Abstract
We intended to investigate the clinicopathological features of intrahepatic intraductal papillary neoplasms of the bile duct (IPNB), especially their malignant features and post-resection prognosis. Forty-three patients who met the definition of IPNB and who underwent liver resection between January 2002 and June 2015 were selected from our institutional database of liver resection cases. The mean age was 63.3 ± 6.9 years and 24 were male. Hepatolithiasis was present in addition in 10 of the patients. Left- and right-sided hepatectomies and concurrent bile duct resection (BDR) were performed in 28, 15, and 10 patients, respectively; R0 resection was performed in 37 patients. The mean tumor diameter was 4.1 ± 2.2 cm. Histological tumor grade was low in 4 cases, intermediate in 6, and malignant in 33. There was no cancer-related recurrence or death in the 10 patients with low-grade or intermediate lesions. In the 33 patients with malignant lesions, rates of tumor recurrence and overall survival were 12.5 and 96.2 % at 1 year, 36.4 and 91.3 % at 3 years, and 47.0 and 68.8 % at 5 years, respectively. Multivariate analysis showed that R1 resection was the only prognostic factor for tumor recurrence and patient survival. BDR was performed in only 2 of 6 patients undergoing R1 resection. Intrahepatic IPNB is a rare type of biliary neoplasm that encompasses a histological spectrum ranging from benign disease to invasive malignancy. Long-term survival was anticipated after curative resection. R1 resection reduced survival outcomes; therefore, we suggest that concurrent BDR should be performed if the resection margin of the bile duct is not reliably free of neoplastic involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraductal papillary neoplasms of the bile duct (IPNB) have been recently associated with certain types of papillary tumor with malignant potential occurring in the extrahepatic and intrahepatic bile ducts.1–3 These papillary tumors are also known as biliary papillomatosis, papillary adenoma, or papillary cholangiocarcinoma.4 Some features of IPNB overlap with intrahepatic cholangiocarcinoma (ICC) of the intraductal growth (IG) type. Because of the imprecise definition of cases, extent of diseases, and date of reports, the reported malignant potential of biliary papillomatosis ranges widely from 19.5 % to as high as 83 %, with conflicting survival outcomes.5 IPNB is classified as a distinct clinical and pathological entity in the 2010 World Health Organization classification.6 These tumors show papillary proliferation in the bile duct with or without mucin secretion and are considered to be IPNB, the biliary counterpart of intraductal papillary mucinous neoplasm (IPMN) of the pancreas.6,7
In this study, we intended to evaluate the clinicopathological features and long-term outcomes of 43 patients with IPNB in the intrahepatic ducts.
Materials and Methods
Definition of IPNB
According to Naito et al.8 and Jung et al.,9 intrahepatic IPNB is defined as a tumor satisfying the following four criteria: localization in the liver, marked dilatation of the bile duct with intraluminal filling defects on radiological imaging, papillary or cast-like growth of a mass predominantly within the bile ducts on gross examination, and papillary or villous tumors showing fibrovascular cores under microscopy.
Patient Selection
After an extensive search of our institutional database of liver resection cases, 43 patients who met the above criteria for intrahepatic IPNB were identified. They underwent liver resection between January 2002 and June 2015 and were followed up until the end of 2015 or until death. Their medical records were reviewed retrospectively after approval of the Institutional Review Board of our institution.
Histologically, the intrahepatic IPNB tumors were mainly well-differentiated papillary adenocarcinoma and/or a papillary epithelial borderline lesion, or adenoma with fine fibrovascular stroma, in addition to fulfilling the IPNB criteria. The exclusion criteria were as follows: borderline or carcinoma composed of considerable tubulopapillary or tubular components protruding into the lumen of the bile duct, moderately to poorly differentiated adenocarcinoma, mucinous cystic neoplasm with ovarian-like stroma of the liver or biliary tract, other types of malignant tumor showing IG, and reactive or hyperplastic biliary epithelial lesions.10
We searched our institutional database of liver resection cases again to identify ICC of the IG type (IG-ICC) because this tumor entity is often confused clinically with intrahepatic IPNB.11 We identified 62 patients with IG-ICC during the period under review and compared them with the patients with intrahepatic IPNB.
Preoperative Imaging Evaluation
Routine preoperative imaging evaluation included abdominal and chest computed tomography (CT), liver magnetic resonance imaging with cholangiopancreatography (MRCP), and 2-18F-fluoro-2-deoxy-d-glucose positron-emission tomography (FDG-PET). The extent of hepatic resection was primarily determined by the future volume of the liver remnant, with consideration of tumor-free resection margin and hepatic functional reserve. If the future liver remnant appeared too small, right portal vein embolization was performed 2–3 weeks before surgery. The process of major hepatectomy has been described elsewhere.11,12
Modalities for bile duct evaluation included endoscopic retrograde cholangiopancreatography (ERCP), MRCP, and percutaneous transhepatic cholangioscopy (PTCS). For PTCS, initial percutaneous transhepatic biliary drainage was performed using a pigtail catheter under fluoroscopic guidance, and the tract was dilated 2 or 3 days after drainage. PTCS was then performed 7 days after tract dilatation. Cholangioscopic evaluation was performed using a cholangioscope with external dimension 4.9 mm (FCN-15X; Pentax, Tokyo, Japan) or 5.1 mm (FCN-1530; Pentax), and the various mucosal appearances of the bile duct tumors and strictures were examined. Multiple targeted biopsies were collected using forceps (KA 1811S; Pentax) under direct cholangioscopic visualization.5,9 PTCS was often helpful in determining the extent of intra- and extrahepatic IPBN.
Surgical Procedures
Hepatic resection was classified as either anatomical or non-anatomical hepatectomy. Anatomical hepatectomy included resection of one or more adjacent hepatic segments along the hepatic vasculature. When involvement of the bile duct was seen in preoperative imaging studies or the resection margin of the bile duct was tumor-positive in intraoperative frozen-section biopsy, concurrent bile duct resection (BDR) was performed. Regional lymphadenectomy beyond lymph node (LN) sampling was not routinely done. If preoperative imaging studies implied regional LN metastasis or if LN metastasis was suspected during surgery, all resectable regional LNs including the peripancreatic area and celiac axis were dissected. Perioperative mortality was defined as patient death from any cause within 1 month of surgery.
Degree of Malignancy of the Intrahepatic Bile Duct
Sections stained with hematoxylin and eosin (H&E) were reviewed and each tumor was categorized into one of three groups according to the degree of malignancy: adenoma (low grade), borderline (intermediate grade), and malignant (carcinoma in situ and high grade). Tumors with microinvasion were categorized as malignant.
Postoperative Surveillance and Treatment of Tumor Recurrence
Patients were followed every 2–4 months during the first year after surgery, depending on pathology and tumor stage; thereafter, the follow-up interval was adjusted and for malignant lesions was every 3–4 months. The general principles of treatment for recurrent cholangiocarcinoma lesions were followed for our patients with malignant IPNB, including locoregional treatment and systemic chemotherapy.11
Statistical Analysis
Numeric variables are presented as means with standard deviations. Continuous variables were compared using Student t test if normally distributed. Categorical variables were compared using the chi-square test and Fisher’s exact test. Tumor recurrence and patient survival rates were estimated using the Kaplan–Meier method and compared with the log-rank test. SPSS version 21.0 for Windows (SPSS, Chicago, IL, USA) and Statistica version 6.0 (StatSoft, OK, USA) were used for the analyses. Data were considered significant at p < 0.05.
Results
Demographic Data and Clinical Characteristics
Of the 43 patients with intrahepatic IPNB, 24 (55.8 %) were male. The mean age was 63.3 ± 6.9 years (range 47–78). Initial clinical manifestations were abdominal pain or discomfort (n = 15), gastrointestinal symptoms (n = 9), and no symptoms with incidental detection (n = 16). Six patients underwent prior cholecystectomy because of gallstone disease. Intrahepatic duct stones were detected in 10 patients (23.3 %) at the time of IPNB diagnosis. No patient was associated with Clonorchis sinensis infection. The mean level of carcinoembryonic antigen (CEA) was 1.9 ± 1.3 U/mL (range 0.5–4.6); the mean level of carbohydrate antigen (CA) 19-9 was 40.7 ± 126.1 U/mL (range 2.5–832.0).
Findings of Preoperative Imaging Studies
All patients underwent radiological examinations including abdominal ultrasonography, abdominal CT scan, and MRCP; 23 patients were evaluated using ERCP; and 8 were evaluated by preoperative PTCS. Detailed imaging findings are summarized in Table 1. Both MRCP and PTCS were useful to determine the gross extents of the involved lesions, by which the extents of resection were decided before operation. PTCS enabled us to identify involvement of the first-order intrahepatic duct or hilar duct confluence portion in 3 patients; thus, they were indicated for concurrent bile duct resection.
Surgical Procedures
Tumors were located in the left liver in 28 patients and right liver in 15; their operations were resection of the involved liver with or without concurrent BDR. The extents of liver resection are summarized in Table 2. BDR was performed concurrently in 10 patients (23.3 %). One patient underwent right hepatectomy after preoperative portal vein embolization. Curative resection (R0 resection) was performed in 37 patients (86.1 %), and another 6 patients had tumor cell-positive resection margins (R1 resection), mainly at the resected bile duct margins. Concurrent BDR was performed in only 2 (33.3 %) of the 6 patients who underwent R1 resection. There was no case of perioperative mortality.
Pathological Findings
Among the 43 patients reviewed, 10 were diagnosed pathologically with benign lesions (low and intermediate grades) of intrahepatic IPNB, and the other 33 had malignant lesions including intraductal papillary adenocarcinoma or intraductal papillary mucinous adenocarcinoma (Fig. 1). The tumor was confined within the bile duct in 23 patients (69.7 %), and the other 10 had periductal or hepatic parenchymal invasion. These 33 patients were further classified into three groups of high-grade tumor (n = 7), microinvasive carcinoma (n = 9), and invasive carcinoma (n = 17). The mean tumor diameter was 4.1 ± 2.2 cm (range 2.0–11.2). Only 1 patient had regional LN metastasis (Table 3).
Photographs of resected liver specimens. a Resected left liver specimen with intraductal papillary neoplasm. b This low-grade dysplasia lesion has no hepatic parenchymal invasion and no lymphovascular emboli (H&E, ×100). c Resected left liver specimen showing a well-differentiated intraductal papillary adenocarcinoma with mucinous ductal rupture and mucin pool formation. d Resected right liver specimen showing intraductal papillary adenocarcinoma with extension to the subepithelial connective tissue and lymphovascular invasion
Comparison of the Clinicopathological Features of Benign and Malignant Lesions
There were no significant differences between the patients with benign (n = 10) and malignant (n = 33) lesions in age, preoperative tumor markers including total bilirubin, or in tumor size. However, the patients with malignant lesions tended to have a higher incidence of mucin pool formation (Table 4).
Survival Outcomes and Risk Factor Analysis
Nine of the 10 patients with benign intrahepatic IPBN remained alive during a mean follow-up period of 44 months. One patient who had undergone R0 resection of a right hepatectomy with BDR for a 4-cm lesion was diagnosed with de novo cancer of the pancreas tail with multiple hepatic metastases at 9 months after surgery; thus, postoperative survival period was 15 months.
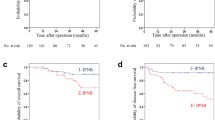
In the 33 patients with malignant intrahepatic IPBN, the mean follow-up period was 53 months. During that period, tumors recurred in 12 patients, the recurrences being intrahepatic, extrahepatic, or both. The sites of extrahepatic recurrence were lung (n = 2), bone (n = 2), stomach (n = 1), abdominal wall (n = 1), and peritoneal seeding (n = 2). All these patients underwent recurrence treatments including systemic chemotherapy and radiotherapy. Their tumor recurrence rates were 12.5 % after 1 year, 36.4 % at 3 years, and 47.0 % at 5 years; their overall survival rates were 96.2 % at 1 year, 91.3 % at 3 years, and 68.8 % at 5 years (Fig. 2).
There was no tumor recurrence or patient death in 6 patients with IPNB of high grade. The tumor recurrence and patient survival rates were 11.1 and 100% at 1 year, 44.4 and 85.7 % at 3 years, and 63.0 and 51.4 % at 5 years in 9 patients with microinvasive carcinoma; and 17.6 and 93.8 % at 1 year, 37.4 and 85.9 % at 3 years, and 44.4 and 77.3 % at 5 years in 17 patients with invasive carcinoma, respectively (p = 0.203 for tumor recurrence and p = 0.120 for patient survival).
Univariate analysis showed that significant risk factors for tumor recurrence were CA 19-9, perineural invasion, and R1 resection; and R1 resection for patient survival (Table 5). In multivariate analysis, R1 resection was the only prognostic factor for tumor recurrence and patient survival (Fig. 3, Table 6).
Overlap of Intrahepatic IPNB and IG-ICC
After comparing the 62 patients showing IG-ICC with the 33 patients with intrahepatic malignant IPNB, 9 (27.3 %) of the 33 were also classified as IG-ICC. The profiles of the majority of the ICC group have been published previously.11
The 62 patients with IG-ICC were divided into IPNB (n = 9) and non-IPNB (n = 53) groups. There was almost no difference between these groups in tumor size (4.3 ± 2.9 vs. 3.7 ± 1.8 cm; p = 0.325), predominant well differentiation (100 vs. 86.8 %; p = 0.053), absence of lymphovascular and perineural invasion (11.1 vs. 13.2 %; p = 1.0), tumor recurrence rate (at 3 years, 31.3 vs. 27.2 %; p = 0.727), or patient survival rate (at 3 years, 66.7 vs. 71.1 %; p = 0.655). The exception was performance of concurrent BDR (55.6 vs. 20.8 %, respectively; p = 0.027).
Discussion
IPNB has been reported sporadically around the world13,14 and has been considered as a precursor lesion of cholangiocarcinoma.1 IPNB was proposed as a new disease entity because of striking similarities to IPMN of the pancreas, where the disease entity and clinicopathological features are well established.15 However, preoperative diagnosis of intrahepatic IPBN is usually difficult in practice. The common clinical manifestations of patients with intrahepatic IPNB are recurrent abdominal pain, repeated episodes of acute cholangitis, and obstructive jaundice, as found in the present study.
The common abnormal finding in imaging studies in patients with intrahepatic IPNB was intrahepatic duct dilatation. When intraductal masses were not detected on ultrasonography or CT scan, they were often diagnosed to be biliary stones, clonorchiasis, or benign biliary strictures. ERCP may be useful in making the diagnosis of intrahepatic IPNB, whose characteristic findings are multiple small filling defects and serrated irregularity of the bile duct wall. On cholangiography, diffuse bile duct dilatation and amorphous filling defects in the bile duct are characteristic. However, a large amount of mucin secretion and obstruction by the tumor prevent complete opacification of the entire biliary tract. As a result, ERCP evaluation of the precise extent of ductal involvement is often suboptimal.16
Cholangioscopic evaluation provided detailed information on the extent of IPNB and enabled the appropriate surgical treatment to be provided. PTCS evaluation has some advantages over conventional radiological imaging: it may visualize the bile duct mucosa directly and detect small or subtle mucosal lesions that are not evident on direct cholangiograms. Because small papillary lesions may not be detected using conventional radiological methods, these undetected lesions, usually remote from the main tumor, may be the foci of recurrence. We prefer PTCS to peroral cholangioscopy because the latter examination involves the difficult use of the remote-controlled baby scope, making the technique inferior to PTCS for complete evaluation of the intrahepatic duct. PTCS examination is therefore an indispensable preoperative procedure for determining treatment modality and the appropriate extent of resection in intrahepatic IPNB. It is also useful in patients with mucin-producing lesions because mucin is observed as filling defects on direct cholangiography.16,17
Intrahepatic IPNB should not be regarded as a benign disease with low malignant potential but as a premalignant lesion with high malignant potential. In the present study, low-grade intrahepatic IPNB was rather rare and the majority of intrahepatic IPNB cases were high-grade IPNB, and invasive IPNB with minimal and considerable invasion. IPNB with different malignant potentials can be ultimately diagnosed as adenoma, borderline tumor, non-invasive carcinoma, or invasive carcinoma,8 leading to the conclusion that the spectrum of IPNB represents a continuum of intraductal neoplastic progression. The progression from benign to malignant disease may follow the adenoma–carcinoma sequence.
Recent studies have revealed striking similarities between IPNB and pancreatic IPMN.18 In both organs, these neoplasms arise within the ductal system and show a predominantly intraductal growth pattern macroscopically and papillary proliferation with delicate fibrovascular cores.19 However, there are several differences between IPNB and IPMN; one important difference is with respect to mucin hypersecretion. Mucin is macroscopically identifiable in most cases of IPMN but in only one third of IPNB cases.7,13,20 Furthermore, mucin pool formation was observed in only 7 of 43 cases (16.3 %) in the present study.
Because patients with intrahepatic IPNB have a better prognosis than patients with usual ICC,3,10,21 surgical resection is regarded as the first-choice treatment for patients with intrahepatic IPNB without distant metastasis. Early and accurate diagnosis is therefore important in this disease entity. In the present study, the only reliable prognostic factor was surgical curability for tumor recurrence and patient survival; thus, the extent of resection should be assessed accurately before and during surgery. Jarnagin et al.22 have recommended regional lymphadenectomy for tumors localized in the hilum or distal bile duct. LN metastasis is less common in patients with malignant intrahepatic IPNB than in usual ICC. In the present study, the main reason for R1 resection was the presence of microscopically residual tumor at the hilar bile duct margins. Considering that only 2 of 6 patients with R1 resection underwent concurrent BDR, we emphasize the role of intraoperative frozen-section biopsy. If the BDR margins are not reliably free of IPNB, we suggest concurrent BDR should be performed because it decreases the possibility of R1 resection.
There are many similarities in the clinicopathological manifestation and prognostic outcomes of malignant intrahepatic IPNB and IG-ICC, as shown in the present study. In a Japanese multi-center study, 81.8 % (126 of 154) of biliary tract carcinomas of papillary growth and IG-ICC fulfilled the criteria for IPNB.10 This proportion is much greater than our finding of 14.5 % (9 of 62), implying that it would be increased by thorough pathological review of our ICC cases. In the Japanese study, the majority of high-grade and invasive IPNBs contained foci of moderately differentiated adenocarcinoma within the intraductal papillary tumor,10 suggesting that a majority of IG-ICC could be regarded as of IPNB lineage and that clinically detectable IPNBs are already a malignant papillary lesion.
There are some limitations to our study. First, this was a retrospective, single-center study. Second, the sample size was not large enough for reliable analysis of survival. Multi-center studies should be performed to identify reliable prognostic factors and to clarify tumorigenesis.
We conclude that intrahepatic IPNB is a rare type of biliary neoplasm and encompasses a histological spectrum ranging from benign disease to invasive malignancy. Long-term survival is anticipated after curative resection, even in patients with malignant intrahepatic IPNB. Since R1 resection reduces survival outcomes, we suggest that concurrent BDR should be performed if the BDR margin is not reliably free of neoplastic involvement.
References
Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010; 2:419–427.
Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch 2008;453:589–598.
Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, et al. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int 2007;27:1174–1184.
Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006;44:1333–1343.
Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer 2004;100:783–793.
Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 2010;60:419–429.
Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D'Angelica MI, et al. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012;56:1352–1360.
Naito Y, Kusano H, Nakashima O, Sadashima E, Hattori S, Taira T, et al. Intraductal neoplasm of the intrahepatic bile duct: clinicopathological study of 24 cases. 2012; World J Gastroenterol 18:3673–3680.
Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol 2012; 57:787–793.
Nakanuma Y, Sato Y, Ojima H, Kanai Y, Aishima S, Yamamoto M, et al.; Hepatolithiasis Subdivision of Intractable Hepatobiliary Diseases Study Group of Japan. Clinicopathological characterization of so-called "cholangiocarcinoma with intraductal papillary growth" with respect to "intraductal papillary neoplasm of bile duct (IPNB)". Int J Clin Exp Pathol 2014;7:3112–3122.
Hwang S, Lee YJ, Song GW, Park KM, Kim KH, Ahn CS, et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. J Gastrointest Surg 2015;19:1291–1304.
Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, Moon DB, et al. Quantified risk assessment for major hepatectomy via the indocyanine green clearance rate and liver volumetry combined with standard liver volume. J Gastrointest Surg 2015;19:1305–1314.
Wu SD, Lu CD, Lu CJ, Huang J, Zhou J. Mucin-producing intrahepatic biliary papillomatosis. Surg Today 2010;40:845–850.
Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, et al. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci 2010;17:211–222.
Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, et al. Intraductal papillary neoplasms of the bile duct. Int J Hepatol 2014;2014:459091.
Yang J, Wang W, Yan L. The clinicopathological features of intraductal papillary neoplasms of the bile duct in a Chinese population. Dig Liver Dis 2012;44:251–256
Jang GW, Hwang S, Lee YJ, Kim KH, Park KM, Ahn CS, et al. Clinicopathological features of the intraductal papillary neoplasms of the intrahepatic bile duct. Korean J Hepatobiliary Pancreat Surg 2012;16:138–141.
Kloek JJ, van der Gaag NA, Erdogan D, Rauws EA, Busch OR, Gouma DJ, et al. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol 2011;42:824–832.
Wan XS, Xu YY, Qian JY, Yang XB, Wang AQ, He L, et al. Intraductal papillary neoplasm of the bile duct. World J Gastroenterol 2013;19:8595–8604.
Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol 2011;35:512–521.
Nakanishi Y, Zen Y, Hirano S, Tanaka E, Takahashi O, Yonemori A, et al. Intraductal oncocytic papillary neoplasm of the bile duct: the first case of peribiliary gland origin. J Hepatobiliary Pancreat Surg 2009;16:869–873.
Jarnagin WR, Bowne W, Klimstra DS, Ben-Porat L, Roggin K, Cymes K, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg 2005; 241:703–712.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, WJ., Hwang, S., Lee, YJ. et al. Clinicopathological Features and Long-Term Outcomes of Intraductal Papillary Neoplasms of the Intrahepatic Bile Duct. J Gastrointest Surg 20, 1368–1375 (2016). https://doi.org/10.1007/s11605-016-3103-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-016-3103-5