Abstract
Background
The aim of this study was to investigate the prognostic impact of the initial serum postoperative CA19-9 levels in patients with extrahepatic bile duct cancer.
Methods
Data of a total of 143 patients of extrahepatic bile duct cancer with elevated preoperative serum CA19-9 levels (>37 U/ml) who underwent surgery with curative intent were reviewed retrospectively. The patients were divided into the “Normalization group” and “Non-normalization group” (initial postoperative serum CA19-9 ≤37 and >37 U/ml, respectively), and the clinicopathological factors and survival outcomes in these groups were comparatively analyzed.
Results
The cumulative 5-year overall survival (OS) rate and median survival time (MST) were 39.2 % and 42.9 months, respectively, in the Normalization group and 17.9 % and 24.0 months, respectively, in the Non-normalization group (P < 0.001). Presence of jaundice, a poorer histological differentiation grade (G3–4), lymph node metastasis, and initial postoperative serum CA19-9 level (>37 U/ml) were significant independent predictors of a poor prognosis on multivariate analysis.
Conclusion
Non-normalization of the serum CA19-9 level in the initial postoperative phase is a strong predictor of a poor prognosis and is a useful marker to identify patients who would need additional treatments and stricter follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Extrahepatic bile duct cancer is defined as adenocarcinoma of the biliary tree and includes hilar bile duct cancer and distal bile duct cancer. Surgical resection remains the only curative treatment. The 5-year overall survival rate after operation is relatively poor, ranging from 20 to 45 %.1–3 As the incidence of this disease is increasing worldwide,4 improvements in therapy and establishment of minimum standards for adequate surgical therapy are clearly warranted. Clarification of the prognostic factors would ensure proper disease staging, which in turn will be useful to target additional therapy at patients who are at the greatest risk of recurrence.
CA19-9 is a carbohydrate tumor-associated antigen, originally isolated from a human colorectal cancer cell line by Koprowski et al.5,6 in 1979. The monoclonal antibody 1116 NS 19-9 reacts with the sialylated Lewis ab blood group substance. Approximately 5 % of the population is Lewis a−/b− type, and the serum CA19-9 levels cannot increase in these individuals.7 Since the development of a radioimmunometric assay for serum CA19-9 measurement by Del Villano et al.8 in 1983, serum CA19-9 has been used for the diagnosis, prediction of prognosis, and clinical monitoring in bile duct cancer patients. The serum CA19-9 concentration is increased in approximately 60–70 % of patients with bile duct cancer.9
Preoperative serum CA19-9 levels have been shown to be correlated with the survival in patients with bile duct cancer.10–12 However, the usefulness of measuring the initial postoperative serum CA19-9 levels for predicting the prognosis in these patients remains unclear. This study was undertaken to examine the significance of the initial postoperative serum CA19-9 level for predicting the prognosis in patients with extrahepatic bile duct cancer. It is of considerable importance to assess the likelihood of recurrence in the early postoperative period to ensure timely identification of patients who would need additional treatments and stricter follow-up.
PATIENTS AND METHODS
Patients
From August 1992 to February 2013, 260 patients underwent operation for extrahepatic bile duct cancer with curative intent at the Hepatobiliary Pancreatic Surgery Division, National Cancer Center Hospital East. In the present study, we retrospectively analyzed the data of 155 of these 260 patients who fulfilled the following conditions for enrollment in this study: (1) aberrant elevation of the preoperative CA19-9 serum values (>37 U/ml); (2) preoperative serum CA19-9 measured within 1 month prior to the operation; (3) initial measurement of the postoperative serum CA19-9 level between 1 and 3 months after the operation (by reason that the half-life of CA 19-9 is approximately 12 h, so postoperative CA 19-9 serum levels will be decreased normally 1 to 2 weeks after the operation); and (4) absence of cholangitis, according to the diagnostic criteria in the Tokyo Guidelines for the Management of Acute Cholangitis (TG13),13 and obstructive jaundice (defined as T-Bil >2.0 mg/dl) at the time of measurement of the serum CA19-9 level. We excluded cases of treatment-related death (n = 4), cases with an R2 resection status (n = 5), and patients with distant metastases (n = 3). Finally, 143 patients were eligible for inclusion in the present study.
Serum CA19-9 concentrations were measured by a commercially available immunoradiometric assay kit (cobas8000e®; Roche Diagnostics Co., Ltd., Japan). The recommended normal upper limit of serum CA19-9 is 37 U/ml. In principle, the initial postoperative serum CA19-9 measurement was performed 1 to 3 months after the surgical resection, and the patients were classified into two groups according to the serum CA19-9 values measured at this first examination after resection: the “Normalization” group (postoperative serum CA19-9 ≤37 U/ml; n = 98) and the “Non-normalization” group (postoperative serum CA19-9 >37 U/ml; n = 45). We compared the clinicopathological factors and survival outcomes between these two groups.
A single investigator (YK) retrospectively reviewed all the patient data from the institutional bile duct tumor database maintained by the Hepatobiliary Pancreatic Surgery Division. The clinicopathological factors and survival outcomes of the patients were analyzed retrospectively.
Surgical Strategy and Operative Procedures
The decision concerning resectability was made preoperatively and was based mainly on the findings on computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (US), cholangiography, and sometimes cholangioscopy and angiography. In the patients with obstructive jaundice, preoperative biliary decompression was performed by percutaneous transhepatic biliary drainage (PTBD), endoscopic retrograde biliary drainage (ERBD), or endoscopic nasobiliary drainage (ENBD). The surgical procedure employed depended on the location of the primary tumor. In principle, in the case of tumors with obvious invasion of the intrapancreatic bile duct, pancreaticoduodenectomy was performed, and in that of tumors with invasion of the hilar bile duct, hemi-hepatectomy with bile duct resection was performed. Bile duct resection was performed only in cases where the tumor was localized to the middle or upper portion of the bile duct (Bismuth type I or II tumors) and did not infiltrate the adjacent organs (cTis, cT1, and cT2). The resection included excision of the tumor with the intent to obtain an adequate macroscopic tumor-free margin. Dissection of the regional lymph nodes was also performed in all of these patients. In cases where the tumor involved the major portal vein, aggressive combined resection of the portal vein with reconstruction was performed. When the tumor involved the future remnant main hepatic artery, hepatic arterial resection with reconstruction was performed under a surgical microscope by plastic surgeons. Presence of distant metastasis, extensive lymph node metastasis, peritoneal metastasis, bilateral extensive intrahepatic bile duct infiltration, bilateral branch involvement of the major vessels, and other systemic poor operative risk factors were considered as contraindications for curative resection. As a rule, no patient received neoadjuvant therapy, postoperative adjuvant chemotherapy, and postoperative radiation.
Histological Evaluation
The histopathological findings were classified according to the WHO Classification of Tumors of the Digestive System, Fourth Edition.14 The staging was principally based on the UICC TNM classification of malignant tumors (7th edition, 2009).15
Statistical Analysis
All data are expressed as median values. We used the chi-square test to compare the categorical variables. Continuous variables were compared by Student’s t test and Mann-Whitney’s U test. Survival data were processed by the Kaplan-Meier method and compared by the log-rank test. P values of less than 0.05 were considered to indicate statistical significance. Factors related to survival were analyzed using a Cox proportional hazards regression model. Only variables that were selected as being statistically significant by univariate analysis (P < 0.05) were included in the multivariate analysis. Statistical analysis was performed using the SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Comparison of the Patient Characteristics Between the Normalization Group and Non-normalization Group
The patient demographic and clinicopathologic features are shown in Table 1. The median follow-up time was 28 months (range 3–216 months). There were 96 men and 47 women, ranging in age from 39 to 88 years (median, 70 years). The percentage of patients with jaundice was 76.2 %. The median preoperative and postoperative serum CA19-9 values were 113.0 U/ml (39–19,750 U/ml) and 24.0 U/ml (0.8–32,006 U/ml), respectively. Comparison of the Normalization and Non-normalization groups revealed no significant differences between the two groups in the mean age, sex ratio, incidence of jaundice, operative methods employed, distribution of the tumor location, mean tumor size, need for blood transfusion, distribution of the histological type, UICC T-factor, UICC N-factor and disease stage, or the preoperative serum CA19-9 values; on the other hand, significant differences in some characteristics were identified between the two groups. As might be expected, the mean postoperative serum CA19-9 value was significantly higher in the Non-normalization group (mean level, 70.0 U/ml) than in the Normalization group (mean level, 15.0 U/ml). In terms of the residual tumor classification, the percentage of cases with R1 resection was significantly higher in the Non-normalization group (46.7 %) than in the Normalization group (19.4 %).
Comparison of the Survival Outcomes Between the Normalization Group and Non-normalization Group
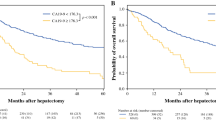
The cumulative 5-year OS and MST for the entire cohort of 143 patients were 32.2 % and 32.5 months, respectively. We performed Kaplan-Meier analyses for the OS, as shown in Fig. 1. The median observation period in the Normalization group was 32 months, while that in the Non-normalization group was 22 months. The cumulative 5-year OS rate and MST were 39.2 % and 42.9 months, respectively, in the Normalization group and 17.9 % and 24.0 months, respectively, in the Non-normalization group, with a significant difference in the survival rate between the two groups (P < 0.001).
Comparison of patient survival according to the postoperative CA19-9 status. The cumulative 5-year OS rate and MST were 39.2 % and 42.9 months, respectively, in the Normalization group and 17.9 % and 24.0 months, respectively, in the Non-normalization group, with the survival rate differing significantly between the two groups (P < 0.001)
The percentage of cases with R1 resection differed significantly between the two groups; therefore, we stratified the results of the Kaplan-Meier analyses according to the residual tumor classification: R0 resection cases (Fig. 2a) and R1 resection cases (Fig. 2b). In the R0 resection cases, the cumulative 5-year OS rate was 39.4 % in the Normalization group and 15.0 % in the Non-normalization group, with a significant difference in the survival rate between the two groups (P = 0.004). In the R1 resection patients, the cumulative 5-year OS rate was 39.0 % in the Normalization group and 19.0 % in the Non-normalization group, with a significant difference in the survival rate between the two groups in the R1 patients also (P = 0.026). Thus, regardless of the residual tumor classification, the survival outcomes were more favorable in the Normalization group than in the Non-normalization group.
Comparison of patient survival according to the postoperative CA19-9 status after adjustment for the residual tumor status. a Comparison of the survival curves in the R0 resection group (n = 103). The 5-year survival rates in the Normalization and Non-normalization groups were 39.4 and 15.0 %, respectively (P = 0.004). b Comparison of the survival curves in the R1 resection group (n = 40). The 5-year survival rates in the Normalization and Non-normalization groups were 39.0 and 19.0 %, respectively (P = 0.026)
Preoperative and Postoperative Serum CA19-9 Levels as Predictors of Survival and Analysis of the Prognostic Factors
To determine the predictive values of the preoperative and postoperative serum CA19-9 levels, categories of serum CA19-9 values were used in the univariate model. The most discriminative cutoff points for the prognosis proved to be a preoperative serum CA19-9 level of 100 U/ml and postoperative serum CA19-9 level of 37 U/ml (Table 2). Table 3 shows the prognostic factors in all the patients. Univariate analysis identified the presence of jaundice, preoperative CA19-9 value >100 U/ml, postoperative serum CA19-9 serum value >37 U/ml, need for blood transfusion, differentiation grade G3–4, and a high T-factor and N-factor as significant predictors of a poor prognosis by univariate analysis. Multivariate analysis carried out using a Cox proportional hazard regression model identified the presence of jaundice (P = 0.016, OR 1.988, 95 % CI 1.137–3.476), elevated postoperative serum CA19-9 value (>37 U/ml) (P < 0.001, OR 2.841, 95 % CI 1.838–4.386), a poor differentiation grade (G3–4) (P < 0.001, OR 4.484, 95 % CI 2.532–7.937), and a high N-factor (P < 0.001, OR 3.086, 95 % CI 1988–4.808) as independent predictors of a poor prognosis. Neither univariate nor multivariate analysis identified the residual tumor classification as a prognostic factor.
Comparison of the Recurrence Site and Recurrence Rate According to the Length of Time after Surgery Between the Normalization Group and Non-normalization Group
Table 4 shows the initial recurrence sites and recurrence rates stratified by the length of time after surgery according to the postoperative serum CA19-9 status. With respect to the rate of initial recurrence in multiple organs and initial recurrence at sites such as the liver, lung, lymph node, peritoneum, local, and others, no significant differences were identified between the two groups. The relapse rate within 1 year after the operation was significantly higher in the Non-normalization group (69.4 %) than in the Normalization group (42.3 %) (P = 0.012). In the Normalization group, the recurrence rate was slightly higher during the first 6–12 months (25.0 %); however, it remained around 20 % in all the other periods examined (Fig. 3). On the other hand, in the Non-normalization group, the recurrence rate was very high during the 0- to 6-month (33.3 %) and 6- to 12-month periods (36.1 %). The relapse rate was significantly higher in the Non-normalization group during the early postoperative periods.
DISCUSSION
In the treatment of the bile duct cancer, surgical resection remains the only curative treatment. Complete tumor resection should be associated with normalization of the initial serum CA19-9 levels after the operation. However, in some cases, the serum CA19-9 levels remain higher than the upper limit of normal. There are several reports suggesting that in patients with pancreatic cancer, non-normalization of the postoperative serum CA19-9 is associated with a poor prognosis.16–19 On the other hand, in patients with bile duct cancer, the clinical significance of non-normalization of the postoperative serum CA19-9 is not yet clear. Serum CA19-9 levels are strongly associated with biliary obstruction and inflammation. Therefore, in our series, patients manifesting the features of cholangitis or obstructive jaundice (T-Bil > 2.0 mg/dl) at the time of measurement of the serum CA19-9 were excluded from the analysis.
Several studies have suggested that the preoperative serum CA19-9 levels are correlated with the survival in patients with bile duct cancer.10 – 12 On the other hand, the prognostic significance of the initial postoperative serum CA19-9 levels remains unclear. Kondo et al. reported that the initial postoperative CA19-9 levels (>37 U/ml) may be predictive of the survival in patients with resectable cholangiocarcinoma.20 However, their report included patients with intrahepatic bile duct cancer (n = 25). In both the TNM classification established by the International Union Against Cancer (UICC)15 and the classification established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS),21 intrahepatic bile duct cancer is identified as a separate entity from extrahepatic bile duct cancer. Therefore, we excluded patients with intrahepatic bile duct cancer from the analyses. To the best of our knowledge, our study is the first to reveal the prognostic impact of the initial postoperative serum CA19-9 levels in patients with extrahepatic bile duct cancer.
In this study, we excluded patients with preoperative serum CA19-9 values <37 U/ml so as to (1) eliminate patients with Lewis blood type a−/b−, in whom the serum CA19-9 level cannot increase, and (2) evaluate the prognostic impact of the postoperative CA19-9 as a “surgical RECIST.” Motoi et al. proposed the concept of “surgical RECIST” based on the Response Evaluation Criteria in Solid Tumors (RECIST) listed in the guidelines for pancreas cancer therapy.19 They defined “surgical RECIST” based on the presence/absence of residual tumor and the postoperative serum CA19-9 level as critical factors affecting the prognosis. A pancreatic cancer patient was defined as a “surgical complete responder” (sCR) if R0 or R1 resection was performed and the serum CA19-9 level returned to normal range in the postoperative period, and as a “surgical partial responder” (sPR) in the case of R2 resection and/or persistently elevated serum CA 19-9 in the postoperative period. Based on the same concept, we evaluated the prognostic impact of the initial postoperative serum CA19-9 level in patients with extrahepatic bile duct cancer. The Normalization group in our study was equivalent to the sCR group in the aforementioned study. Similar to the case in the pancreatic cancer patients, persistently elevated initial postoperative serum CA19-9 levels were found to be associated with a significantly poor prognosis in patients with bile duct cancer.
While, as mentioned above, preoperative serum CA19-9 levels have been shown to be correlated with the survival in patients with bile duct cancer,10 – 12 our analyses in this study did not identify the preoperative serum CA19-9 level as an independent prognostic factor in patients with extrahepatic bile duct cancer. We speculate the following reasons for this discrepant finding. First, the cohort in this study was limited to cases with abnormal elevation of the preoperative serum CA19-9 value (>37 U/ml). If the cohort had also included cases with normal preoperative CA19-9 levels (0–37 U/ml), then the preoperative serum CA19-9 may have been identified as an independent prognostic factor. Second, the serum CA19-9 level is known to be strongly associated with pressure and inflammation in the bile duct; therefore, we excluded patients with cholangitis and jaundice from the study. Notwithstanding such exclusion, the effect of cholangitis and jaundice on elevation of the preoperative serum CA19-9 value may not have been eliminated completely. In the preoperative period, a drainage tube is inserted into the bile duct; therefore, mechanical irritation of the duct wall by the tube may lead to inflammation. Furthermore, among patients with hilar bile duct cancer, many have inadequate drainage area of the bile ducts because we ordinarily insert a tube only into the bile duct of the future remnant liver. Therefore, the preoperative serum CA19-9 levels may not be truly reflective of the amount of CA19-9 produced by the tumor.
Many previous reports have suggested the residual tumor status (R status) as an independent prognostic factor in patients with bile duct cancer.22 – 24 In this study, the proportion of patients with R1 resection was significantly higher in the Non-normalization group than in the Normalization group (Table 1); therefore, R1 was considered as a potential poor prognostic factor. However, comparison of the Kaplan-Meier survival curves between the Normalization group and Non-normalization group according to the R status showed that the survival outcomes were significantly more favorable in the Normalization group irrespective of the R status (R0 or R1 resection) (Fig. 2). In addition, multivariate analysis carried out using a Cox proportional hazard regression model identified the presence of jaundice, an elevated postoperative serum CA19-9 value (>37 U/ml), a poorer histological differentiation grade (G3–4), and a higher UICC N stage (N1) as independent prognostic factors. Neither univariate analysis nor multivariate analysis identified the residual tumor classification status as a significant prognostic factor. On the basis of these findings, it was concluded that non-normalization of the initial postoperative CA19-9 level was a significant predictor of a poor prognosis, irrespective of the R status.
In conclusion, postoperative normalization/non-normalization of the serum CA19-9 level is strongly predictive of the prognosis in patients with extrahepatic biliary duct cancer, and postoperative CA19-9 measurement can help identify patients who would need additional treatments, such as adjuvant chemotherapy, and stricter follow-up. In the study group with persistent elevation of the serum CA19-9 levels after the operation, many patients developed recurrence within a year of the surgical resection. Therefore, stricter follow-up of these patients is necessary.
References
Akoad M, Jenkins R Proximal biliary malignancy. Surg Clin North Am. 2008; 88: 1409–28, x-xi.
Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001; 234: 507–17; discussion 17–9.
Veillette G, Castillo CF Distal biliary malignancy. Surg Clin North Am. 2008; 88: 1429–47, xi.
Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009; 208: 134–47.
Koprowski H, Herlyn M, Steplewski Z, Sears HF Specific antigen in serum of patients with colon carcinoma. Science. 1981; 212: 53–5.
Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979; 5: 957–71.
Magnani JL, Steplewski Z, Koprowski H, Ginsburg V Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19–9 in the sera of patients as a mucin. Cancer Res. 1983; 43: 5489–92.
Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19–9. Clin Chem. 1983; 29: 549–52.
Duffy MJ CA 19-9 as a marker for gastrointestinal cancers: a review. Ann Clin Biochem. 1998; 35 (Pt 3): 364–70.
Hatzaras I, Schmidt C, Muscarella P, Melvin WS, Ellison EC, Bloomston M Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB (Oxford). 2010; 12: 134–8
Kau SY, Shyr YM, Su CH, Wu CW, Lui WY Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg. 1999; 188: 415–20.
Liu SL, Song ZF, Hu QG, Shan D, Hu SB, Li J, et al. Serum carbohydrate antigen (CA) 19-9 as a prognostic factor in cholangiocarcinoma: a meta-analysis. Front Med China. 2010; 4: 457–62.
Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013; 20: 24–34.
Bosman FT, Carneiro F, Hruban RH, Theise ND, eds. WHO Classification of Tumours of the Digestive System, Fourth Edition. Lyon: IARC Press; 2010.
International Union Against Cancer (UICC). TNM Classification of Malignant Tumors (2009). 7 ed, Wiley-Liss, New York.
Ferrone CR, Finkelstein DM, Thayer SP, Muzikansky A, Fernandez-delCastillo C, Warshaw AL Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006; 24: 2897–902.
Kinsella TJ, Seo Y, Willis J, Stellato TA, Siegel CT, Harpp D, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol. 2008; 31: 446–53.
Montgomery RC, Hoffman JP, Riley LB, Rogatko A, Ridge JA, Eisenberg BL Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997; 4: 551–6.
Motoi F, Rikiyama T, Katayose Y, Egawa S, Unno M Retrospective evaluation of the influence of postoperative tumor marker status on survival and patterns of recurrence after surgery for pancreatic cancer based on RECIST guidelines. Ann Surg Oncol. 2011; 18: 371–9.
Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Sasaki H, et al. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014; 110: 422–9.
Japanese Society of Hepato-Biliary-Pancreatic Surgery. General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tract (2013). 6 ed.
Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007; 31: 1256–63.
Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, et al. Perineural invasion in extrahepatic cholangiocarcinoma: prognostic impact and treatment strategies. J Gastrointest Surg. 2013; 17: 1429–39.
Wakai T, Shirai Y, Moroda T, Yokoyama N, Hatakeyama K Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005; 103: 1210–6.
Acknowledgments
No special comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Source of Financial Support
None
Conflict of Interest
Nothing to declare
Additional information
Permissions
We do not use previously published materials in this article.
Rights and permissions
About this article
Cite this article
Kato, Y., Takahashi, S., Gotohda, N. et al. Prognostic Impact of the Initial Postoperative CA19-9 Level in Patients with Extrahepatic Bile Duct Cancer. J Gastrointest Surg 20, 1435–1443 (2016). https://doi.org/10.1007/s11605-016-3180-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3180-5