansen MC, Junggeburt JM, van Hillegersberg R, Rijken AM, van Coevorden F et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol 2006;13:651-8.
Authors’ Contributions
Criteria #1:
Design and study conception: BCO, SY, JNV
Acquisition of data: BCO, SY, LF, SG, SYH
Analysis and interpretation: BCO, SY, JNV, MEH, SEK, TAA, YSC, KA, SG
Criteria #2:
Drafting: BCO, SY, JNV
Revising: BCO, SY, LF, SYH, SEK, KA, YSC, TAA, MEH, SG, JNV
Criteria #3:
All authors approved the final version.
Criteria #4:
All authors agreed to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Odisio, B.C., Yamashita, S., Frota, L. et al. Planned Treatment of Advanced Metastatic Disease with Completion Ablation After Hepatic Resection. J Gastrointest Surg 21, 628–635 (2017). https://doi.org/10.1007/s11605-016-3324-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3324-7