Abstract
Although the indication of locally advanced pancreatic cancer with arterial involvement is controversial, the outcome of the patients with such disease treated by combined resection and reconstruction of the invaded artery has improved recently. For pancreatic body carcinoma invading the celiac axis, distal pancreatectomy with celiac axis resection has been safely performed. However, in case of pancreatic body carcinoma with involvement of the celiac axis, the common hepatic artery and the gastroduodenal artery, margin-negative resection requires total pancreatectomy with celiac axis resection and restoration of hepatic arterial flow. Here, we describe an interposition grafting technique using the splenic artery harvested from the resected specimen. This technique is effective and may widen the resectability of pancreatic cancer in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery remains the only potentially curative treatment for patients with pancreatic cancer, and en bloc resection with sufficient surgical margin was reported to be associated with long-term survival.1 , 2 Currently, an invasion of the portal-superior mesenteric vein does not preclude patients from surgery because combined venous resection and reconstruction of these vessels can be safely performed. The survival of patients with such a procedure performed by experienced surgeons compares favorably with those who undergo standard resections.3 Recently, resection and reconstruction of invaded arteries has been reportedly associated with no significant increase in mortality and may contribute to improved prognosis in carefully selected patients.4 – 6 For pancreatic body carcinoma invading the celiac axis (CA), distal pancreatectomy combined with celiac axis resection (DP-CAR) has been reported to be safely performed with complete cancer removal by en bloc resection of the nerve plexus.7 , 8 Also, we previously reported that preserving the left gastric artery (LGA) during DP-CAR can result in similar curability without an increased risk of ischemic complications of the stomach or liver (modified DP-CAR).9 Pancreaticoduodenectomy combined with hepatic artery resection and reconstruction can be performed for invasion to the short segment of the common hepatic artery (CHA) and proper hepatic artery (PHA) due to pancreatic head carcinoma.10 However, in case of pancreatic body carcinoma with cancer involvement of the CA, the CHA, and the gastroduodenal artery (GDA), margin-negative resection requires total pancreatectomy with restoration of hepatic arterial flow. For reconstruction of the hepatic artery, an autologous venous graft or a prosthetic graft can be used for interposition. However, the use of a venous graft is not appropriate for arterial reconstruction and requires additional harvesting from another location, whereas the use of a prosthetic graft should be avoided because of the potential risk of postoperative infections. Here, we describe an interposition grafting technique using the splenic artery (SPA) harvested from the resected specimen.
Surgical Techniques
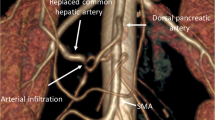
To evaluate the indication of surgery, we routinely performed three-dimensional computed tomography (3D-CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT. The operative procedure then begins with a thorough exploration to confirm the absence of contraindications for resection. Following cholecystectomy, the PHA is identified and taped. Following dissection of the distal part of the left gastric vein and identification of the LGA, the dissection proceeds to the origin of the LGA. The confirmation of no cancer cells by intraoperative pathological diagnosis at the nerve plexus around the LGA root and of the position where PHA will be severed is required for margin-negative resection.
Lifting the transverse colon upward, the peritoneum of the duodenal recess is incised and the aorta, the inferior vena cava, and the left renal vein are exposed using the left posterior approach by mobilizing and rotating the fourth portion of the duodenum and the uncinate process of the pancreas. After the transection of the proximal jejunum, the mesentery base is incised, and the ligament of Treitz is opened and divided on the left and anterior side of the mesenteric root. The superior mesenteric artery (SMA) is taped, and the dissection proceeds to the origin of the SMA with consequent division of the common trunk of the first jejunal artery and the inferior pancreaticoduodenal artery, which generally arises from the left dorsal aspect of the SMA. Thus, the posterior and right aspects of the SMA are exposed, and the uncinate process of the pancreatic head is separated from the SMA (Fig. 1). These procedures were previously described as the artery-first approach to pancreaticoduodenectomy but may be difficult to perform in obese patients.11 , 12
After transection of the distal part of the stomach, the dissection proceeds to the CA, the avascular plane is opened to reach the aorta, and the diaphragmatic slips around the aorta are excised. Thus, the root of the CA is identified and taped.9 Complete lymphadenectomy of the lower hepatoduodenal ligament is performed, and the common bile duct is divided proximal to the cystic duct. The GDA need not be identified because it will be resected en bloc with the CHA and CA. After transposing the upper jejunum to the right of the mesenteric pedicle, the remaining nerve plexus of the pancreatic head is dissected, and the portal vein is skeletonized, thereby completely isolating the pancreatic head from the SMA and CA. The splenorenal ligament is divided, and the spleen is drawn medially together with the tail of the pancreas after retroperitoneal dissection. After division of the short gastric vessels and the inferior mesenteric vein, the splenic vein is divided, and the dissection proceeds to the left. The adrenal vein and the adrenal gland become part of the posterior plane, and the renal vein becomes the inferior border of dissection.
After division of the CA distal to the branching of the LGA and the PHA with clamping of each artery as an important final step, en bloc resection is performed together with the pancreas, duodenum, peripancreatic lymph nodes, and the CA, including the CHA and the SPA. If the portal-superior mesenteric vein is invaded with cancer, venous resection and reconstruction is conducted (Fig. 2).
The SPA is harvested starting at a site sufficiently distal from the tumor invasion and mobilized as far as possible toward the splenic hilum (Fig. 3). The SPA can then be cut on both sides and flushed with heparin solution. An end-to-end anastomosis with a microvascular technique between the PHA and CA with the SPA graft is performed at both sides by an experienced vascular surgeon (Fig. 4). To restore gastrointestinal continuity, choledochojejunal and gastrojejunal anastomoses are performed.
Discussion
Pancreatic cancer has an extremely poor prognosis. Surgery is the only curative option, and prolonged survival is achieved only by resection with macroscopic tumor clearance. The advancement in surgical techniques in the modern era has led to improved results and long-term survival for patients with pancreatic cancer following surgery.1 , 2 Meanwhile, the indication of locally advanced pancreatic cancer with arterial involvement of the hepatic artery and SMA remains controversial because of the high rates of postoperative mortality and morbidity.13 , 14 Hirano et al.7 reported an excellent outcome of locally advanced cancer of the pancreatic body with involvement of the CA, while Bachellier et al.15 reported that pancreatic resection combined with arterial resection may prolong survival in carefully selected patients; the criteria for resectability have gradually expanded.
In case of combined resection of the hepatic artery, great care is needed to maintain intraoperative arterial blood flow to the liver before the CHA is severed. Although arterial blood flow through the GDA can nourish the liver in DP-CAR, the combined resection of GDA abolishes arterial blood supply to the liver. Yoshidome at al.16 reported that PHA embolization prior to pancreaticoduodenectomy immediately develops collateral circulation; the left liver was perfused by the inferior phrenic artery or the artery running thorough the lesser omentum, and the right liver was perfused by the retroperitoneal collateral artery. Thus, pancreaticoduodenectomy combined with resection of the hepatic artery without reconstruction might be a feasible procedure in selected patients. However, complete removal by en bloc resection of the retroperitoneal tissue, including the nerve plexus around the CA, may alter the collateral pathway. Therefore, reconstruction of the hepatic artery is almost mandatory. Suzuki et al.17 reported the reconstruction of the hepatic artery using the middle colic artery, while Seelig et al.5 and Hackhert et al.18 reported the use of the SPA. The use of the SPA harvested from the resected specimen does not sacrifice any other blood supply, and the caliber of the SPA is well matched to that of the CA and PHA. Such graft interposition between the PHA and the CA distal to the LGA branching does not jeopardize arterial supply to the stomach. Care should be taken to prevent a kinking of the artery, which may cause early thrombosis and liver ischemia. Although venous stagnation of the stomach due to the lack of the left gastric vein is a matter of concern, no problems with delayed gastric emptying or gastric ulcer is experienced with probable drainage via the cranial remnants of the lesser omentum and the distal esophagus.
A total of 105 patients with pancreatic ductal carcinoma underwent pancreatectomy from 2008 to 2014. Among these patients, 11 patients underwent pancreatectomy combined with artery resection; pancreaticoduodenectomy combined with hepatic artery resection and reconstruction was performed in three patients, DP-CAR was performed in six patients, and the procedure described here was performed in two patients. Postoperative 3D-CT angiography revealed that patency was maintained in the anastomosis between the PHA and CA with the SPA graft. The postoperative courses were uneventful. Pathological findings showed a cancer invasion to the resected artery and free of surgical margin from the stump of cancer invasion. Both patients underwent adjuvant chemotherapy with S-1. One patient had a recurrence of paraaortic lymph node 36 months since surgery, and another patient had lung metastasis 14 months since surgery.
In conclusion, hepatic artery reconstruction using SPA graft interposition is effective and may widen the resectability of pancreatic cancer in selected patients. Furthermore, this technique may be useful in cases with insufficient blood flow to the liver in DP-CAR because of nontumorous reasons such as arteriosclerotic stenosis.
References
Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, Schurr PG, Liebl L, Thieltges S, Gawad KA, Schneider C, Izbicki JR En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg 2008;247:300–309
Butturini G, Stocken DD, Wente MN, Jeekel H, Klinkenbijl JH, Bakkevold KE, Takada T, Amano H, Dervenis C, Bassi C, Buchler MW, Neoptolemos JP, Pancreatic Cancer Meta-Analysis G Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75–83
Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004;8:935–949
Bockhorn M, Burdelski C, Bogoevski D, Sgourakis G, Yekebas EF, Izbicki JR Arterial en bloc resection for pancreatic carcinoma. Br J Surg 2011;98:86–92
Seelig MH, Belyaev O, Uhl W Reconstruction of the common hepatic artery at the time of total pancreatectomy using a splenohepatic bypass. J Gastrointest Surg 2010;14:913–915
Amano H, Miura F, Toyota N, Wada K, Katoh K, Hayano K, Kadowaki S, Shibuya M, Maeno S, Eguchi T, Takada T, Asano T Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg 2009;16:850–857
Hirano S, Kondo S, Hara T, Ambo Y, Tanaka E, Shichinohe T, Suzuki O, Hazama K Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg 2007;246:46–51
Mayumi T, Nimura Y, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Hamaguchi K, Hayakawa N Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol 1997;22:15–21
Kimura A, Yamamoto J, Aosasa S, Hatsuse K, Nishikawa M, Nishiyama K, Tsujimoto H, Moriya T, Hase K, Shinmoto H, Kaji T Importance of Maintaining Left Gastric Arterial Flow at Appleby Operation Preserving Whole Stomach for Central Pancreatic Cancer. Hepatogastroenterology 2012;59:2650–2652
Amano H, Miura F, Toyota N, Wada K, Katoh K, Hayano K, Kadowaki S, Shibuya M, Maeno S, Eguchi T, Takada T, Asano T Pancreatectomy with reconstruction of the right and left hepatic arteries for locally advanced pancreatic cancer. J Hepatobiliary Pancreat Surg 2009;16:777–780
Sanjay P, Takaori K, Govil S, Shrikhande SV, Windsor JA ‘Artery-first’ approaches to pancreatoduodenectomy. Br J Surg 2012;99:1027–1035
Kurosaki I, Minagawa M, Takano K, Takizawa K, Hatakeyama K Left posterior approach to the superior mesenteric vascular pedicle in pancreaticoduodenectomy for cancer of the pancreatic head. JOP : Journal of the pancreas 2011;12:220–229
Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Buchler MW, Weitz J Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882–893
Martin RC, 2nd, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg 2009;144:154–159
Bachellier P, Rosso E, Lucescu I, Oussoultzoglou E, Tracey J, Pessaux P, Ferreira N, Jaeck D Is the need for an arterial resection a contraindication to pancreatic resection for locally advanced pancreatic adenocarcinoma? A case-matched controlled study. J Surg Oncol 2011;103:75–84
Yoshidome H, Shimizu H, Ohtsuka M, Yoshitomi H, Kato A, Furukawa K, Miyazaki M Pancreaticoduodenetomy combined with hepatic artery resection following preoperative hepatic arterial embolization. Journal of hepato-biliary-pancreatic sciences 2014;21:850–855
Suzuki H, Hosouchi Y, Sasaki S, Araki K, Kubo N, Watanabe A, Kuwano H Reconstruction of the hepatic artery with the middle colic artery is feasible in distal pancreatectomy with celiac axis resection: A case report. World J Gastrointest Surg 2013;5:224–228
Hackert T, Weitz J, Buchler MW Splenic artery use for arterial reconstruction in pancreatic surgery. Langenbecks Arch Surg 2014;399:667–671
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aosasa, S., Nishikawa, M., Noro, T. et al. Total Pancreatectomy with Celiac Axis Resection and Hepatic Artery Restoration Using Splenic Artery Autograft Interposition. J Gastrointest Surg 20, 644–647 (2016). https://doi.org/10.1007/s11605-015-2991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-015-2991-0