Abstract
Introduction
Radiofrequency ablation (RFA) ± endoscopic resection (EMR) is an established treatment strategy for neoplastic Barrett’s and intramucosal cancer. Most patients are managed with proton pump inhibitors. The incidence of recurrent Barrett’s metaplasia, dysplasia, or cancer after complete eradication is up to 43 % using this strategy. We hypothesize the addition of fundoplication should result in a lower recurrence rates after complete eradication.
Methods
Multi-institutional retrospective review of patients undergoing endotherapy followed by Nissen fundoplication
Results
A total of 49 patients underwent RFA ± EMR followed by Nissen fundoplication. Complete remission of intestinal metaplasia (CR-IM) was achieved in 26 (53 %) patients, complete remission of dysplasia (CR-D) in 16 (33 %) patients, and 7 (14 %) had persistent neoplastic Barrett’s. After fundoplication, 18/26 (70 %) remained in CR-IM. An additional 10/16 CR-D achieved CR-IM and 4/7 with persistent dysplasia achieved CR-IM. One patient progressed to LGD while no patient developed HGD or cancer.
Conclusion
Endoscopic therapy for Barrett’s dysplasia and/or intramucosal cancer followed by fundoplication results in similar durability of CR-IM to patients being managed with PPIs alone after endoscopic therapy. However, fundoplication may be superior in preventing further progression of disease and the development of cancer. Fundoplication is an important strategy to achieve and maintain CR-IM, and facilitate eradication of persistent dysplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic therapy using radiofrequency ablation (RFA), with or without endoscopic mucosal resection (EMR) is the dominant treatment strategy for the management of dysplastic Barrett’s esophagus and intramucosal adenocarcinoma.1 , 2 The majority of patients undergoing treatment are managed with maximal dose proton pump inhibitors (PPIs) following endoscopic intervention. This treatment strategy has been proven to eradicate dysplasia and early cancers in up to 87 % of patients at early follow-up. However, recurrence rates of up to 43 % at 24 month after initial eradication have been reported and as many as 8 % of patients progress to develop cancer.3 – 6 One hypothesis to explain the high rate of recurrence after endoscopic therapy is ineffective long-term acid suppression and in particular incomplete control of weakly acidic reflux.7 Laparoscopic Nissen fundoplication is an attractive alternative to long-term PPI therapy because it provides effective control of all types of reflux regardless of pH,8 , 9 addresses the pathophysiology of GERD, restores the pH gradient thus impacting goblet cell density and bile acid-induced carcinogenesis,10 and may resolve deleterious genomic changes secondary to reflux.11 Prior studies combining laparoscopic fundoplication with endoscopic therapy have demonstrated a lower recurrence rate when compared to RFA with or without EMR and long-term PPI therapy or showed no difference in recurrence.12 , 13 However, fundoplication in these studies was applied prior to, concomitantly with, or after endoscopic treatment. We hypothesized that laparoscopic fundoplication rather than long-term PPI treatment after endoscopic therapy should result in a lower rate of recurrence and lower rate of progression compared to historical controls.
Materials and Methods
We performed a multi-institutional, retrospective review of patients with Barrett’s metaplasia, dysplasia, and/or intramucosal cancer that underwent endoscopic therapy (RFA ± EMR) followed by laparoscopic fundoplication. Five separate surgical units with a subspecialty interest in esophageal disease and experience in endoscopic therapy agreed to participate: Swedish Cancer Institute, The Oregon Clinic, University of Southern California Divisions of General and Thoracic Surgery, and the University of Rochester. The institutional review board at each participating unit approved the study. Individual consent was waived due to the retrospective nature of the study.
Patients were included in the study if they had biopsy-proven Barrett’s esophagus with or without dysplasia or intramucosal adenocarcinoma. All patients were required to undergo endoscopic therapy with radiofrequency ablation to eradicate the Barrett’s segment. In addition, patients with nodular or raised Barrett’s esophagus underwent endoscopic mucosal resection at the discretion of the treating surgeon. Endoscopic therapy was stopped at the discretion of the treating surgeon and the patient then underwent laparoscopic fundoplication. Patients were excluded if they had a fundoplication prior to endoscopic therapy.
Data collected included demographic and baseline GERD characteristics. Pathology reports were reviewed to determine the presence or absence of intestinal metaplasia, dysplasia, or intramucosal cancer. When available, a combination of upper endoscopy, post-operative pH testing, or barium esophagograms were reviewed to determine if reflux was present, if a recurrent hernia existed, or if the fundoplication had failed. Student’s t test was used for continuous variables and chi squared for categorical variables. Durability was calculated using the Kaplan–Meier method on SPSS 19.
Definitions and Endpoints
Patients were grouped after endoscopic therapy by the clearance of disease. Complete remission of intestinal metaplasia (CR-IM) was defined as the absence of all dysplasia and intestinal metaplasia on at least one set of biopsies following endoscopic therapy and continuing to the most recent biopsy prior to fundoplication. Complete remission of dysplasia (CR-D) was defined as the absence of dysplasia but the persistence of intestinal metaplasia on the most recent biopsies before fundoplication was performed. Patients who were indefinite for dysplasia or who achieved CR-IM but were then discovered to have recurrent intestinal metaplasia prior to fundoplication were included in this group. Patients with persistent low-grade (LGD) or high-grade (HGD) dysplasia despite endoscopic therapy and who underwent fundoplication as “salvage” therapy comprised the last group.
Durability was measured from the date of the biopsies demonstrating CR-IM after endoscopic therapy and before fundoplication to the most recent biopsies. Recurrence was diagnosed if intestinal metaplasia or dysplasia was identified after CR-IM. For patients who had CR-D or persistent dysplasia before fundoplication, durability was not ascertained, but the absence of progression to a worse histological diagnosis and the ability to achieve CR-IM or CR-D on last biopsy were considered as additional endpoints.
Endoscopic Treatment Protocols
The application of EMR and RFA were not standardized in the study; however, each institution’s protocol was very similar and several general principles were followed.14 – 16 First, nodular or raised areas of Barrett’s esophagus usually underwent aggressive EMR to determine the depth of involvement and eradicate visible disease. Second, radiofrequency ablation was applied using 12 kJ for the circumferential device and 12–15 kJ using the paddle device at 8- to 12-week intervals as described in the AIM-dysplasia trial.1 Third, a period of surveillance after endoscopic therapy and before fundoplication occurred to ensure that the dysplastic cells were not going to reoccur immediately and necessitate esophagectomy for progressive disease. Fourth, patients were treated with ablation and not biopsied until all endoscopically visible Barrett’s was replaced with neosquamous epithelium or endoscopic findings necessitated biopsy. A specific biopsy schedule was not mandated but in general after eradication, surveillance endoscopies were undertaken at 8- to 10-week intervals and biopsies using the Seattle protocol were taken if touch-up ablation was not performed.17 Fifth, touch-up ablations after fundoplication were performed at the discretion of the treating surgeon. Lastly, patients who developed a stricture after endoscopic therapy were managed with endoscopic dilation.
Operative Techniques
Laparoscopic fundoplication was conducted with similar techniques and principles as the majority of the surgeon’s involved either were colleagues or had trained together. Briefly, the hiatus was dissected to restore adequate intra-abdominal length. Collis gastroplasty was used sparingly to avoid placing gastric mucosa against the neosquamous lining. The hiatus was reconstructed primarily to approximate the size of the patient’s esophagus. The use of bio-absorbable mesh was left to the discretion of the attending surgeon. Nissen fundoplications were completed over a 56–60 Fr bougie and generally measured 2 to 2.5 cm in length. PPI use post-operatively was center-dependent but over 90 % discontinued them after fundoplication and resumed therapy only for symptoms or pH positive findings.
Results
A total of 49 patients underwent RFA followed by Nissen fundoplication. The patients were predominantly Caucasian males with a mean age of 61 years with longstanding GERD complicated by long-segment Barrett’s esophagus and hiatal hernia (Table 1). Initial histology was primarily high-grade dysplasia or intramucosal cancer as described in Table 2.
After diagnosis and before fundoplication, EMR was performed in 53 % followed by an average of 3.7 RFA treatments per patient (range 1–11) (Table 3). There were no serious complications; five patients (10 %) developed a mild stricture after RFA. Of the 49 patients studied, CR-IM was achieved in 26 (53 %), CR-D in 16 (33 %), and 7 (14 %) had persistent dysplasia following endoscopic therapy (Tables 1, 2, and 3).
In the 26 patients who achieved CR-IM and then underwent fundoplication, 22 (85 %) had CR-IM at the most recent biopsies with a mean follow-up of 26.1 months (range 5.2 to 62.3). This follow-up is broken up into 5.5 months from achievement of CR-IM to the time of fundoplication and 20.6 months from fundoplication to the time of the most recent biopsies. Four patients developed recurrent Barrett’s metaplasia after fundoplication and an additional four patients had transient Barrett’s metaplasia before returning to CR-IM on all subsequent biopsies. All eight patients recurred at the gastroesophageal junction or cardia with five undergoing 1–2 additional RFA treatments. Thus, the true recurrence rate is 30.7 % (8/26) at over 2 years of follow-up even though only 15 % (4/26) had recurrent disease on their most recent biopsies
In the 16 patients who achieved CR-D at the time of fundoplication, 10 (62.5 %) ultimately achieved CR-IM at their most recent biopsy, 5 patients remained stable with Barrett’s metaplasia at last biopsy, and 1 patient who had high grade dysplasia on entry then achieved CR-D at fundoplication and ultimately had LGD at the most recent biopsy. No patient progressed beyond their entry histology at a mean follow-up of 24 months, which includes a 7-months mean surveillance from the achievement of CR-D to the time of fundoplication.
Additional RFA after fundoplication was delivered to seven patients in the CR-D group. Four patients needed RFA to move from CR-D to CR-IM and two patients needed RFA to maintain intestinal metaplasia. One patient entered the study with HGD and achieved CR-D at the time of fundoplication but at last biopsy recurred with LGD at the gastroesophageal junction, on the gastric side of the fundoplication.
In the seven patients who had persistent dysplasia after endoscopic therapy, the fundoplication was considered salvage therapy to achieve better GERD control. These patients differed from the CR-IM group with a longer mean duration of GERD, a long Barrett’s segment, a higher BMI, and a larger hiatal hernia (Table 1). Despite worse baseline characteristics and entry pathology, four (57 %) achieved CRIM at their most recent biopsy after a mean of 5.25 RFA treatments, two (29 %) achieved CR-D, and one (14 %) patient who had intramucosal carcinoma on entry histology continues to have low-grade dysplasia.
Only three patients had a failed fundoplication noted on follow-up EGD or videoesophagram. All three of these patients were in the group with CR-IM before fundoplication. All three continued to have CR-IM at their last biopsy.
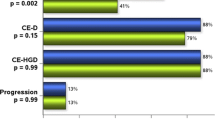
At the most recent surveillance biopsy, 73.5 % of patients had achieved or maintained CR-IM (Fig. 1). Compared to their entry histology, none of the 49 patients progressed to a worse histology at mean of 25 months of follow-up (Table 4). There were no patients who progressed to HGD or intramucosal cancer. One patient recurred to their baseline histology of intestinal metaplasia and two patients with HGD/IMC currently have LGD. Similarly, when the histology immediately prior to fundoplication was compared to the most recent biopsies (Table 5), only one patient progressed after endoscopic therapy and fundoplication from intestinal metaplasia to low-grade dysplasia.
Discussion
The treatment of Barrett’s esophagus with dysplasia or intramucosal adenocarcinoma has been changed radically by the application of radiofrequency ablation and endoscopic mucosal resection to eradicate the neoplastic cells. Despite its initial effectiveness, there are multiple reports showing that the durability of ablation deteriorates over time, with up to 43 % of patients having biopsies showing recurrent intestinal metaplasia, dysplasia, or frank cancer. Nearly all of these reports utilized twice daily, maximal dose PPIs to control symptoms of GERD. The primary finding in this study demonstrates that laparoscopic fundoplication after endoscopic therapy results in similar recurrence rates when compared to historic outcomes using PPIs alone.
Even though we hypothesized that endoscopic therapy followed by fundoplication should provide superior durability and thus lower recurrence rates than endoscopic therapy with PPI therapy, our 30 % recurrence rate after CR-IM is similar to those reported by a large U.S. multicenter consortium (33 % at 24 months of follow-up4), a large single U.S. center series (43 % at 33 months of follow-up5) and the U.S. RFA Registry (28 % at 26 months of follow-up).18 Our results differ from O’Connell and Velanovich12 who reported a 5.3 % recurrence/persistence rate with RFA and Nissen compared to a 25 % recurrence/persistence rate with RFA and PPIs; however, their surgical results may not be comparable since no data was provided on the patient’s baseline histology, longer segments of Barrett’s had a maximum of 6 cm ablated regardless of total length, and concomitant RFA and fundoplication was attempted.
Importantly, the definition of recurrence plays a key role in reported rates. Recurrence rates of 6 % have been reported in a single center study with 5 year follow-up from the Netherlands, but they excluded biopsies from the gastric cardia which were included in the other series and would have resulted in additional recurrences totaling 35 %.19 Similarly, the UK National Halo RFA Registry reported a 9 % recurrence rate at 12 months of follow-up based on biopsies taken from above the gastroesophageal junction.3 Using this definition of recurrence, our study would have reported no recurrences.
The importance of intestinal metaplasia found in the gastric cardia or on the cardia side of the gastroesophageal junction is unclear. In our series and the study from the Netherlands,19 a number of patients had transient focal intestinal metaplasia in this region that was not found on subsequent surveillance biopsies. Whether these biopsies should be excluded from recurrence analysis is unclear. On one hand, the presence of intestinal metaplasia has a small risk of transformation to cancer but in 5 years of follow-up, Phoa et al.19 demonstrated that no cancers were found in this region. This region may also be protected regardless of fundoplication or PPI management because it resides at the lower end of the pH gradient and is potentially less impacted by weakly acidic reflux.10 On the other hand, the risk of transformation to cancer cannot be ignored, and therefore reporting recurrences in this region should arguably become standard.
There are several explanations why the durability of endoscopic therapy with fundoplication should be similar to that of endoscopic therapy with PPI’s. First, it appears that the majority of recurrences in most series occur at the GE junction or cardia. It has been suggested this might be due to using focal paddle ablation at the gastroesophageal junction,20 but notably the study using this technique still had a recurrence rate of 35 % in this region.19 It seems more likely that the dynamic characteristics of this region lead to its continued exposure to gastric contents even after fundoplication. Second, it is plausible that the acquired genetic abnormalities driving the development of neoplastic change remain despite ablation and are responsible for recurrence.21
Even though fundoplication does not appear to reduce the recurrence of Barrett’s metaplasia after endoscopic therapy in comparison to PPI therapy, this strategy did demonstrate that the rate of progression of disease and cancer development was lower than historic PPI series. None of our patients progressed or recurred with HGD or cancer and we had only 1 patient who progressed to LGD after achieving CR-IM. Comparatively, progression to HGD occurs in 8 to 11 % of patients in the series reporting this outcome.4 , 5 Similarly, these series have demonstrated a risk of developing not only intramucosal cancer but also invasive cancers in 3 to 8 % of patients, some ultimately requiring esophagectomy.3 – 5 , 19
The differences in disease progression and cancer risk may be related to ongoing reflux seen in the historic studies using PPIs.7 In a randomized trial comparing the management of Barrett’s esophagus with PPIs versus fundoplication, Parrilla and colleagues showed that a successful fundoplication not only reduced the rate of de novo development of dysplastic Barrett’s esophagus but that progression to worse dysplasia was also less common.8 Similarly, successful outcomes of endoscopic therapy were associated with a fundoplication when compared to patients who failed.14 In addition, there are other effects of fundoplication on the neosquamous lining that may be responsible for these differences such as reversal of genomic DNA changes caused by reflux,11 reduction in inflammatory mediators such as COX-2,22 and reduction cdx-2 by better control of GERD.23
The use of fundoplication after endoscopic therapy has several applications. First, it can be simply used to control ongoing symptoms of GERD despite maximal medical therapy as evidenced by the presenting symptoms of patients in this study. Second, it may also be used to reduce the time to CR-IM or to achieve CR-IM if patients have residual intestinal metaplasia. Our initial rate of CR-IM was only 53 % but at 2 years of follow-up increased to 73.5 % with the additional 18 patients achieving CR-IM with only one to two additional RFA treatments and in some cases without treatment. Comparatively, Gupta et al.4 showed that incremental portions of patients achieving CR-IM took longer than the current series with 26 % at 1 year, 56 % at 2 years, and 71 % at 3 years of treatment. Lastly, fundoplication can be used to salvage endoscopic therapy when clearance of dysplasia is in question. This strategy resulted in 6 out 7 patients achieving CR-IM or CR-D only after fundoplication, presumably by improved control of GERD. Although the limited number of patients in this study prevents us from drawing any conclusions, we hope to use the same cohort of institutions to examine this concept in greater depth in a follow-up study.
Despite the known benefits of fundoplication in the management of GERD, the number of patients being referred after endoscopic therapy appears to be very small. In the U.S. RFA registry,13 only 5.4 % of patients had undergone fundoplication. There are several possible reasons for this utilization. First, the majority of patients undergoing endoscopic therapy are managed by gastroenterology and are not referred, likely because there has been no reported difference in cancer prevention when medical and surgical therapy are compared,24 , 25 or because fundoplication for Barrett’s esophagus and cancer management is neither considered nor indicated in most gastroenterology articles.26 Second, there may be reluctance on the part of surgeons and gastroenterologists to complicate a future esophagectomy if progressive disease is encountered or cancer reoccurs. This may be justified given the results of several series showing a higher complication rate.27 , 28 Lastly, there may be concerns about surveillance biopsies after fundoplication and the degree of difficulty of performing fundoplication after endoscopic therapy. In our experience, performing endoscopic biopsy after fundoplication is equivalent if not easier than biopsy in the non-surgical patient. However, fundoplication can be more challenging after endoscopic therapy. Adhesions in the mediastinum may be denser, and establishing adequate intra-abdominal esophageal length requires persistence.
There are several limitations to this study. First, the numbers are small compared to larger medical trials and registries, but the proportion of patients and entry histology are similar to these other trials and the recurrence results are likewise similar. Nevertheless, a larger trial with a control group is necessary. Second, we did not centralize pathology review to assess histology. However, each participating center has a dedicated esophageal unit that works with experienced pathologists. Third, the retrospective nature of the study and the lack of a standardize treatment protocol create biases especially a selection bias, though this is no different than the challenges of using data from the UK Barrett’s registry or the US RFA registry. Lastly, although our follow-up is similar to other series, it is relatively short and outcomes may change with longer follow-up.
Conclusion
The strategy of endoscopic therapy for Barrett’s metaplasia, dysplasia and/or intramucosal cancer followed by fundoplication results in similar durability and recurrence rates when compared to patients being managed with PPIs following endoscopic therapy. However, fundoplication may be superior to PPIs after endoscopic therapy in preventing further progression of disease and the development of cancer, particularly in refractory patients. Fundoplication is an important strategy after endoscopic therapy for Barrett’s to achieve and maintain CR-IM, and to facilitate the eradication of persistent dysplasia.
References
Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency Ablation in Barrett’s Esophagus With Dysplasia. N Engl J Med. 2009 May 28;360(22):2277–88.
Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. Clin Gastroenterol Hepatol. Elsevier Inc.; 2010 Jan;8(1):23–9.
Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013 Jul;145(1):87–95.
Gupta M, Iyer PG, Lutzke L, Gorospe EC, Abrams J a, Falk GW, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology. Elsevier, Inc; 2013 Jul;145(1):79–86.e1.
Guarner-Argente C, Buoncristiano T, Furth EE, Falk GW, Ginsberg GG. Long-term outcomes of patients with Barrett’s esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointest Endosc. Elsevier Inc.; 2013 Feb;77(2):190–9.
Shaheen NJ, Overholt BF, Sampliner RE, Wolfsen HC, Wang KK, Fleischer DE, et al. Durability of Radiofrequency Ablation in Barrett’s Esophagus with Dysplasia. Gastroenterology. 2011 Aug;141(2):460–8.
Krishnan K, Pandolfino JE, Kahrilas PJ, Keefer L, Boris L, Komanduri S. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology. Elsevier Inc.; 2012 Sep;143(3):576–81.
Parrilla P, Martínez de Haro LF, Ortiz A, Munitiz V, Molina J, Bermejo J, et al. Long-Term Results of a Randomized Prospective Study Comparing Medical and Surgical Treatment of Barrett’s Esophagus. Ann Surg. 2003 Mar;237(3):291–8.
Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: Effect of omeprazole. Gastroenterology. 2001 Jun;120(7):1599–606.
Theodorou D, Ayazi S, DeMeester SR, Zehetner J, Peyre CG, Grant KS, et al. Intraluminal pH and goblet cell density in Barrett’s esophagus. J Gastrointest Surg. 2012 Mar;16(3):469–74.
Smith E, Kelly JJ, Ruskiewicz AR, Sullivan T, Jamieson GG, Drew P a. The effect of long-term control of reflux by fundoplication on aberrant deoxyribonucleic acid methylation in patients with Barrett esophagus. Ann Surg. 2010 Jul;252(1):63–9.
O’Connell K, Velanovich V. Effects of Nissen fundoplication on endoscopic endoluminal radiofrequency ablation of Barrett’s esophagus. Surg Endosc. 2011 Mar;25(3):830–4.
Shaheen NJ, Kim HP, Bulsiewicz WJ, Lyday WD, Triadafilopoulos G, Wolfsen HC, et al. Prior fundoplication does not improve safety or efficacy outcomes of radiofrequency ablation: results from the U.S. RFA Registry. J Gastrointest Surg. 2013 Jan;17(1):21–8; discussion p.28–9.
Hunt BM, Louie BE, Schembre DB, Bohorfoush AG, Farivar AS, Aye RW. Outcomes in Patients Who Have Failed Endoscopic Therapy for Dysplastic Barrett’s Metaplasia or Early Esophageal Cancer. Ann Thorac Surg. 2013 May 2;95(5):1734–40.
Zehetner J, DeMeester SR, Hagen J a, Ayazi S, Augustin F, Lipham JC, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg. 2011 Jan;141(1):39–47.
Lada M, Watson TJ, Shakoor A, Nieman DR, Han M, Tschoner A, et al. Eliminating a Need for Esophagectomy: Endoscopic Treatment of Barrett’s Esophagus with Early Esophageal Neoplasia. J Thorac Cardiovasc. 2014;In press.
Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’sesophagus. Gastroenterology 1993;105:40–50.
Bulsiewicz WJ, Dellon ES, Lyday WD, Ertan A, Camara DS, Komanduri S, et al. Predictors of recurrent Barret’s esophagus after successful ablation in a nationwide, multicenter cohort: Results from the U.S. RFA Registry. Gastroenterology. 2013;144(S-1).
Phoa KN, Pouw RE, van Vilsteren FGI, Sondermeijer CMT, Ten Kate FJW, Visser M, et al. Remission of Barrett’s esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology. Elsevier, Inc; 2013 Jul;145(1):96–104.
Corley DA. Can You Stop Surveillance After Radiofrequency Ablation of Barrett’s Esophagus? A Glass Half Full. Gastroenterology. 2013 Jul;145(1):39–42.
Fleischer DE, Odze R, Overholt BF, Carroll J, Chang KJ, Das A, et al. The case for endoscopic treatment of non-dysplastic and low-grade dysplastic Barrett’s esophagus. Dig Dis Sci. 2010 Jul;55(7):1918–31.
Vallböhmer D, DeMeester SR, Oh DS, Banki F, Kuramochi H, Shimizu D, et al. Antireflux surgery normalizes cyclooxygenase-2 expression in squamous epithelium of the distal esophagus. Am J Gastroenterol. 2006 Jul;101(7):1458–66.
Vallböhmer D, DeMeester SR, Peters JH, Oh DS, Kuramochi H, Shimizu D, et al. Cdx-2 expression in squamous and metaplastic columnar epithelia of the esophagus. Dis Esophagus. 2006 Jan;19(4):260–6.
Chang EY, Morris CD, Seltman AK, O’Rourke RW, Chan BK, Hunter JG, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with barrett esophagus: a systematic review. Ann Surg. 2007 Jul;246(1):11–21.
Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol. 2003 Nov;98(11):2390–4.
Vakil N. Review article: the role of surgery in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007 Jun 15;25(12):1365–72.
Shen KR, Harrison-Phipps KM, Cassivi SD, Wigle D, Nichols FC, Allen MS, et al. Esophagectomy after anti-reflux surgery. J Thorac Cardiovasc Surg. United States; 2010 Apr;139(4):969–75.
Chang AC, Lee JS, Sawicki KT, Pickens A, Orringer MB. Outcomes after esophagectomy in patients with prior antireflux or hiatal hernia surgery. Ann Thorac Surg. Elsevier Inc.; 2010 Apr;89(4):1015–21; discussion 1022–3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, C.S., Louie, B.E., Wille, A. et al. The Durability of Endoscopic Therapy for Treatment of Barrett’s Metaplasia, Dysplasia, and Mucosal Cancer After Nissen Fundoplication. J Gastrointest Surg 19, 799–805 (2015). https://doi.org/10.1007/s11605-015-2783-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2783-6