Abstract
Background
Postoperative pancreatic fistula (POPF) is a serious complication of pancreaticoduodenectomy (PD). Sarcopenia is a newly identified marker of frailty. We performed this study to assess whether preoperative sarcopenia has an impact on clinically relevant POPF formation.
Methods
A total of 266 consecutive patients who underwent a PD between 2010 and 2014 were enrolled in this retrospective study. Skeletal muscle mass was measured using preoperative computed tomography images. The impact of preoperative sarcopenia on clinically relevant POPF formation was analyzed using univariate and multivariate analyses.
Results
Of the 266 patients, 132 (49.6 %) were classified as having preoperative sarcopenia. The rate of clinically relevant POPF formation was significantly higher in the sarcopenia group (22.0 vs. 10.4 %; P = 0.011). A multivariate logistic regression analysis showed that sarcopenia (odds ratio, 2.869; P = 0.007) was an independent risk factor for the development of clinically relevant POPF, along with a soft pancreas and a parenchymal thickness at the pancreatic resection site of ≥8 mm.
Conclusions
Preoperative sarcopenia was identified as a strong and independent risk factor for clinically relevant POPF formation after PD. Perioperative rehabilitation and nutrition therapy may contribute to the prevention of POPF formation and a safer PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite recent advances in surgical procedures and perioperative management techniques, pancreaticoduodenectomy (PD) is still associated with major morbidity and prolonged hospitalization.1 – 3 Postoperative pancreatic fistula (POPF) is the most serious complication after PD, since it is often associated with a fatal outcome. A recent Japanese population-based study demonstrated that 30-day postoperative and in-hospital mortality rates of PD patients have been improved (1.2 and 2.8 %, respectively). However, the overall morbidity rate after PD remains high in the 40 to 55 % range, as does the rate of clinically relevant POPF that ranges from 10 to 30 %.1 - 3
Sarcopenia, a newly identified marker of frailty, is characterized by progressive and generalized deterioration of the skeletal muscle mass and strength.4,5 Sarcopenia is known to be an important factor influencing morbidity, recovery, and survival after surgery.6 – 9 Sarcopenia is known to have negative impacts on the postoperative morbidity and the prognosis of cancer patients undergoing PD.10 – 13 No detailed analyses have been performed to determine the relationship between sarcopenia and the development of clinically relevant POPF.
To examine the significance of preoperative sarcopenia as a risk factor for the development of POPF after PD, we reviewed data of 266 consecutive patients that had undergone PD at our institution. Our goals were to identify POPF risk factors and to improve perioperative management techniques.
Materials and Methods
Patient and Data Collection
We retrospectively reviewed the data of 266 consecutive patients who had undergone PD at the National Cancer Center Hospital East, Chiba, Japan, between January 2010 and December 2014. We excluded those patients who had undergone hepato-pancreaticoduodenectomy.
For each patient, we reviewed the preoperative routine CT images and collected the clinical data, including the preoperative characteristics, postoperative complications, and histopathological diagnosis, from a prospectively maintained computer database. The data collected included the age and sex of the patients, height, weight, body mass index (BMI), presence/absence of diabetes, American Society of Anesthesiologists classification (ASA) score, serum albumin, total peripheral blood lymphocyte count, histopathological diagnosis, presence/absence of lymph node metastasis, type of operation, operation time, estimated blood loss, presence/absence of transfusion, main pancreatic duct (MPD) diameter, stump thickness, stump width, consistency of the pancreas (soft or hard) at the resection site, presence/absence of need for reoperation, 30-day readmission rate, mortality, postoperative hospital stay, preoperative biliary drainage (yes/no), and preoperative therapy. The prognostic nutritional index (PNI), an indicator of the nutritional status, was assessed in accordance with the following equation, described previously14: PNI = 10 × serum albumin [mg/dL] + 0.005 × total lymphocyte count. The parenchymal thickness and width were calculated using the respective formulae, described previously3 (Fig. 1). The postoperative complications, including POPF formation (ISGPF definition15), surgical site infection (SSI), and delayed gastric emptying (DGE), were scored using the Clavien-Dindo classification.16
Surgical Techniques and Perioperative Management
We performed a subtotal stomach-preserving PD in most of the patients. The operative procedures and perioperative management were described previously by Sugimoto et al.3 Briefly, we divided the pancreas using a scalpel, an ultrasonically activated device, or a combination of both at the surgeon’s discretion. For the reconstruction, we performed an end-to-side pancreaticojejunostomy using the modified technique first described by Kakita et al.17 For the outer layer, we placed two to four interrupted sutures penetrating the pancreatic parenchyma and picking up the seromuscular layer of the jejunum using a 3–0 non-absorbable monofilament. Next, we fixed the pancreatic duct and full thickness of the jejunal wall as the inner layer with 8 to 14 interrupted sutures using 5–0 absorbable monofilament sutures, depending on the size of the MPD. Then, we accomplished an approximation of the jejunal wall and the pancreatic stump with ligation of the outer-layer stitches to cover the cut surface of the pancreas completely. We then placed a 5-Fr or a 6-Fr short internal drainage tube through the pancreatic duct. We did not perform external pancreatic or biliary drainage. Closed suction drains were placed near the pancreaticojejunal and choledochojejunal anastomoses. We measured the MPD diameter (MPDd), stump thickness, and stump width at the pancreatic resection site using ultrasound and evaluated the consistency of the pancreas (soft or hard) subjectively during the operation.
We managed all the patients using the same perioperative schedule. Briefly, we administered piperacillin prophylactically for 3 days. We did not administer octreotide postoperatively. We evaluated the amylase level in the drainage fluid and cultured the drainage fluid on postoperative days (POD) 1, 3, and 5. A routine postoperative computed tomography (CT) examination was not planned. The drains were removed on POD 3–6 once the drainage fluid did not show a high amylase level or any signs of infection. In patients with signs of infection in the drainage fluid, we performed a drain replacement via the ordinary tract created during the operation under fluorography on POD 7–10. In principle, the oral intake of water and solid food was restarted on POD 1 and 3, respectively.
Computed Tomography Image Analysis and Sarcopenia
We used a three-dimensional image analysis system (Volume Analyzer SYNAPSE VINCENT; Fujifilm Medical, Tokyo, Japan) to analyze the CT images. Skeletal muscle at the level of the L3 vertebra was identified and quantified using HU thresholds of −29 to +15018 (Fig. 2). All the measurements and calculations described above were performed by the same examiner (YN), who was blinded to the surgical outcomes at the time of the quantification.
In this study, we applied the sarcopenia definition proposed by Martin et al.19 According to this definition, sarcopenia is defined as a skeletal muscle index (SMI) = ([skeletal muscle area at L3]/[height]2)20 of <43 cm2/m2 in men with a BMI of <25 kg/m2, <53 cm2/m2 in men with a BMI of ≥25 kg2/m2, and <41 cm2/m2 in women.
Statistical Analyses
We compared the clinical characteristics between the two groups using a chi-square analysis for non-continuous variables and the t test or the Mann-Whitney U test for continuous variables. We conducted a logistic regression analysis to determine the associations between clinicopathologic factors and the development of clinically relevant POPF. All the reported P values are two-sided, and P < 0.05 was considered as denoting statistical significance. All the analyses were performed using the IBM SPSS Statistics 21 software package (SPSS Inc., Tokyo, Japan).
The study protocol was approved by the Institutional Review Board of the National Cancer Center, Japan.
Results
Patient Characteristics and Sarcopenia
Based on Martin’s definition,19 132 patients (49.6 %) were classified as having sarcopenia in our study cohort. The patient characteristics and the presence or absence of sarcopenia are shown in Table 1. The median patient age was 69 years, and 181 patients (68.0 %) were men. Pancreatic tumor was the most common disease to be treated with a PD (61.3 % of the patients), and lymph node metastasis was diagnosed in 140 patients (52.6 %).
Sarcopenia was more common in women and older patients and was associated with a significantly lower height (P = 0.001), body weight (P < 0.001), BMI (P < 0.001), serum albumin level (P < 0.001), and PNI (P = 0.001). Other factors such as the presence/absence of diabetes, the ASA score, the need or the lack of preoperative biliary drainage, and preoperative therapy were not correlated with the presence of sarcopenia.
The median (range) SMI (cm2/m2) was 45.4 (28.4–72.2) in the male subjects and 38.5 (28.2–51.0) in the female subjects. An identical SMI was observed for each BMI level and age (Fig. 3).
Operative Characteristics and Pancreatic Configuration Data
The operative characteristics and their relationships with sarcopenia and non-sarcopenia are shown in Table 2. No differences in the type of operation, the need for portal vein or superior mesenteric vein (PV/SMV) resection, the operation time, or the need for transfusion were seen between the two groups, while the estimated blood loss was higher in the non-sarcopenia group (P = 0.046). Overall, 147 patients (55.3 %) had a soft pancreas, which is widely acknowledged as a strong risk factor for POPF formation. Regarding the configuration and consistency data for the remnant pancreas, no significant differences were observed between patients with and those without sarcopenia.
Postoperative Complications and Recovery
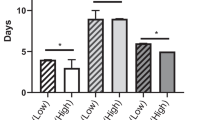
The postoperative complications and their relationships with sarcopenia and non-sarcopenia are shown in Table 3. Clinically relevant POPF formation occurred in 43 patients (16.2 %), and major complications (including grades III, IV, and V of the Clavien-Dindo classification) occurred in 66 patients (24.8 %). The rate of formation of clinically relevant POPF in the patient group with sarcopenia was significantly higher than that in the non-sarcopenia group (22.0 vs. 10.4 %; P = 0.011). Similarly, the rate of major complications was significantly higher in the sarcopenia group (34.1 vs. 15.7 %; P = 0.001). The total rates of DGE (13 patients, 4.9 %) and incisional SSI (17 patients, 6.4 %) were very low, showing no significant differences between the patient groups with and without sarcopenia. The postoperative reoperation, 30-day readmission, and mortality rates in this study were 0.4, 1.1, and 0.4 %, respectively, with no significant differences observed between patients with and those without sarcopenia. The postoperative hospital stay was 14.0 days, which is nearly acceptable. The postoperative hospital stay in the group with sarcopenia was significantly longer than that in the patient group without sarcopenia (15.0 vs. 13.0 days; P = 0.001) because of the difference in the morbidity rate. Regarding the adjuvant therapy, a total of 112 patients with pancreatic adenocarcinoma were suitable for adjuvant chemotherapy. Twelve patients (10.7 %) failed to start adjuvant chemotherapy because of their insufficient recovery; nine patients (75.0 %) were sarcopenic preoperatively. The time from surgery to the first administration of adjuvant chemotherapy in the sarcopenia group was significantly longer than that in the non-sarcopenia group (37.0 vs. 34.0 days; P = 0.043).
Risk Factors for Clinically Relevant POPF
The preoperative and intraoperative risk factors, including sarcopenia that may be associated with the development of clinically relevant POPF, were evaluated. A univariate analysis identified the body weight, BMI, diagnosis of pancreatic tumor, presence/absence of sarcopenia, presence/absence of need for a PV/SMV resection, MPDd, parenchymal thickness, parenchymal width, and consistency of the pancreas as being significantly associated with the development of clinically relevant POPF (Table 4). A multivariate logistic regression analysis performed using the aforementioned factors identified a soft pancreas (P < 0.001), the presence of sarcopenia (P = 0.007), and parenchymal thickness at the site of resection of ≥8 mm (P = 0.016) as being significant independent risk factors for the development of clinically relevant POPF (Table 5). The odds ratio (OR) of sarcopenia for the development of clinically relevant POPF was 2.896 (95 % confidence interval, 1.329–6.197), that of a soft pancreas was 15.951 (95 % CI, 3.375–75.383), and that of a parenchymal thickness ≥8 mm at the site of resection was 3.142 (95 % CI, 1.236–7.986).
Discussion
This study demonstrated that preoperative skeletal muscle depletion, otherwise known as sarcopenia, was a strong and independent risk factor for the development of clinically relevant POPF (OR, 2.896; 95 % CI, 1.329–6.197; P = 0.007), which is one of the most important complications after PD.
PD is still associated with a relatively high morbidity, and the reported rate of the development of clinically relevant POPF after PD is approximately 10–30 %.1 – 3 We previously reported that POPF grade B occurred in 84 patients (26.4 %) and POPF grade C occurred in 6 patients (1.9 %) in our cohort.3 Several studies have investigated the risk factors for the development of POPF.2, 21 In our previous study, we investigated several factors in relation to the risk of development of POPF, including the consistency of the pancreas and the pancreatic configuration data, and reported that the risk factors for the development of POPF after PD were a decreased MPDd and an increased parenchymal thickness of the pancreas at the resection site, in addition to a soft pancreas.3 In the present study, we demonstrated, using a detailed analysis of several potentially related factors, that sarcopenia was the second strongest risk factor for the development of clinically relevant POPF after PD, while the consistency of the soft pancreas and pancreatic configuration data are widely acknowledged as being strong and important risk factors. The incidence of major complications (24.8 %) and clinically relevant POPF (16.2 %) in this study were relatively high but were consistent with previous reports.1, 2, 21 Therefore, further modifications and improvements of the surgical technique and perioperative management of PD are needed to reduce the incidence of complications after PD. Because skeletal muscle depletion can be prevented preoperatively thorough rehabilitation and nutrition therapy, we postulated that sarcopenia might be a novel indicator for lowering the risk of major complications and POPF. In addition, the reported overall mortality after PD is approximately 3.0 %.1,2 In our series of 266 consecutive patients who underwent PD, however, the postoperative reoperation, 30-day readmission, and mortality rates were 0.4, 1.1, and 0.4 %, respectively, which are nearly acceptable.
Sarcopenia is associated with aging, chronic disease, and cancer and reflects many clinical conditions such as nutritional status and a compromised immune status. Sarcopenia is an integrated and quantitative marker of frailty.5, 6, 22 Regarding the relationships between sarcopenia and the postoperative outcomes, many studies have demonstrated that preoperative sarcopenia has a strong impact on the morbidity and mortality associated with surgery.7 – 10 In the field of pancreatic surgery, Peng et al. examined 557 pancreatic cancer patients by measuring the total psoas area as a marker of sarcopenia and found that sarcopenia was a predictor of survival following pancreatic surgery.12 They also reported that sarcopenia was not associated with the overall morbidity.12 Joglekar et al. reported that sarcopenia was an independent prognostic factor of the surgical outcomes.14 However, in both studies, the method of muscle mass measurement based on the total psoas area was not optimal, and the evaluated patients also included those who had undergone distal pancreatectomy, in addition to those who had received a PD. The risk of the development of POPF after PD had not been studied since the acknowledgement of the validity of evaluations of sarcopenia based on the lumbar skeletal muscle index in the current consensus statement.6 The present study is the first to demonstrate that preoperative sarcopenia is related to the development of clinically relevant POPF after PD using a detailed analysis of the clinicopathologic factors and the measurement of sarcopenia based on CT images.
The pathophysiological mechanisms underlying the association between preoperative sarcopenia and the risk of postoperative complications and prognosis have not yet been established definitively. Lutz et al. demonstrated that as the skeletal muscle mass decreases and the adipose tissue mass increases, the production of anti-inflammatory cytokines and adiponectin decreases and the production of pro-inflammatory molecules such as leptin, chemerin, resistin, TNF-α, IL-1, and IL-6 increases.22 It is our assumption that the pro-inflammatory state in sarcopenic patients leads to a weakening of the immune system and poor wound healing after surgery, thereby exerting an impact on the risk of postoperative complications. Moreover, they demonstrated that myokines released by the skeletal muscle affected the immune system with special reference to natural killer (NK) cells,22 thereby impacting the prognosis of cancer patients after surgery.
We demonstrated that sarcopenia is a risk factor for POPF after PD. Although a soft pancreas and an increased parenchymal thickness at the pancreatic resection site are strong risk factors, it should be noted that we can potentially prevent skeletal muscle depletion and improve the skeletal muscle mass preoperatively through rehabilitation and nutrition therapy. Kim et al. demonstrated that the combination of low-intensity exercise and essential amino acid supplementation improved the skeletal muscle mass, strength, and walking speed in sarcopenic women.23 Singh et al. demonstrated in his review that presurgical exercise may benefit cancer patients through positive effects on function and physical capacity.24 However, the effects of rehabilitation and nutrition therapy on preoperative sarcopenic patients have not been investigated. Additional study is needed to prevent skeletal muscle depletion preoperatively and enable safe surgery.
The present study has some potential limitations. First, several SMI cutoff values have been reported for the diagnosis of sarcopenia. Prado’s definition (SMI of <52.4 cm2/m2 in men and <38.5 cm2/m2 in women) was constructed using a Canadian cohort of 250 patients with respiratory and gastrointestinal tract cancer.5 In the present study, we adopted Martin’s definition,19 which was constructed using a large cohort of >1000 cancer patients. We have assumed that Martin’s definition is more suitable for a low-BMI population, such as an Asian cohort, because the definition stratifies sarcopenia based on the BMI. According to Martin’s definition, 132 patients (49.6 %) were classified as having sarcopenia in our study cohort, which was consistent with the frequency reported from other sarcopenia studies in Japan.25 In addition, there are various measuring methods for the assessment of sarcopenia other than measuring skeletal muscle mass using abdominal CT: grip strength, gait speed, functional performance defined by the short physical performance battery26, and the timed get-and-go test27. We did not perform such measuring methods in this retrospective study; therefore, future studies should investigate preoperative sarcopenia using multiple measuring methods. Second, the cutoff values for the pancreatic configuration data in this study (MPDd of 2 mm and parenchymal thickness of 8 mm) are not accepted worldwide. However, we adopted these cutoff values based on the results of our previous report3 under the assumption that the values would be appropriate for our cohort. Finally, this study was a single-institution, retrospective study with a relatively small number of patients. However, it should be noted that notwithstanding the cutoff values adopted or the tools used to assess sarcopenia, this study clearly demonstrated the existence of a relationship between sarcopenia and the postoperative course. Additional studies on perioperative interventions (rehabilitation and nutrition therapy) are needed to improve the postoperative course after PD. Our findings could serve as the basis for studies in the future.
Conclusion
Preoperative sarcopenia was identified as a strong and independent risk factor for the development of clinically relevant POPF, which is one of the most important complications after PD. Perioperative rehabilitation and nutrition therapy for sarcopenic patients may contribute to reducing the incidence of POPF and ensuring the safety of PD.
References
Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, Baba H, Tomita N, Nakagoe T, Sugihara K, Mori M. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773–80.
Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber PR, Sauvanet A, Sa Cunha A, Le Treut YP, Adham M, Mabrut JY, Chiche L, Bachellier P; French Surgical Association (AFC). Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HBP 2014;16:46–55.
Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, Konishi M. Schematic pancreatic configuration: a risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastrointest Surg 2013;17:1744–51.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95.
Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2015;261:1173–83.
Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, Stewart SB, Thapa P, Tarrell RF, Cheville JC, Tollefson MK. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer 2014;120:2910–8.
Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, Nagino M. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg 2015;39:1494–500.
Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 2015;261:345–52.
Amini N, Spolverato G, Gupta R, Margonis GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, Kamel IR, Pawlik TM. Impact Total Psoas Volume on Short- and Long-Term Outcomes in Patients Undergoing Curative Resection for Pancreatic Adenocarcinoma: a New Tool to Assess Sarcopenia. J Gastrointest Surg 2015;19:1593–602.
Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, Makary M, Hirose K, Edil B, Choti MA, Herman J, Cameron JL, Wolfgang CL, Pawlik TM. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478–86.
Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973–9.
Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol 2015;111:771–5.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5. (in Japanese with English abstract).
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8–13.
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96.
Kakita A, Takahashi T, Yoshida M, Furuta K. A simpler and more reliable technique of pancreatojejunal anastomosis. Surg Today 1996;26:532–35.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–47.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, Heymsfield SB, Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–38.
Yamamoto Y, Sakamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. A preoperative predictive scoring system for postoperative pancreatic fistula after pancreaticoduodenectomy. World J Surg 2011;35:2747–55.
Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–46.
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16–23.
Singh F, Newton RU, Galvão DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol 2013;22:92–104.
Fukushima H, Yokoyama M, Nakanishi Y, Tobisu K, Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 2015;10:e0115895.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94.
Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Institutional Review Board of the National Cancer Center, Japan.
Grant Support and Other Assistance
None
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nishida, Y., Kato, Y., Kudo, M. et al. Preoperative Sarcopenia Strongly Influences the Risk of Postoperative Pancreatic Fistula Formation After Pancreaticoduodenectomy. J Gastrointest Surg 20, 1586–1594 (2016). https://doi.org/10.1007/s11605-016-3146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3146-7