Abstract
Background
Our aim was to establish a new pN staging system for gastric cancer based on the number and location of metastatic lymph nodes (MLNs) and to compare it with other systems.
Methods
We retrospectively analyzed the prognostic data of 521 gastric cancer patients who underwent curative resection. Survival analyses were used to establish a pN staging system that considers both the number and location of MLNs and to compare discriminatory ability and monotonicity of gradients (linear trend χ 2 score), homogeneity ability (likelihood ratio test), and prognostic stratification ability (Akaike information criterion) between Japanese Gastric Cancer Association (JGCA) and Union for International Cancer Control (UICC) systems.
Results
Cut-point survival analysis divided pN+ patients into two groups: Nxn1~6 and Nxn≥7. N0, N1, N2, and N3 (the previous classifications) were replaced by N0, N1n1~6, N2n1~6, and N1n≥7 + N2n≥7 + N3n1~6 + N3n≥7, respectively. Compared with two widely used staging systems, the new system had the highest likelihood ratio test [106.06 (new) vs 95.09 (JGCA) vs 94.33 (UICC)] and linear trend χ 2 scores [102.30 (new) vs 89.12 (JGCA) vs 86.97(UICC)] and the lowest Akaike information criterion (AIC) score [2,283.88 (new) vs 2,285.31 (JGCA) vs 2,299.88 (UICC)].
Conclusion
A new pN staging system based on the number and location of MLNs is an efficient prognostic indicator of the survival of patients with gastric cancer following radical surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although its incidence has steadily declined over the past few decades, gastric carcinoma is still one of the most common malignancies worldwide. An estimated 934,000 new cases are diagnosed each year, with the highest incidence rate in Northeast Asia, intermediate incidence rates in Europe and South America, and the lowest incidence rates in North America, Africa, South Asia, and Oceania.1,2 In China, there are more than 40,000 estimated new cases of stomach cancer per year,3 most of which are diagnosed at an advanced stage with lymph node metastasis. The depth of tumor invasion and nodal involvement is thought to be the most important prognostic factors in gastric cancer in both the hemispheres.4,5 Since variations in pN stage distribution significantly affect the predicted outcome of gastric cancer patients, a greater reliability of staging in gastric cancer is warranted.
There are two main pN staging systems for gastric cancer in the world: the TNM system of the Union for International Cancer Control (UICC), which is based on the number of metastatic lymph nodes (MLNs), and the former system of the Japanese Gastric Cancer Association (JGCA) (13th edition and earlier), which stresses the location (anatomic position of MLNs and their distance from primary tumors) of MLNs. Both staging systems provide prognostic information for gastric cancer patients with lymph node metastasis, and both have great clinical value. pN staging based on the number of MLNs is simple, reliable, and reproducible but does not provide information about the extent of lymph node metastasis. Conversely, pN staging based on the location of MLNs is complex and tedious but does provide information on the extent of lymph node metastasis, as well as guidance and appraisal of standard lymphadenectomies.
Our goal was to incorporate the advantages of the two staging systems into a single staging system. Here, we propose a new pN classification system based on both the number and location of MLNs. We evaluated its prognostic value in terms of the survival of patients with gastric cancer following radical and in comparison with the JGCA (13th edition) and UICC (7th edition) staging systems.
Materials and Methods
Patients
From May 1998 to June 2008, 811 consecutive gastric cancer patients underwent curative gastrectomy at the Division of Gastrointestinal Surgery, the First Affiliated Hospital, Sun Yat-sen University in China. All patients had routinely received preoperative chest X-rays and abdominal computer tomography scans to exclude liver and lung metastasis. In some instances, they received bone or positron emission topography scans to exclude bone or other distant metastases.
The inclusion criteria of our study were as follows: (1) Patients were diagnosed with primary gastric adenocarcinoma via a histopathologic examination, (2) patients underwent radical gastrectomy with D2 lymphadenectomy or D2+ if necessary, (3) at least 15 lymph nodes were dissected, and (4) the death of the patient was cancer-associated. The exclusion criteria were as follows: (1) a history of other primary malignant tumors; (2) patients had a distant metastasis, such as peritoneal, liver, bone, or extraregional lymph node metastases; (3) patients were diagnosed with gastric stump cancer after gastric resection for benign disease; (4) patients received neo-adjuvant chemotherapy; and (5) patients died during their initial hospitalization.
Among the 811 patients who underwent surgery at our hospital, 160 had an insufficient number of lymph nodes harvested, 207 were diagnosed with a distant metastasis (130 patients had peritoneal metastasis, 36 had liver metastasis, and 47 had extraregional lymph node metastasis) and had received palliative resection, and 34 were diagnosed with remnant gastric cancer. Ultimately, 521 eligible patients were included in our study.
Perioperative Treatment
The depth of tumor involvement, the state of lymph node metastasis and distant metastasis, histological type, and degree of differentiation were evaluated by expert pathologists. Curative operations were performed by surgeons trained by the standardized radical operation program. En bloc resection of the primary tumor and its lymphatic drainage area was recommended as the standard procedure. D2 lymphadenectomy was performed according to the 14th edition of the JGCA guidelines and was routinely based on tumor location. An extension of lymphadenectomy (D2+ or D3) to further stations was optional for patients suspected of having lymph node metastases based on preoperative examinations and operative exploration, such as the station nos. 12b, 12p, 13, and 16. Extraregional lymph node included the station nos. 14a, 14v, 15, 17, 18, 19, 20, 110, 111, and 112. D2 lymphadenectomy in the hepatoduodenal ligament and along the common hepatic artery and D3 lymphadenectomy in no. 16a2 and no. 16b1 areas are shown in Fig. 1a, b. Depending on their pN classification (according to the staging system in the 5th edition of the UICC),6 patients received postoperative chemotherapy based on a 5-fluorouracil and calcium leucovorin regimen.
Lymph Node Evaluation
Nodal involvement was evaluated according to the pN classifications of the 7th edition of the UICC guidelines7 and the 3rd edition of the Japanese gastric cancer treatment guidelines.8 The number of MLNs was determined as according to the 7th edition of the UICC guidelines, which define pN classes on the basis of the number of regional MLNs as follows: N0, 0; pN1, 1–2; pN2, 3–6; and pN3, >6. The anatomic location of MLNs was determined according to the 3rd edition of the Japanese gastric cancer treatment guidelines, and the extent of lymph node involvement was based on the type of gastrectomy.
Surveillance
The postoperative follow-up schedule was every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter until the patient died. During the follow-up period, all patients received gastroscopic examinations, tumor markers tests, chest X-rays, and computed tomography scans or abdominal ultrasound examinations. The most recent follow-up date was December 2013. The follow-up rate was 95.1 %.
Statistical Analysis
The postoperative cumulative survival rate was determined according to the life-table method, and the log-rank test was used to assess statistical differences between groups. Survival curves were constructed according to the Kaplan-Meier method. Parameters with statistical significance in a univariate analysis were further analyzed by a multivariate analysis using a Cox proportional hazard model. The most significant statistical cutoff point for the number of MLNs was determined by a cut-point survival analysis. Homogeneity was measured using the likelihood ratio χ 2 test, which is related to the Cox regression model. Discriminatory ability and monotonicity of gradients were measured using the linear trend χ 2 test. The Akaike information criterion (AIC) within the Cox proportional hazard model was used to minimize potential bias in comparing different prognostic systems. The AIC was defined by a −2 log maximum likelihood +2 multiplied by the number of parameters in the model. The accepted level of significance was P < 0.05. The statistical analysis package (SPSS 16.0, SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
The mean age of the 521 patients in our cohort was 56.64 ± 12.34 years (range, 25–87). The cohort consisted of 359 (68.9 %) men and 162 (31.1 %) women. One hundred fifty-nine patients (30.5 %) had adenocarcinoma of the proximal stomach, and 362 (69.5 %) had adenocarcinoma of the distal stomach. A total of 12,511 lymph nodes were retrieved from the 521 patients (mean number of lymph nodes per patient, 24.01 ± 0.59; range, 15–78). Three hundred and four patients were lymph node-positive (N+), and 1,738 MLNs were present in these patients (mean number of MLNs per N+ patient, 5.72; range, 1–55). The incidence of lymph node metastasis was 58.3 % (304 of 521 patients).
Survival Analysis for Prognostic Factors
Statistically significant prognostic factors for the survival of patients with gastric cancer were identified by a univariate analysis using the Cox proportional hazard model and were as follows: sex, tumor size, tumor location, histological type, depth of invasion, number of MLNs, and location of MLNs (based on the JGCA pN classification system). Using a Cox proportional hazard model and a forward LR procedure, a multivariate analysis of the results obtained in the univariate analysis was performed. Of the factors identified in the univariate analysis, both the number of MLNs [hazard ratio (HR), 1.046; 95 % confidence interval (CI), 1.024–1.069; P < 0.05] and the location of MLNs (HR, 1.427; 95 % CI, 1.186–1.717; P < 0.05) were significant independent prognostic factors. Sex, tumor size, and depth of invasion were also independent prognostic factors.
Determination of the Most Appropriate Cut-point of Metastatic Lymph Nodes
To determine the most suitable cut-point for the number of MLNs, we compared the survival hazard between groups with different numbers of MLNs using a Cox proportional hazard model. The highest chi-square value was deemed the cut-point. As indicated in Table 1, the threshold of the group with seven MLNs had the highest chi-square value and therefore was the cut-point used in our study (χ 2 value, 34.126; HR, 19.734; 95 % CI, 7.255–53.676).
Establishment of a New pN Stage
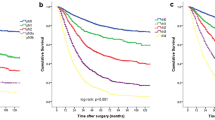
According to the results of the cut-point analysis, N+ patients could be divided into two groups: Nxn1~6 (number of MLNs ≤6) and Nxn≥7 (number of MLNs ≥7). As determined by the log-rank test of the Kaplan-Meier method, N1n1~6 and N2n1~6 were significantly associated with higher overall postoperative survival rates than were N1n≥7 and N2n≥7, respectively (N1n1~6, 125.3 months and N1n≥7, 23.0 months; N2n1~6, 45.1 months and N2n≥7, 25.5 months; P < 0.05) (Fig. 1b, c). There were no statistically significant differences in survival between N1n≥7, N2n≥7, N3n1~6, and N3n≥7 (23.0, 25.5, 22.5, and 18.0 months, respectively; P > 0.05). Each subgroup except the ones shown in Fig. 1d had statistically significant differences in survival with other subgroups. On the basis of the survival results, the pN stages of patients with gastric cancer were reclassified. The new designations were N0, N1n1~6, N2n1~6, and N1n≥7 + N2n≥7 + N3n1~6 + N3n≥7, which correspond to the previous classifications of N0, N1, N2, and N3, respectively (Fig. 2).
Comparison of Three Staging Systems
-
1. Performance
The performance of the pN staging systems was assessed by the likelihood ratio test (which assesses homogeneity) and the linear trend test (which assesses discriminatory ability and monotonicity of gradients). In the linear trend test, the χ 2 scores for the new system, the JGCA system, and the UICC system were 102.30, 89.12, and 86.97, respectively. The likelihood ratio χ 2 scores showed a similar trend: Scores for the new pN system, the JGCA system, and the UICC system were 106.06, 95.09, and 94.33, respectively. Therefore, the new system had the best discriminatory ability and monotonicity of gradients and the best homogeneity of the three systems.
-
2. Prognostic Ability
Postoperative cumulative survival rates and comparisons between each subgroup of each pN stage were determined by the life table and the Kaplan-Meier methods. As shown in Fig. 3a–c, the new, JGCA, and UICC pN staging systems were able to access differences in survival among each subgroup (P < 0.05).
Fig. 3 a Survival curves according to the stages based on the number of metastatic lymph nodes (7th edition of UICC/AJCC system). b Survival curves according to the stages based on the anatomical location of metastatic lymph nodes referred to the 3rd JGCA treatment guideline. c Survival curves according to the new pN stages. The differences between each group in the three different staging systems were statistically significant (P < 0.05)
The results of a univariate analysis showed that the new pN staging system was a statistically significant prognostic factor. As shown in Fig. 3, the three pN staging systems predicted survival differences among each pN category and thus were highly correlated. Three separate multivariate tests were applied to the three systems to avoid problems resulting from multicollinearity (Table 2). The multivariate results showed that the new pN staging system had the lowest AIC value (2,283.88), followed by the UICC system (2,285.31), and the JGCA system (2,299.88). This indicates that the new pN staging system provides the most accurate prognostic stratification of the three systems.
Table 2 Multivariate survival analysis results
Discussion
The most important characteristic of a cancer staging system is accuracy for prognostic stratification,9 especially for gastric cancer. At present, the pN classification system for gastric cancer is under extensive evaluation and investigation. The current Japanese classification system for pN stage is no longer based on the anatomical location of MLNs10; it has been revised to match the more widely used UICC classification7 and other systems based on the number of MLNs (Table 3). In the revised Japanese classification system, there are four pN stages based on the number of MLNs, but not the extent of lymph node metastasis. However, basing pN classification on the number of MLNs, regardless of the extent of MLNs, remains controversial.
The latest pN classifications in the 7th edition of the UICC guidelines and the 14th edition of the JGCA guidelines are based on the number of MLNs and are simple, objective, reproducible, and easily compared worldwide and have better prognostic ability than other node-staging systems.12–17 Kunisaki et al.11 retrospectively analyzed 1,244 gastric cancer patients and found that the UICC pN classification system was more rational and homogenous and a more accurate predictor of prognosis than was the former JGCA classification system. Many researchers agree with this assessment.14–17 A major concern was that the number of harvested lymph nodes and the extent of lymphadenectomy skew the outcomes predicted by the UICC system. Since the extent of lymphadenectomy varies greatly among different gastric cancer centers, and especially between western and eastern countries, the number of lymph nodes retrieved also varies. In institutions in western countries, extended lymphadenectomy is not a standard procedure,18,19 and the number of dissected lymph nodes may be lower in these institutions than in Japanese and Chinese institutions, where extended lymphadenectomy is routinely performed. This disparity was the reason for decreasing the number of involved lymph nodes that contribute to stage migration.20 Moreover, the UICC pN staging system does not provide information about the location of MLNs, which is an important prognostic factor for patients with gastric cancer, even those with the same number of MLNs. As shown in our study, there were significant differences in the survival in patients classified as N1 and N2 by the former JGCA system when the number of MLNs was less than 7.
The former JGCA pN classification system (13th edition and earlier) was based on the anatomical location of MLNs because Japanese surgeons believed that node metastases progressed through the lymph node stations in a stepwise manner.21 It also considered tumor spread and operative strategies. Although less popular than the UICC system, the former JGCA system was superior to the UICC system in terms of the prognostic stratification of stages III and IV gastric cancer patients.22,23 However, it was criticized because its sophisticated and meticulous mapping procedure for dissecting lymph nodes was difficult, tedious, and a burden to surgeons and pathologists.
Our study was conducted at a gastric cancer center where D2 lymphadenectomy is considered the standard procedure. Our results using the Cox proportional hazard model showed that both the number and anatomical location of MLNs were important independent prognostic factors, indispensable for pN staging. It was therefore necessary to establish a new pN classification system in which both parameters were taken into consideration.
Although the guidelines for pN classification in the 7th edition of the UICC are widely accepted, some researchers argue that they do not provide an appropriate cut-point for the number of MLNs.24–26 Using the Cox proportional hazard model, we defined the cut-point as the threshold of the subgroup with seven MLNs, and we used this value in a survival analysis of each subgroup. On the basis of the results of this analysis, the pN stages of patients with gastric cancer were reclassified, and there were significant survival differences among the four new pN stages, which were designated N0, N1n1~6, N2n1~6, and N1n≥7 + N2n≥7 + N3n1~6 + N3n≥7.
According to Ueno et al.,27 the performance of a prognostic system is assessed by the following criteria: (1) homogeneity (the survival differences of patients at the same stage are small within the system), (2) discriminatory ability (the survival differences of patients between different stages were small within the system), and (3) monotonicity of gradients (an earlier stage was associated with a better survival time than an advanced stage in the system). We used the linear trend test (for discriminatory ability and monotonicity of gradients) and likelihood ratio test (for homogeneity) to compare the performances of the new, former JGCA, and UICC classification systems. As described above, the new pN staging system had the highest linear trend χ 2 score and likelihood ratio χ 2 score of the three systems and therefore the best homogeneity and discriminatory ability and monotonicity of gradients.
All three pN staging systems were independent prognostic factors as determined by a multivariate analysis. The AIC value was used to minimize the bias and complexity of the model and to improve the accuracy of correlations between the staging system and the survival data. As described above, the new pN staging system had the lowest AIC value of the three systems and therefore the best prognostic ability with minimal bias and complexity in this model.
We emphasize that number-based lymph node staging is suitable only when harvested lymph nodes are meticulously examined. Fifteen or more lymph nodes are needed for an accurate number-based staging of lymph node metastasis. Operative curability in respect to the extent of lymph node dissection cannot be assessed by number-based staging systems. The extent of lymph node metastasis must be determined by the anatomical distribution of the involved lymph nodes, and the completeness of lymph node dissection should be evaluated at the anatomical location of the removed lymph nodes.28 Hence, the ideal pN staging system should consider both the location of MLNs and extent of lymph node metastasis, as does the new pN classification system.
On the basis of the results of our study, we recommend the new pN classification system because of its easy performance and excellent prognostic ability. In the new system, classification of lymph nodes into different complicated stations is no longer necessary nor is subdivision of biopsy tissues according to the regions of lymphadenectomy such as D1, D2, or D3. The new system therefore overcomes previous disadvantages and inconveniences and increases reproducibility.
In conclusion, both the number and anatomical location of MLNs are important and indispensable for pN staging, as shown not only in our study but also in other retrospective studies. The new pN classification system had greater prognostic stratification strength than did two routinely used node-staging systems. We believe that the new pN classification system, which includes both the number and location of MLNs, will serve as an appropriate and comprehensive staging system for gastric cancer.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24(14):2137–50.
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96
Wang YC, Wei LJ, Liu JT, et al. Comparison of Cancer Incidence between China and the USA. Cancer Biol Med. 2012;9(2):128–132.
Deng JY, Liang H.. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20(14):3967–3975.
Cambruzzi E, Azeredo AM, Kronhart A et al. The presence of metastases in regional lymph nodes is associated with tumor size and depth of invasion in sporadic gastric adenocarcinoma. Arq Bras Cir Dig. 2014;27(1):18–21.
Fleming ID, Cooper JS, Henderson DE. AJCC cancer staging manual, 5th edition. New York: Springer, 1997.
Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 7th ed. Chicago: Springer, 2010.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011. 14(2):113–123.
Fielding LP, Fenoglio-Preiser CM, Freedman LS. The future of prognostic factors in outcome prediction for patients with cancer. Cancer. 1992;70(9):2367–77.
Japanese Gastric Cancer Association. Japanese classification of Gastric Cancer-14th edition (2010) -. KANEHARA & CO., LTD; Tokyo (Japanese)
Kunisaki C, Shimada H, Nomura M, et al. Comparative evaluation of gastric carcinoma staging: Japanese classification versus new american joint committee on cancer/international union against cancer classification. Ann Surg Oncol. 2004;11(2):203–6.
Hayashi H, Ochiai T, Suzuki T, Shimada H, Hori S, Takeda A, et al. Superiority of a new UICC-TNM staging system for gastric carcinoma. Surgery. 2000;127(2):129–35.
Hidaka H, Eto T, Maehara N, Jimi S, Hotokezaka M, Chijiiwa K. Comparative effect of lymph node metastasis classified by the anatomical site or by the number of nodes involved on prognosis of patients with gastric cancer. Hepatogastroenterology. 2008;55(88):2269–72.
Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, et al. Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification. Cancer. 1999;86(4):553–8.
Celen O, Yildirim E, Gülben K, Berberoğlu U. Prediction of survival in gastric carcinoma related to lymph node grading by the new American Joint Committee on Cancer/Union International Contre le Cancer System or the Japanese system. Eur J Surg Suppl. 2003;(588):33–9.
Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345(8952):745–8.
Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: Preliminary results of the MRC randomized controlled surgical trial. Lancet. 1996;347(9007):995–9.
Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–8.
Ohno S, Tachibana M, Shibakita M, Dhar DK, Yoshimura H, Kinugasa S, et al. Prognostic significance of Fas and Fas ligand system-associated apoptosis in gastric cancer. Ann Surg Oncol. 2000;7(10):750–7.
Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Validation of staging systems for gastric cancer. Gastric Cancer. 2008;11(2):111–8.
Ikeguchi M, Murakami D, Kanaji S, Ohro S, Maeta Y, Yamaguchi K, et al. Lymph node metastasis of gastric cancer: comparison of Union International Contra Cancer and Japanese systems. ANZ J Surg. 2004;74(10):852–4.
Tong JH, Sun Z, Zhu Z, et al. Prognostic significance of lymph node station 7 for patients with gastric cancers underwent radical surgery. J Surg Oncol. 2012. 105(8):805–812.
Kim JP, Yang HK, Oh ST. Is the new UICC staging system of gastric cancer reasonable? (Comparison of 5-year survival rate of gastric cancer by old and new UICC stage classification). Surg Oncol. 1992;1(3):209–13.
Li F, Zhang R, Liang H, et al. The pattern of lymph node metastasis and the suitability of 7th UICC N stage in predicting prognosis of remnant gastric cancer. J Cancer Res Clin Oncol. 2012. 138(1):111–117.
Deng J, Liang H, Sun D, et al. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol. 2010. 17(5):1259–1266.
Deng JY, Liang H, Sun D, et al. The most appropriate category of metastatic lymph nodes to evaluate overall survival of gastric cancer following curative resection. J Surg Oncol. 2008;98(5):343–8.
Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34(3):529–34.
Adachi Y, Kitano S, Sugimachi K. Surgery for gastric cancer:10-year experience worldwide. Gastric Cancer. 2001;4(4):166–74.
Grant Support
This study was supported by research grants from the National Nature Science Foundation of China (No. 30972883)
Conflict of Interest
The authors declare they have no conflict of interests (financial, professional, or personal) which are relevant to the manuscript.
Author Contributions
Dr. Chen Jianhui and Dr. Chen Chuangqi contributed equally to this study, including study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Dr. He Yulong, Wu KaiMing, and Wu Hui participated in the acquisition of data and statistical analysis. Dr. Cai Shirong supervised the whole study as well as monitored the standard surgical operations. All the authors took part in the surgical operations for gastric cancer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jianhui, C., Chuangqi, C., Yulong, H. et al. A New pN Staging System Based on Both the Number and Anatomic Location of Metastatic Lymph Nodes in Gastric Cancer. J Gastrointest Surg 18, 2080–2088 (2014). https://doi.org/10.1007/s11605-014-2663-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2663-5