Abstract
Introduction
Endoscopic therapy has revolutionized the treatment of Barrett’s esophagus with high-grade dysplasia (HGD) or intramucosal adenocarcinoma by allowing preservation of the esophagus in many patients who would previously have had an esophagectomy. This paradigm shift initially occurred at high-volume centers in North America and Europe but now is becoming mainstream therapy. There is a lack of uniform guidelines and algorithms for the management of these patients. Our aim was to review important concepts and pitfalls in the endoscopic management of superficial esophageal adenocarcinoma.
Methods
A small group colloquium consisting of gastroenterologists, surgeons, and pathologists reviewed published data and discussed personal and institutional experiences with endotherapy for HGD and superficial esophageal adenocarcinoma.
Results
The group reviewed data and provided recommendations and management algorithms for seven areas pertaining to endoscopic therapy for Barrett’s HGD and superficial adenocarcinoma: (1) patient selection and evaluation; (2) imaging and biopsy techniques; (3) devices; (4) indications for resection versus ablation; (5) ER specimen handling, processing, and pathologic evaluation; (6) patient care and follow-up after endoscopic therapy; and (7) complications of endoscopic therapy and treatment options.
Conclusions
Endoscopic therapy is preferred over esophagectomy for most patients with HGD or intramucosal adenocarcinoma, and may be applicable to select patients with submucosal tumors. Clear guidelines and management algorithms will aid physicians and centers embarking on endoscopic therapy and enable a standardized approach to the management of these patients that is applicable internationally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic resection (ER) and mucosal ablation for Barrett’s esophagus with high-grade dysplasia (HGD) or superficial adenocarcinoma has revolutionized the treatment for these complications of gastroesophageal reflux. Given the safety and efficacy of endoscopic therapy, a paradigm shift has occurred at high-volume centers worldwide. However, since these centers were often simultaneously developing protocols and strategies, there has been no uniform approach to the management of these patients. Development of a standardized approach would allow better comparison of results between centers and countries. Further, as this paradigm shift percolates into community and lower-volume centers, it is important that the lessons learned are shared. Toward this end, we assembled a group of surgeons, gastroenterologist, and pathologists with experience in this disease to review the literature and share personal and center experiences with endotherapy. Our aim was to define important management concepts, share lessons learned, and describe an approach useful for the evaluation and treatment of patients with high-grade dysplasia and intramucosal adenocarcinoma of the esophagus.
Methods
A grant was provided by the Borchard Foundation to host a small group colloquium on the topic of endoscopic therapy for HGD and superficial adenocarcinoma. The specifications of the grant limited the participants to 12, and in the final group, there were 4 surgeons, 4 pathologists, and 3 gastroenterologists. All participants had extensive experience with Barrett’s esophagus and superficial esophageal adenocarcinoma. Attendees were asked to review the published literature and be prepared to discuss their personal and center experience with endotherapy for esophageal adenocarcinoma. To facilitate the discussion, a series of questions were provided prior to the colloquium to each attendee focused in seven areas: (1) patient selection and evaluation; (2) imaging and biopsy techniques; (3) devices for ER and ablation; (4) indications for resection versus ablation; (5) ER specimen handling, processing, and pathologic evaluation; (6) patient care and follow-up after endoscopic therapy; and (7) complications of endoscopic therapy and treatment options for those complications. The responses and the discussion that followed were used to develop this manuscript.
Results
Patient Selection and Evaluation
Patient Selection
Endotherapy and esophagectomy are similarly effective cancer therapies for patients with HGD or intramucosal cancer.1 , 2 It would be inappropriate to proceed with either therapy without informing a patient about the benefits and drawbacks of both options. Endotherapy will be preferred by most patients given its minimal complication rate and the opportunity to preserve the esophagus.3 However, endotherapy failure will necessitate an esophagectomy in some patients. Rarely, patients best treated by esophagectomy are not surgical candidates. Esophagectomy, when indicated, should be performed at a high-volume tertiary care center by an experienced esophageal surgeon in order to minimize morbidity and maximize quality of life in these patients who are expected to be cured of their disease.4 – 8
There are no absolute exclusion criteria for endotherapy although esophagectomy might be more appropriate in selected individuals, including those with poor esophageal body function, severe, uncontrollable reflux symptoms, dysphagia, or frequent aspiration.3 Poor candidates for endotherapy are those not committed to the often prolonged length of treatment or those unable or unwilling to undergo repeated upper endoscopies for treatment or surveillance. Ideal candidates for endotherapy are those with short lengths of Barrett’s, normal esophageal motility and no esophagitis, stricture, or large hiatal hernia. Factors that predict a reduced likelihood of successful endotherapy include long lengths of Barrett’s, particularly ultra-long (≥8 cm) Barrett’s, high-grade (poor) differentiation within an adenocarcinoma, lesions ≥2 cm in size, a large hiatal hernia, and poorly controlled reflux despite aggressive proton pump inhibitor therapy.9 – 11
Patient Evaluation
When endoscopic biopsies show HGD or adenocarcinoma, the slides should be reviewed by a pathologist with expertise in Barrett’s esophagus. If the initial endoscopy did not include a Seattle biopsy protocol, a repeat endoscopy should be promptly scheduled. During that endoscopy, biopsies should be taken from all suspicious areas and then from four quadrants every 1–2 cm throughout the length of the columnar segment. It is recommended that the evaluation be done with high-definition white light and narrow band imaging (NBI) or similar electronic enhancement.12 Spraying the mucosa with dilute (1.5–2 %) acetic acid can facilitate identification of subtle abnormalities within the columnar mucosa.13 , 14 Other stains and new technologies may be useful in specific circumstances. The length and circumference of the columnar mucosa should be measured using the Prague system, the presence and size of a hiatal hernia recorded, and the location, size, and Paris characteristics of any lesions noted.15 The Paris classification, although designed for squamous tumors, has clinical utility for lesions within Barrett’s and is recommended.16 – 18
In patients with a visible lesion or suspicious area within the columnar mucosa, the critical first step is to perform an ER of the lesion to determine if adenocarcinoma is present, and to evaluate the depth of invasion and tumor characteristics including grade and lymphovascular invasion. Endoscopic ultrasound (EUS) is not reliable for determining depth of invasion in small (<2 cm) lesions and is not routinely necessary.19 In larger lesions, EUS is helpful to evaluate for invasion into the muscularis propria and to stage the lymph nodes. In patients with confirmed HGD or intramucosal adenocarcinoma, CT and PET scans are of minimal value unless the pathology of the ER specimen suggests a “high risk” lesion and esophagectomy is being considered.20 High risk features include lymphovascular invasion (LVI) in any lesion, tumor invasion beyond 500 microns into the submucosa, or any submucosal invasion by a high grade (poorly differentiated) adenocarcinoma.21 – 23 Barium swallow and/or esophageal manometry are recommended only in patients with poorly controlled reflux symptoms or erosive esophagitis despite high-dose acid suppression medications. These patients should be considered for antireflux surgery prior to or early in the course of endotherapy.11
Devices, Indications, and Techniques for Endoscopic Resection and Ablation
Confirmed HGD or adenocarcinoma should prompt intervention in nearly all patients. A visible abnormality or nodule must be excised by ER, and the ability to perform ER is fundamental to a program embarking on endotherapy. Even flat or minimally raised lesions can harbor adenocarcinoma invasive beyond the mucosa that potentially would be inadequately treated with ablation. An improperly performed ER can leave the patient with no choice but an esophagectomy, so it is essential that the treating physician be adequately trained for this procedure.24 The goal of ER is to excise the full thickness of the mucosa and submucosa to allow histologic determination of the depth of invasion. There is no maximum tumor size for ER, but lesions ≥2 cm are more likely to have invaded into the submucosa or have lymphovascular invasion.23 It is useful to photograph the site of a planned ER and carefully document the precise location of any lesions. For ER, both the band (Duette, Cook Group Inc, Bloomington, IN) and cap techniques (Olympus Corporation, Tokyo, Japan) are acceptable, and are preferred over endoscopic submucosal dissection (ESD) for most lesions.25 , 26 An advantage of the band technique is that it does not require submucosal saline injection to “lift” the lesion. However, for larger lesions, injection is recommended since failure to lift is indicative of invasion beyond the submucosa. When using the band technique, the snare should be applied below the band in order to avoid leaving part of the specimen behind, and potentially compromising the ability to assess the true depth of invasion. Cutting below the band is safe since the bands generally do not have sufficient strength to include the muscularis propria.27
When a lesion is present, every effort should be made to completely excise the lesion by applying suction when the cap is over the central-most portion of the lesion. Piecemeal resection of larger lesions is acceptable but leads to more artifact and prohibits accurate assessment of the mucosal resection margins. If piecemeal resection is necessary, the band technique is recommended since it allows safe overlapping resections. Although ESD makes en-bloc resection of larger lesions possible, it is rarely required since a piecemeal resection does not compromise the ability to evaluate the most critical issues: (1) the maximal depth of tumor invasion and (2) the status of the submucosal resection margin.
While ER is most often used to excise a lesion, it can also be used to remove flat Barrett’s. Short, non-circumferential tongues of columnar mucosa can be fully excised with ER, but ER of circumferential segments or those >3 cm in length should be avoided to reduce the risk of significant stricture.3 , 28 Typically the ER site is not closed, but if there is concern about perforation or the patient needs to be anticoagulated the opposing mucosal edges can be clipped or sewn together. Resection specimens from adjacent sites may be marked and sent in a single pathology container, while those from separate sites should be sent in individual containers. In most circumstances, orientation of the specimen is not necessary, but a description of the ER technique (band vs. cap), whether saline or other material was injected prior to resection, and the location of the ER (esophagus or gastroesophageal junction) should be included in the pathology requisition.
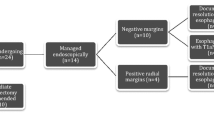
Ablation is the preferred option for patients with long-segment Barrett’s esophagus, no mucosal irregularities on endoscopy, and only HGD present on biopsies using the Seattle biopsy protocol.29 Importantly, ablation does not produce a specimen, and the elimination of disease is based on statistical likelihood rather than histologic confirmation. In patients with flat HGD, RFA is preferred given the clinical data supporting efficacy and safety with this approach.30 Alternatives include cryoablation, argon beam ablation, and rarely photodynamic therapy.31 – 33 Typically, ablation should not be done in the same setting as ER due to the risk of perforation, but if there is sufficient Barrett’s tissue separate from the ER site, simultaneous ablation can be performed. Patients with a biopsy showing adenocarcinoma without a visible lesion represent a special group. When there is a short segment of Barrett’s, the preferred approach is complete ER of all the columnar mucosa. With long-segment Barrett’s, treatment at a tertiary center is recommended so that techniques such as confocal or zoom microscopy can localize the focus of adenocarcinoma and allow targeted ER of that area. Suggested management algorithms are shown in Figs. 1 and 2.
The Pathology of ER Specimens: Specimen Fixation, Processing, and Cutting
An ER specimen should be placed in a marked container that is filled with an adequate volume of 4 % neutral buffered formalin. Prior to insertion into formalin, the specimen can be pinned out on an appropriate surface (e.g., a cork-board) if desired, but this is not necessary and is best done only in units with technical experience in pinning the specimen or when the pathologist is immediately available for handling the fresh specimen. Importantly, the tissue will shrink with fixation, and if pinning is chosen, the tissue should not be under tension, and pins must not go through the lesion or a close margin. Once placed into formalin, the specimen must remain there for a minimum of 6 h to ensure adequate fixation. Specimens should not be placed in saline since they will rapidly degrade. Frozen section of an ER specimen is not recommended in routine practice.
Gross examination of the ER specimen by a pathologist should include notation of the size of the specimen in three dimensions and the presence or absence of a lesion. The nature and size of any lesion, the distance from the edge of the lesion to the nearest mucosal margin, and whether the lesion appears to grossly involve any margin should be documented. If available, photographing the specimen on a grid is useful.
Cutting the specimen for embedding is a critical step for precise pathologic evaluation of an ER specimen. When a lesion is present, it should not be assumed that the apex of the ER specimen represents the central portion of the lesion. Instead, the thickest portion of the ER specimen that contains the lesion should be the focal point for sectioning. The recommended initial step is to take a 2-mm section through the lesion at the deepest point of the resection perpendicular to the closest margin (Fig. 3a, supplement). This is best done with the ER specimen placed mucosal side down to ensure that the deepest point of the ER specimen is included. This central section should be placed in an individual cassette. The remaining two sides of the specimen are cut into 2 mm sections parallel to the central section and placed either in separate cassettes or with at most two to three sections per cassette. When no lesion is present on gross examination, the initial 2-mm central section should include the maximum longitudinal dimension of the specimen and again go through the deepest point of the resection (Fig. 3b, supplement). The remaining two sides are managed as for specimens with a lesion. In specimens that are too small for sectioning in the manner described above (6 mm and thinner), the specimen is simply bisected at the deepest point of the resection, through a lesion if present, and then the halves are laid cut-edge down within the same cassette. Hematoxylin and eosin staining is routinely used for evaluation.
(Supplement): cutting an ER specimen. a When a lesion is present, it is recommended that an initial 2 mm cut is placed through the lesion at the deepest part of the specimen perpendicular to the closest margin. b When no lesion is present, the 2-mm cut goes through the deepest part of the specimen in the longest dimension of the specimen
Pathologic Evaluation and Reporting of ER Specimens
The evaluation of an ER specimen is complex. The cap and suction used to perform an ER distort the mucosal histology and causes the muscularis mucosae to be divided tangentially with the snare. Further, patients with Barrett’s have variable but sometimes marked hyperplasia and duplication of the muscularis mucosae. During fixation, the specimen will shrink, and if the specimen is not pinned, then the mucosal edges will usually roll under the resection margin of the specimen. These changes can complicate pathologic evaluation of the specimen. It is recommended that all ER specimens undergo second review by an expert gastrointestinal pathologist given evidence of frequent misinterpretation of ER specimens.34
Pathologic evaluation of an ER specimen should commence with the central section since this typically has the least artifact. The serial sections made from the lateral parts of the specimen on either side of the initial central section frequently do not contain submucosa and are often tangential sections through the hyperplastic, rolled up muscularis mucosae. The first step is to define the plane between mucosa and submucosa. This is best done by drawing a line between the deepest fibers of the muscularis mucosa and the submucosa (Fig. 4). The submucosa is defined by three elements that are not present in the mucosa: thick-walled muscular blood vessels, adipose tissue, and submucosal glands. Of these, the most consistent are thick-walled blood vessels that are often dilated and almost always present immediately below the deepest muscle fibers of the muscularis mucosae. Adipose tissue is found less frequently. Submucosal glands are present in only a minority of cases.
An ER specimen after unpinned fixation. Note how the mucosal edges curl around under the specimen. The black line outlines the deepest fibers of the muscularis mucosa and defines the mucosal-submucosal junction. In Barrett’s esophagus, the muscularis mucosae is seldom a single layer, but instead is nearly always duplicated and often irregularly hyperplastic, causing this line to be irregular. Importantly, the line between the mucosa and submucosa in an ER specimen that is not pinned out will usually curve around toward the center of the section
Next, it is critical to understand the margins of the ER specimen. The resection margin is defined by the line of passage of the snare through the esophageal tissue. This is typically tangential through the muscularis mucosae around the periphery and through the submucosa at the deepest portion of the specimen. A line drawn between the mucosa and submucosa will define three margins. Two of the margins are mucosal margins, defined as the points between the surface of the specimen and the plane between the mucosa and submucosa on either side, and the third is the submucosal margin which is the area of submucosa between the points where the line between the mucosa and submucosa reaches the resection margin on each side (Fig. 5). Recognizing that the “deepest” portion of the section may actually be the mucosa that has rolled under during fixation, the term “deep margin” is potentially confusing. Instead, use of the terms “mucosal margin” and “submucosal margin” are preferred to the vague terms “lateral margin” and “deep margin.” Extreme care is necessary to not confuse the mucosal and submucosal margins. The treatment implications of mucosal versus submucosal margin involvement are considerable. A technically adequate ER specimen should contain submucosa, but the extent of resected submucosa varies, and it is not possible from an ER specimen to know whether all the submucosa was removed unless the specimen contains muscularis propria. If muscularis propria is present, it may indicate a full-thickness resection and a potential perforation.
The black dots demonstrate the junction of the mucosa and the submucosa in this unpinned ER specimen. The mucosal margins are marked as is the submucosal margin, note how the mucosal margins are rolled up under the specimen adjacent to the submucosal margin. To avoid confusion, the terms “submucosal margin” and “mucosal margin” are recommended over non-specific terms such as “deep margin” and “lateral margin”
Once the line separating the mucosa from the submucosa has been drawn and the margins determined, the remaining key elements of the analysis can be performed with accuracy and reproducibility.
-
1.
Description for all ER specimens
-
(a)
The surface epithelium including the presence of adenocarcinoma, high grade dysplasia, low grade dysplasia, intestinal metaplasia, non-intestinalized columnar epithelium, squamous mucosa, or erosion
-
(b)
The tissue present at each mucosal margin
-
(c)
The presence or absence of submucosa
-
(a)
-
2.
Description for ER specimens containing an adenocarcinoma
-
(a)
A description of the depth of invasion
-
(i)
Adenocarcinomas that are confined to the mucosa (i.e., completely above the line between mucosa and submucosa) need not be subclassified further (m1, m2, m3, etc.). A description of the depth of invasion into the mucosa may be useful but is not essential. In cases where an intramucosal cancer is very close to the line between the mucosa and submucosa, deeper sections are recommended to evaluate for invasion into the submucosa.
-
(ii)
Adenocarcinomas that invade into the submucosa must be carefully evaluated. Since the pathologist cannot determine if all the submucosa was excised with the ER procedure, division of the existing submucosa into thirds (sm1, sm2, and sm3) is artificial and is not recommended. Instead, the depth of tumor invasion into the submucosa should be measured in microns. This measurement is taken from the deepest fibers of the muscularis mucosae to the deepest point of submucosal invasion by the tumor (Fig. 6). Submucosal adenocarcinomas are classified into those with invasion ≤500 μm from the bottom of the muscularis mucosae and those that invade deeper. In addition, the total distance from the bottom of the muscularis mucosae to the submucosal resection margin should be measured in microns. Lastly, the extent of lateral involvement of the submucosa at the line between mucosa and submucosa should be measured in microns.
-
(iii)
In ER specimens that do not contain any submucosa, the pathology report should indicate whether the resection margin within the mucosa is involved by invasive adenocarcinoma. In patients in whom the resection margin is free of carcinoma, the distance from the deepest point of intramucosal tumor to the resection margin should be measured in microns.
Fig. 6 Recommended measurements for tumors invasive into the submucosa. X in micrometer = lateral extent of submucosal involvement just below the muscularis mucosa. Y in micrometer = depth of tumor invasion into submucosa below the lowermost fibers of muscularis mucosa. Z in micrometer = total distance from lowermost fibers of muscularis mucosa to submucosal resection margin. By subtraction, Z minus Y will be the depth of uninvolved submucosa below the lesion to the resection margin
-
(i)
-
(b)
The degree of differentiation, graded as low (well and moderately differentiated) or high grade (poorly differentiated, including signet ring cell carcinoma)
-
(c)
The presence of lymphovascular invasion
-
(d)
The presence of perineural invasion
-
(e)
The status of the submucosal margin of resection (involved or negative)
-
(f)
The status of the mucosal margins of resection (involved or negative)
A recommended style for pathologic reporting of an ER specimen is shown (Table 1).
Clinical Implications of the Pathologic Findings on ER Specimens
Adenocarcinomas limited to the mucosa have minimal potential for metastases and can be safely and reliably treated by ER ± ablation.1 , 10 , 35 , 36 Intramucosal tumors that are 2 cm or larger in size or show LVI may have an increased risk for lymph node metastases.23 , 37 These cases should be referred to a tertiary center and consideration given to an esophagectomy, particularly in young healthy patients. An intramucosal adenocarcinoma with high histologic grade (poor differentiation) is associated with an increased risk for failure of endotherapy, but is not associated with an increased risk for lymph node metastases.22 , 23 An adenocarcinoma invasive into the submucosa with LVI or poor differentiation is best treated by esophagectomy because of the significant risk of lymph node involvement.22 There is continued controversy about the safety of endoscopic therapy for a “low risk” submucosal adenocarcinoma. Low risk is defined as a lesion that is low grade (well or moderately differentiated), has no LVI, and invades ≤500 μm below the muscularis mucosa into the submucosa. The data supporting this approach are limited and extreme caution is advised, particularly in young healthy patients who are otherwise good candidates for an esophagectomy.21 , 22
Management of Patients Undergoing Endoscopic Therapy
Endoscopic ablation seldom eliminates all the intestinal metaplasia in one session, particularly with long-segment Barrett’s.38 , 39 While ER of all the columnar mucosa is most likely to accomplish complete eradication, to avoid the risk of stricture, it is best reserved for patients with short segment or non-circumferential Barrett’s. A positive mucosal margin after ER can be treated with further ER or ablation, but a positive submucosal margin is an indication for esophagectomy.22 Prior to embarking on endotherapy, it is essential that the endoscopist set the expectation with the patient that several sessions are likely to be necessary to achieve complete eradication of all the intestinal metaplasia. Importantly, the endpoint for treatment is (1) the absence of columnar mucosa in the esophagus on endoscopy and (2) the lack of intestinal metaplasia on biopsies including those from the gastroesophageal junction (GEJ). Endotherapy sessions continue every 8–10 weeks until this endpoint has been achieved given the high risk for metachronous cancer in these patients. There is no upper limit on the number of treatment sessions provided progress is being made, but consideration should be given to changing strategies for: (a) no change in the length of the columnar segment after two to three ablation sessions, (b) return of columnar mucosa rather than squamous mucosa at the site of an ER or ablation, (c) development of submucosal cancer, or (d) scarring or development of a stricture proximal to residual Barrett’s that makes endotherapy difficult.1 , 39 In these patients, referral to a tertiary center for consideration of an alternative endotherapy technique or an esophagectomy is recommended. Suggested management algorithms are shown in Fig. 7.
Algorithm for patient management after incomplete response to ER or RFA. Asterisk, if ER, then go to clinical algorithm #2. Double asterisks, complete response = no endoscopic evidence of columnar mucosa in the esophagus and complete eradication of IM including from biopsies at the neo-squamo columnar junction
One potential cause for failure to make progress with endoscopic therapy is continued gastroesophageal reflux, particularly weak acid reflux events.11 During endotherapy, patients should be maintained on twice daily PPI ± carafate. The gastric pH can be checked during endoscopy and the medical regimen adjusted if the pH is still acidic. In patients with ongoing reflux and poor response to endotherapy, an antireflux procedure should be considered. A fundoplication does not impair the ability to perform ER or ablation, and in fact elimination of the hiatal hernia may make ablation at the difficult to control GEJ region easier.40 After successful endotherapy and eradication of all intestinal metaplasia, PPI therapy can be weaned down based on reflux symptoms, or the patient can be evaluated for antireflux surgery. There is no clearly defined role for pH monitoring on therapy after ablation at this time.
When the endpoint of therapy has been reached, the patient enters surveillance. The recommended interval for surveillance is every 3 months for the first year, every 6 months for the second year, and then annually to 5 years. Beyond 5 years, surveillance should remain annual or every other year given evidence of the potential for recurrence of intestinal metaplasia long-term after ablation.41 If a patient develops IM or LGD during surveillance, it should be treated and the surveillance is then reset to every 6 months for the next year. If a patient recurs with HGD or intramucosal adenocarcinoma, the clock is reset after re-treatment to every 3-month surveillance endoscopies for the next year. These recommendations do not change based on the interval from reaching the endpoint of endotherapy to the time of the recurrence, but most recurrences are found within the first 2 years. Cancer recurrence beyond 5 years is unusual.39
Recurrence as an island or short tongue of columnar mucosa is commonly ablated; however, if a biopsy shows dysplasia, ER is preferred to avoid undertreating a potential focus of recurrent adenocarcinoma. During endoscopic surveillance, the mucosa should be carefully evaluated using NBI ± acetic acid chromoendoscopy. Biopsies should be taken at four quadrants around the GEJ and from any areas of residual columnar mucosa. The neosquamous mucosa should be carefully examined and biopsies obtained from any abnormal appearing areas. Random biopsies from otherwise normal appearing neosquamous mucosa are seldom useful and are not required.41 – 43 New technology such as optical coherence tomography may allow identification of buried Barrett’s glands below the neosquamous mucosa. The absolute risk related to buried Barrett’s glands after endotherapy is unknown, but adenocarcinomas have developed below the neosquamous mucosa, presumably from buried Barrett’s glands.44 Consequently, patients found to have buried Barrett’s glands should be treated by ER or ablation. Patients found to have buried Barrett’s on a random biopsy without a visible abnormality in the neosquamous mucosa should be referred to a tertiary center for further evaluation.
After successful endotherapy for HGD or intramucosal adenocarcinoma, there is no role for routine EUS or CT-PET scans during follow-up. However, after endotherapy for an adenocarcinoma with ≤500 μm invasion into the submucosa, patients should undergo EUS during each surveillance endoscopy looking for abnormal lymph nodes. If an abnormal node is found, it should be biopsied using EUS-FNA, and if negative, re-checked and re-biopsied in 3 months.45 A stricture after endotherapy should be carefully evaluated to rule out a subtle malignancy, but these are usually benign and can be treated with balloon dilatation and, if recurrent, steroid injection. Stenting is rarely necessary.
Conclusions
Over the past decade, there has been a paradigm shift in the management of HGD and intramucosal adenocarcinoma away from esophagectomy given the similar oncologic outcome with significantly less morbidity and mortality with endotherapy. The requirements for successful endotherapy include a commitment to the process by the patient, vigilance and competency in surveillance and endotherapy on the part of the endoscopist, and accuracy in the evaluation of ER specimens by an experienced gastrointestinal pathologist. Lapses in any of these may lead to death from what is usually a curable stage of disease and negate the benefits of endoscopic therapy. It is hoped that these guidelines and management algorithms will facilitate safe and effective endotherapy by physicians and centers committed to state-of-the-art care for patients with Barrett’s esophagus, and enable a standardized approach that is applicable internationally.
References
Zehetner J, DeMeester SR, Hagen JA et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011; 141(1):39–47.
Schembre DB, Huang JL, Lin OS, et al. Treatment of Barrett’s esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc. 2008;67(4):595–601.
DeMeester SR. Evaluation and treatment of superficial esophageal cancer. J Gastroinst Surg. 2010; 14 (Suppl 1):S94-100.
Peyre CG, DeMeester SR, Rizzetto C, et al. Vagal-sparing esophagectomy: the ideal operation for intramucosal adenocarcinoma and Barrett’s with high-grade dysplasia. Ann Surg 2007: 246(4): 665–71.
Bennett C, Vakil N, Bergman J. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology 2012 ; 143 :336–346.
Fitzgerald RC, Pietro MD, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014 ;63 :7–42.
Greene CL, DeMeester SR, Worrell SG, et al. Alimentary satisfaction, gastrointestinal symptoms, and quality of life 10 or more years after esophagectomy with gastric pull-up. J Thorac Cardivasc Surg. 2014 ;147(3): 909–914.
Greene CL, DeMeester SR, Augustin F, et al. Long-term quality of life and alimentary satisfaction after esophagectomy with colon interposition. Ann Thorac Surg 2014 ;98 :1713–1720.
Phoa KN, Pouw RE, van Vilsteren FG, et al. Remission of Barrett’s Esophagus with Early Neoplasia 5 years after Radiofrequency Ablation with Endoscopic Resection: A Netherlands Cohort Study. Gastroenterology 2013; 145(1):96–104.
Pech O, Behrens A, May A, et al. Long-term results and risk factors analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008; 57(9):1200–6.
Krishnan K, Pandolfino J, Kahrilas P, et al. Increased Risk for Persistent Intestinal Metaplasia in Patients With Barrett’s Esophagus and Uncontrolled Reflux Exposure Before Radiofrequency Ablation. Gastroenterology 2012; 143:576–581.
Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Endoscopy 2010;42:351–359.
Fortun PJ, Anagnostopoulos GK, Kaye P, et al. Acetic acid-enhancing magnification endoscopy in the diagnosis of specialized intestinal metaplasia, dysplasia, and early cancer in Barrett’s oesophagus. Aliment Pharmacol Ther 2006;23:735–742.
Longcroft-Wheaton G, Duku M, Mead R, et al. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol 2010;8:843–847.
Sharma P, Dent J, Armstrong D et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C &M criteria. Gastroenterology 2006; 131(5):1392–1399.
Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005; 37(6): 570–8.
Peters FP, Brakenhoff KP, Curvers WL et al. Histologic evaluation of resection specimens obtained at 293 endoscopic resections in Barrett’s esophagus. Gastrointest Endosc 2008; 67(4):604–9.
Pech O, Gossner L, Manner H et al. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy 2007; 39:588–593.
Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037–1041.
Keswani RN, Early DS, Edmundowicz SA, et al. Routine positron emission tomography does not alter nodal staging in patients undergoing EUS-guided FNA for esophageal cancer. Gastrointest Endosc 2009;69:1210–1217.
Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11(6):630–635.
Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas: a multi-center study. J Gastrointest Surg 2015. Publication in press.
Lorenz D, Origer J, Pauthner M et al. Prognostic Risk Factors of Early Esophageal Adenocarcinomas. Ann Surg 2014; 259(3):469–476.
Van Vilsteren FG, Pouw RE, Herrero LA, et al. Learning to perform endoscopic resection of esophageal neoplasia is associated with significant complications even within a structured training program. Endoscopy 2012;44:4–12.
Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection for early Barrett’s neoplasia. Gastrointest Endosc 2011;74:35–43.
May A, Gossner L, Behrens A, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc 2003;58:167–175.
Tomizawa Y, Iyer PG, Wong Kee Song LM et al. Safety of Endoscopic Mucosal Resection for Barrett’s Esopahgus. Am J Gastroenterol 2013;108:1440–1447.
Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett’s oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010;59:1169–1177.
Pouw RE, Sharma VK, Bergman JJ, et al. Radiofrequency ablation for total Barrett’s eradication: a description of the endoscopic technique, its clinical results and future prospects. Endoscopy 2008;40:1033–1040.
Shaheen NJ, Overholt BF, Sampliner RE, et al. Durability of radiofrequency ablation in Barrett’s esophagus with dysplasia. Gastroenterology 2011;141:460–468.
Shaheen NJ, Greenwald BD, Peery AF et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endoscopy 2010; 71(4):680–5.
Overholt BF, Wang KK, Burdick JS, et al. Five-year efficacy and safety of photodynamic therapy with photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc 2007;66:460–468.
Ragunath K, Krasner N, Raman VS, et al. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroeterol 2005;40:750–758.
Worrell SG, Boys JA, Chandrasoma P, et al. Inter-observer variability in the Interpretation of Endoscopic Mucosal Resection Specimens of Esophageal Adenocarcinoma. J Gastrointest Surg 2015. Publication in press.
Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815–823.
Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma: a retrospective review of esophagectomy specimens. Ann Surg 2011;253(2):271–278.
Barbour AP, Jones M, Brown I, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol 2010;17:2494–2502.
Herrero LA, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett’s esophagus longer than 10 cm. Gastrointest Endosc 2011;73:682–690.
Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146(3):652–660.
Johnson CS, Louie BE, Willie A, et al. The durability of endoscopic therapy for treatment of Barrett’s metaplasia, dysplasia, and mucosal cancer after Nissen fundoplication. J Gastrointest Surg. 2015; 19(5): 799–805.
Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s esophagus with radiofrequency ablation. Dig Dis Sci 2011;56:1996–2000.
Pouw RE, Gondrie JJ, Rygiel AM, et al. Properties of the neosquamous epithelium after radiofrequency ablation of Barrett’s esophagus containing neoplasia. Am J Gastroenterol 2009; 104:1366–1373.
Shaheen NJ, Peery AF, Overholt BF, et al. Biopsy depth after radiofrequency ablation of dysplastic Barrett’s esophagus. Gastroinest Endosc 2010;72:490–496.
Titi M, Overhiser A, Ulusarac O, et al. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett’s esophagus. Gastroenterology 2012;143:564–566.
Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589–2597.
Acknowledgments
The meeting that made this manuscript possible was funded by a grant from the Borchard Foundation. In particular, we thank the director, Dr. Kristen Beling, for her support and hospitality throughout the process. Additional support was provided by a much appreciated educational grant from Olympus Endoscopy. Lastly, this would not have been possible without generous donations by individuals committed to advancing the care of patients with esophageal disease including Ms. Jo Skibby, Mr. and Mrs. Michael Greenberg, Mr. and Mrs. Darrell Anderson, Ms. Judy Engell, and Mr. Donald Hunn.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greene, C.L., Worrell, S.G., Attwood, S.E. et al. Emerging Concepts for the Endoscopic Management of Superficial Esophageal Adenocarcinoma. J Gastrointest Surg 20, 851–860 (2016). https://doi.org/10.1007/s11605-015-3056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-3056-0