Abstract
Purpose
The aim of this study was to evaluate the intraoperative detection rate of residual liver metastases after chemotherapy and to assess the correlation between disappeared liver metastases (DLMs) upon preoperative imaging and complete pathological response.
Methods
Between February 2004 and December 2008 clinicopathological data of 292 consecutive patients who underwent liver resection for colorectal liver metastases were prospectively collected and analyzed in a “per lesion” study. Thirty-three patients with 67 DLMs were included.
Results
During laparotomy, we identified 45 out of 67 DLMs (67%). Six DLMs were detected by macroscopic liver examination (9%) and 39 (58%) by intraoperative ultrasound (IOUS). Overall, persistent microscopic residual disease at pathological examination of the resected specimen or recurrence in situ identified during the follow-up were observed in 41 (61.2%) of 67 LMs that had shown a complete response by imaging. At multivariate analysis moderate or severe hepatic steatosis (p = 0.016), subglissonian localization of nodules (p = 0.019) and residual microscopic disease (p = 0.0006) were associated with IOUS detection of residual metastases. Preoperative chemotherapy with more than six cycles (p = 0.022) and intraoperative detection of nodules by IOUS (p = 0.001) were independent predictors of residual disease.
Conclusions
Systematic US exploration of the liver leads to increase the intraoperative detection rate of DLMs. Furthermore, the majority of DLMs identified by IOUS presents residual disease at pathological examination and should be treated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Outcome improvements of patients with colorectal liver metastases have been attributed not only to advances in surgical technique but also to the emergence of more effective chemotherapeutic regimens1,2 and targeted therapies.3,4 Therefore, there is an increasing trend to administer preoperative chemotherapy also in patients with resectable colorectal liver metastases5 with a growing number of patients showing high radiologic response rates and disappearing liver metastases (DLM).6
However, complete clinical response (CCR) of colorectal metastases has been shown to be of limited predictive value for complete pathological response (CPR),7 which is associated with considerable overall survival.8–10
Nevertheless, previous studies have reported conflicting results about the correlation between CCR and CPR. In particular, DLM may represent a true complete remission in17% to 69% of cases.11–15
Currently, the management of DLMs consists of a surgical exploration aiming to completely resect all sites of DLMs.16,17 The impossibility to intraoperatively detect residual disease may lead to leave metastases untreated.
The aim of this study was to evaluate the intraoperative detection rate of residual liver metastases and to assess the correlation between DLMs upon preoperative imaging and complete pathological response.
Patients and Methods
Between February 2004 and December 2008, 292 consecutive patients underwent liver resection for colorectal liver metastases at our institution and were considered for the study. Their clinicopathological data were prospectively collected and retrospectively analyzed in a “per lesion” study.
Patients were included according the following criteria: preoperative chemotherapy, fewer than 12 liver metastases (LM) before chemotherapy, disappearance at least of one LM on all preoperative imaging, surgery with intraoperative ultrasound IOUS within 4 weeks from last imaging, at least 1 year follow-up after surgery.
Preoperative Chemotherapy
Preoperative chemotherapy was performed both in patients with initially unresectable LMs or resectable LMs with poor prognostic factors. The chemotherapy administered before LM disappearance included fluorouracil and leucovorin (FU/LV) or capecitabine associated to oxaliplatin and/or irinotecan. Targeted therapy with biologic agents was included in selected cases.
Preoperative Imaging
All patients were staged before chemotherapy with abdominal ultrasound and abdomen and chest multidetector computed tomography (CT). Following chemotherapy all patients were evaluated with CT and/or magnetic resonance imaging (MRI).
Computed tomographic scans were performed with a multislice helical CT, using a collimation of 3 mm and reconstruction at 1 and 2.5 mm. Images were acquired with a triphasic hepatic protocol following a noncontrast evaluation of the liver. Images were obtained 11, 80, and 180 s after the start of intravenous injection of iopromide (Ultravist ® 370) at a rate of 3.5 mL/s.
MRI was carried out on a 1.5-T superconducting system using liver specific contrast agent (gadoxetic agent). Fluorodeoxyglucose positron emission tomography (FDG-PET) was additionally performed in selected cases.
Radiologists were aware of DLMs, and pre-chemotherapy imaging was available for review and comparison.
Last imaging was performed within 4 weeks prior to surgery.
Surgical Management
Abdominal exploration and intraoperative liver ultrasonography (Aloka SSD1200 with 7.5 MHz intraoperative linear T-probe and Aloka Prosound Alpha 5 with 7.5 MHz intraoperative mini-convex probe; Aloka Co., Tokyo, Japan) were always performed as the first step to assess the site and extent of the disease, together with the relationship of the tumor with major intrahepatic vessels, and in order to define the extension of the required resection. In all patients, the sites of DLM were carefully examined by IOUS carried out by the surgeon according to a standardized protocol.
Since 2007, constrast-enhanced intraoperative ultrasound (CEIOUS) was additionally performed in selected cases and every time the DLMs were not detected by IOUS. CEIOUS was achieved with a convex 2 to 6 MHz harmonic frequency transducer. In all patients, 2.4 mL of sulfur hexafluoride microbubbles (SonoVue, Bracco Imaging, Milan, Italy) were injected intravenously through a peripheral vein by the anesthesiologist.
The standard surgical procedure was to completely resect all DLMs detected intraoperatively. When DLMs were missed at intraoperative exploration, the initial sites of DLMs were resected according to the following criteria: site clearly detectable, easy resection, small parenchymal sacrifice.
Histopathologic Examination
Specimens were fixed, embedded in paraffin, and stained with hematoxylin–eosin. Slices of 0.3 cm were carried out for the microscopic examination of metastatic deposits. The pathologist was informed about the location of DLMs.
Follow-up
Systemic chemotherapy was continued postoperatively at discretion of the oncologists. Patients were followed every 3 months after the operation with US or CT scan. In selected cases additional imaging like MRI and FDG-PET were required. Follow-up imaging of patients with DLM untreated at surgery was carefully examined looking for recurrence in situ and compared with initial CT scan.
Definitions
Disappearing liver metastasis was defined as a lesion undetectable at any imaging modality following chemotherapy. The number of DLMs was defined according to the preoperative imaging modality that detected the higher number of lesions.
LMs were categorized as hypoechoic, isoechoic, or hyperechoic according to the intraoperative ultrasonographic pattern. When DLMs were not detected intraoperatively by IOUS, the ultrasonographic pattern of the missed lesions was defined along with the appearance of other detectectable LMs.18
Complete pathological response corresponded to no viable microscopic residual cancer cells at the site of missed metastases. A durable clinical response was considered in patients with no recurrences in situ within 12 months. CPR for DLMs included in the specimen or a durable clinical response for the DLMs left in situ were both considered as a complete response (CR).
Sub-glissonian metastasis was defined as lesion within 2 cm from liver surface.
Types of hepatectomies were classified according to the Brisbane 2000 terminology.19
Steatosis was estimated as the percentage of involved hepatocytes and categorized as defined by Kleiner et al.20: no fatty change (<5%), mild (5 to <33%), moderate (33 to <66%) or severe (≥66%).
Statistical Analysis
Univariate statistical comparisons between groups were performed using the Student's t test for continuous variables and the chi-square test for discrete variables. A p value of less than 0.05 was considered significant for all tests. All significant or borderline significant variables at the univariate analysis were entered into a Cox regression model for the multivariate analysis.
Results
Preoperative and Operative Data
Among 292 patients who underwent hepatectomy for LMs, 171 (58.5%) received preoperative chemotherapy. Overall, preoperative imaging showed a total of 624 LMs in these171 patients.
Thirty-three patients that presented DLMs at preoperative imaging and met all inclusion criteria were included in this study. In these 33 patients, 153 LMs were detected before chemotherapy, with a median number of 4 ± 2.7 LMs per patient. After chemotherapy, 67 liver metastases disappeared at preoperative imaging, corresponding to 10.7% of the entire series (67/624).
Preoperative and operative characteristics are summarized in Table 1. Twenty-eight patients (85%) presented synchronous LMs. At diagnosis, disease was unresectable in eight patients (24.2%). Concomitant extrahepatic disease was present in seven patients (21.2%).
All patients were preoperatively evaluated with a median of three hepatic imaging techniques (US, CT scan, MRI, and FDG-PET). All patients were staged with US. CT, MRI, and FDG-PET were undertaken in 30 (90.9%), 26 (78.8%), and 22 (66.6%) patients, respectively. Only three patients were staged with MRI and FDG-PET, without a preoperative CT scan (Table 2).
Sixteen patients were staged preoperatively with all imaging techniques (US, CT, MRI, and FDG-PET). In these patients, 79 LMs were detected before chemotherapy and 30 LMs disappeared at all preoperative imaging modalities. Furthermore, 40 (81.6%) out of the 49 remaining LMs were detected by CT scan, 49 (100%) by MRI, and only 10 (20.4%) by FDG-PET. Based on these findings, MRI was significantly more accurate to detect LMs after chemotherapy compared to CT scan (p = 0.0013) and FDG-PET (p < 0.0001).
The median number of preoperative chemotherapy cycles was 8 ± 3.5. Post-disappearance chemotherapy was administered in nine patients with 6 ± 4.5 median number of cycles. Eleven patients (33%) received biologic agents (bevacizumab or cetuximab) as part of their chemotherapy regimen before LM disappearance. Adjuvant chemotherapy was continued in 20 patients (60%).
After chemotherapy, the median number of DLMs per patient was 1 ± 1.5 with a median pre-treatment size of 10.5 ± 4.8 mm. Two patients among 33 showed complete disappearance of all liver lesions upon imaging. The median size of DLMs detected intraoperatively was 6 ± 3.8 mm; the localization were sub-glissonian (within 2 cm) in 40 (60%) and deep in 27 (40%). At IOUS 36 DLMs were hypoechoic, 20 isoechoic, and 11 hyperechoic. Steatosis moderate or severe was assessed in nine patients (16 LMs). Among these patients DLMs were hypoechoic in 87% of cases (14/16 DLMs).
Intraoperative Detection of Residual Disease
During laparotomy, we identified 45 out of 67 DLMs (67%). Six DLMs were detected by macroscopic liver examination (9%) and 39 by IOUS (58%). Ultrasonographic appearance of DLMs detected by IOUS was hypoechoic in 49%, isoechoic in 26%, and hyperechoic in 26% of cases. We performed CEIOUS in 11 patients and we did not detect any new lesion. As such, we confirmed that only 22 LMs (33%) had disappeared from the site of metastasis, as indicated by preoperative imaging. The median size of the 45 residual LMs seen at surgery was 6 mm (range, 1 to 15 mm). The size of residual lesions was less than 10 mm in 30 cases and less than 5 mm in nine cases.
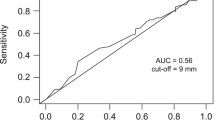
Predictors of intraoperative detection of residual disease by IOUS at univariate and multivariate analysis are reported in Table 3. At multivariate analysis, moderate or severe hepatic steatosis (p = 0.016), subglissonian localization of nodules (p = 0.019), and a residual microscopic disease (p = 0.0006) were independently associated with intraoperative detection of residual metastases by IOUS (Fig. 1).
a CT scan from a 64-year-old male with colorectal liver metastases. Smaller metastasis is arrowed. b MRI after 12 cycles of chemotherapy with 5-fluorouracil, oxaliplatin, and bevacizumab showing a remarkable decrease of the largest metastasis and the disappearance of the smaller metastasis (white arrow). c Intraoperative detection of the DLM by IOUS (white arrow)
Analysis of Complete Pathological Response
Pathological examination of 45 residual LMs included in the surgical resection specimen demonstrated viable carcinoma in 33 cases (73%) and CPR in 12 cases (27%).
Liver resection including the sites of LMs that disappeared was performed for 12 of 22 DLMs. Microscopic examination of the sites of LMs showing complete response on preoperative imaging and not visible disease at surgery showed presence of viable tumor cells in 2 of the 12 resection sites of initial metastases (16%). The remnant ten DLMs that were not resected were followed with serial imaging and four proved to be durable clinical complete response. Six DLMs recurred within 12 months and two were resected.
Results of univariate and multivariate analysis of factors predictive of complete response are reported in Table 4. Preoperative chemotherapy with more than 6 cycles (p = 0.022) and intraoperative detection of nodules by IOUS (p = 0.001) are independent predictors of residual disease.
The type of preoperative chemotherapy was not correlated to the presence of complete response.
In summary, persistent microscopic residual disease or recurrence in situ were observed in 41 (61.2%) of 67 LMs that had shown a complete response by imaging. Complete pathological response or a durable clinical complete response was noted in 26/67 cases (38.8%) (Fig. 2).
Discussion
Recent progresses in systemic and even more intra-arterial chemotherapy for colorectal LMs may result in the disappearance of some LM on liver imaging11–15; rates of DLMs range from 9% to 24% of patients.11–15 Despite recent improvements in imaging technique, correlation between CCR and CPR is still debated. As such, surgical exploration in patients with DLMs aims to find all LMs present at diagnosis in order to perform a radical resection. Few papers reporting data on intraoperative detection of DLMs have been published.11–15 The intraoperative detection rate of DLMs is extremely variable in the published series. Benoist et al.11 reported 30% detection rate of DLMs at surgical exploration and similar results were showed by Tanaka et al.13 By contrast, Van Vledder et al.15 recently assessed an overall 55% of intraoperative detection rate of DLMs, but data about IOUS findings are missing. Surprisingly, a recent analysis by Auer et al.14 reported 11% of DLM found at surgical exploration and only 0.9% detected by IOUS. This reported low IOUS detection rate can be explained by the surgeon's operative policy. In fact, most of patients underwent major or extended hepatic resections, probably leading to perform a less careful ultrasonographic exploration of the liver to be resected. In our series, the rate of intraoperative detection of DLMs was 67% at surgical exploration and after IOUS; this percentage was higher than those reported in literature. These conflicting results can be only partially explained by differences in imaging modalities, chemotherapy regimens, timing and type of surgery. We report the results of a short series, carried out in the recent years. Chemotherapy regimens included targeted therapy in selected cases. Patients were carefully studied in the preoperative workup and additional imaging like MRI and FDG-PET was undertaken in 87% of patients. Moreover, last imaging was always undertaken within 4 weeks before operation. Furthermore, in all patients, IOUS was systematically performed at the beginning of operation by an expert surgeon, using excellent ultrasound technologies and devices and steadily comparing the intraoperative findings with those of preoperative imaging modalities. Parenchymal sparing resections were aimed in all patients when possible, leading to a major hepatectomy rate of 30%.
To our knowledge, this is the first study that analyzed the predictive factors of intraoperative detection of DLMs. Factors like site of LMs and high grade of hepatic steatosis were useful in determining the probability to detect DLMs by IOUS.
The detection of liver metastases is more difficult following chemotherapy because of pathological changes in liver parenchyma and modification in size and appearance of LMs.21,22 In particular, preoperative imaging modalities significantly miss lesions small in size, adjacent to the falciform ligament or localized on the surface of the liver.23 Better evaluation of sub-glissonian LMs intraoperatively may be explained by technical aspects. IOUS is performed at the beginning of the operation and it is usually repeated after the liver mobilization improving the assessment of LMs case by case. Above all, the superficial liver surface could be examined by placing the probe posteriorly, increasing the depth of ultrasound beam penetration and performing a complete assessment using overlapping fields.24
Several studies have shown an increased incidence of hepatic steatosis in patients undergoing chemotherapy for colorectal liver metastases as a manifestation of drug hepatotoxicity.25–27 Although the technological progress in imaging equipment, accuracy of preoperative imaging techniques is reduced by steatosis, resulting in a low signal attenuation of liver parenchyma.
On CT scan the liver appears less dense and the signal intensity of the LMs becomes isodense thus difficult to delineate and detect.28 Furthermore, even the accuracy of FDG-PET is markedly lowered by systemic chemotherapy because of metabolic inhibition by chemotherapeutic drugs.29–31 Several studies reported that MRI with liver-specific contrast agents allows better detection and accurate differentiation of metastases even in fatty liver infiltration.32,33 These findings can be explained by the increased tissue contrast and the higher spatial resolution now available with the new generation of 3T MRI scanners.34 Nevertheless, IOUS provides more useful additional informations on hepatic lesions in postchemotherapy “bright liver”, despite the improvements in preoperative MRI.35 Therefore, Van Vledder et al.36 recently assessed that hepatic steatosis significantly decreases echogenity of colorectal LMs making easier their intraoperative detection. Accordingly, in our series, we found that LMs were hypoechoic in 87% of patients with moderate or severe steatosis. Overall, these data suggest that IOUS could improve intraoperative staging in patients with fatty liver despite an overall poor image quality.
In the present series, the ability of IOUS to find DLMs was not improved by using CEIOUS. These findings are in contrast with previous studies37,38 that suggest CEIOUS may significantly impact on surgical staging and management of patients with colorectal LMs. Nevertheless, these studies were based on preliminary experiences in a small number of patients and with different criteria in patients' selection and data analysis. Recently, Torzilli et al.39 reported that the intraoperative detection rate of new nodules by CEIOUS in patients with colorectal metastases decreased in the last years from 21% to 9%.
After intensive preoperative chemotherapy, liver usually becomes hyperechoic, increasing the contrast between the healthy parenchyma and the metastasis. The natural increase of liver contrast after chemotherapy can probably explain the poor results of CEIOUS in our series. We believe that the use of dedicated intraoperative transducers, which have been recently released, and future availability of new liver specific contrast agents will improve the accuracy of CEIOUS.
In the present series, results on predictive factors of intraoperative IOUS detection of DLMs showed that a CPR strongly correlated with missing DLMs at IOUS exploration. Unfortunately, this finding is not useful as a predictor because requires the pathological examination. On the other hand, our results demonstrate that DLMs missed by IOUS are more likely to present a complete response at histopathology.
Complete pathological disappearance of all liver metastases after chemotherapy is associated with considerable overall survival and is a strong predictor of both prolonged survival and disease cure.7
However, CCR has been shown to be of limited predictive value for CPR7; disagreeing results have been published in different series with a correlation between DLMs and CR ranging from 17% to 69%.11–15 According to a recent series by Van Vledder et al.,15 we observed that only 26 out of 67 DLMs (38.8%) presented a true complete response.
Nowadays, there is no imaging modality reliable to diagnose a CPR6,7,16,17,29–33 upsetting surgical management of DLMs. As such, it could be important to know the factors that may predict a CPR or the presence of residual disease of DLMs detected intraoperatively. We assessed that extended chemotherapy and IOUS findings are independent predictors of residual disease.
Chemotherapy longer than six cycles was independently associated with microscopic residual disease at pathology examination. This may suggest that long-course chemotherapy included patients with more aggressive metastases. In the study of Blazer et al.,9 response to preoperative systemic therapy was the only independent predictor of survival after hepatic resection, along with margin status. Of note, although further investigations are needed, the data thus far indicate that the extent of tumor regression is independent to the duration of chemotherapy. As we previously reported,40 extended chemotherapy for colorectal metastases does not improve pathological response increasing the risk of hepatotoxicity. Therefore, a short course of preoperative chemotherapy might retain all the “survival” advantages while reducing the possible detrimental effects.
According to current management of patients with DLMs,16,17 surgical exploration should be undertaken evaluating all sites of previous disease. As previously reported,11–15 resection of all initial sites of DLMs should be performed when feasible. However, the exact site could not be localized accurately and the resection could not be safe or possible in all cases. In our series, IOUS detection of DLMs is an independent predictor of residual disease. As such, hepatic resection is mandatory when IOUS detects DLMs even when major or extended resection is required. Conversely, if DLMs are missed by IOUS, the initial site should be resected only when the required hepatectomy is easy and feasible in a parenchymal sparing strategy. According to previous studies, our findings confirm the importance of intraoperative detection of all macroscopic disease. Therefore, a careful and systematic IOUS exploration improves the yield of intraoperative assessment of DLMs.
The limits of the study are related to the retrospective design of the analysis. In particular, although the data were collected prospectively, patients did not undergo the same preoperative imaging modalities and chemotherapy regimens were not standardized.
In conclusion, systematic US exploration of the liver leads to increase the intraoperative detection rate of DLMs. Furthermore, the majority of DLMs identified by IOUS presents residual disease at pathological examination and should be treated.
References
Nordlinger B, Sorbye H, Glimelius B, et. al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371:1007–1016.
Adam R, Wicherts DA, de Haas RJ, et. al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009; 27:1829–1835.
Ribero D, Wang H, Donadon M et. al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007; 110:2761–7.
Folprecht G, Gruenberger T, Bechstein WO, et. al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010; 11:38–47.
Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol 2010; 17:492–501.
Chun YS, Vauthey JN, Boonsirikamchai P, et. al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 2009; 302:2338–2344.
Adam R, Wicherts DA, de Haas RJ, et. al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 2008; 26:1635–41.
Rubbia-Brandt L, Giostra E, Brezault C, et. al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neoadjuvant chemotherapy followed by liver surgery. Ann Oncol 2007; 18:299–304.
Blazer DG III, Kishi Y, Maru DM, et. al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008; 26:5344–5351.
Dy GK, Krook JE, Green EM, et. al. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: Results from Intergroup N9741. J Clin Oncol 2007; 25:3469–3474.
Benoist S, Brouquet A, Penna C, et. al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006; 24:3939–3945.
Elias D, Goere D, Boige V, et. al. Outcome of posthepatectomy missing colorectal liver metastases after complete response to chemotherapy: impact of adjuvant intra-arterial hepatic oxaliplatin. Ann Surg Oncol 2007; 14:3188–3194.
Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg 2009; 250:935–942.
Auer RC, White RR, Kemeny NE, et. al. Predictors of a true complete response among disappearing liver metastases from colorectal cancer after chemotherapy. Cancer 2010; 116:1502–1509.
van Vledder MG, de Jong MC, Pawlik TM, Schulick RD, Diaz LA, Choti MA. Disappearing Colorectal Liver Metastases after Chemotherapy: Should we be Concerned? J Gastrointest Surg 2010; 14:1691–700.
Carpenter S, Fong Y. Management of disappearing colorectal hepatic metastases. Adv Surg 2010; 44:269–79.
Thomay AA, Charpentier KP. Optimizing resection for “responding” hepatic metastases after neoadjuvant chemotherapy. J Surg Oncol 2010; 102:1002–8.
Choti MA, Kaloma F, de Oliveira ML, Nour S, Garrett-Mayer ES, Sheth S, Pawlik TM. Patient variability in intraoperative ultrasonographic characteristics of colorectal liver metastases. Arch Surg 2008; 143:29–34.
The Brisbane 2000 Terminology of Liver Anatomy and Resection. Terminology Committee of The International Hepato-Pancreato-Biliary Association. HPB 2000; 2:333–339.
Kleiner DE, Brunt EM, Van Natta M, et. al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 4:1313–1321.
Robinson PJA. The effects of cancer chemotherapy on liver imaging. Eur Radiol 2009; 19:1752–1762.
Znajda TL, Hayashi S, Horton PJ, et. al. Postchemotherapy characteristics of hepatic colorectal metastases: remnants of uncertain malignant potential. J Gastrointest Surg 2006; 10:483–9.
Foroutani A, Garland AM, Berber E, et. al. Laparoscopic ultrasound vs triphasic computed tomography for detecting liver tumors. Arch Surg 2000; 135:933–8.
Torzilli G, Makuuchi M. Intraoperative ultrasonography in liver cancer. Surg Oncol Clin N Am 2003; 12:91–103.
Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer 1998; 77:2008–2011.
Miyake K, Hayakawa K, Nishino M, Morimoto T, Mukaihara S. Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad Radiol 2005; 12:722–7.
Vauthey JN, Pawlik TM, Ribero D, et. al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006; 24:2065–2072.
Angliviel B, Benoist S, Penna C, et. al. Impact of chemotherapy on the accuracy of computed tomography scan for the evaluation of colorectal liver metastases. Ann Surg Oncol 2009; 16:1247–53.
Tan MC, Linehan DC, Hawkins WG, Siegel BA, Strasberg SM. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg 2007; 11:1112–1119.
Glazer ES, Beaty K, Abdalla EK, Vauthey JN, Curley SA. Effectiveness of positron emission tomography for predicting chemotherapy response in colorectal cancer liver metastases. Arch Surg 2010; 145:340–5.
Lubezky N, Metser U, Geva R, et. al. The role and limitations of 18-fluoro-2-deoxy-d -glucose positron emission tomography (FDG-PET) scan and computerized tomography (CT) in restaging patients with hepatic colorectal metastases following neoadjuvant chemotherapy: comparison with operative and pathological findings. J Gastrointest Surg 2007; 11:472–8.
Bipat S, van Leewen MS, Comans EFI, Pijl MEJ, Bossuyt BMM, Zwinderman AH, Stoker J. Colorectal liver metastases: CT, MR imaging and PET for diagnosis—meta-analysis. Radiology 2005; 237:123–31.
Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, Giovagnoni A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging 2010; 31:19–31.
Kulemann V, Schima W, Tamandl D, et. al. Preoperative detection of colorectal liver metastases: MDCT or MRI? Eur J Radiol 2011; 79:e1-6.
Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J of Ultrasound 2003; 16:21–16.
van Vledder MG, Torbenson MS, Pawlik TM, Boctor EM, Hamper UM, Olino K, Choti MA. The effect of steatosis on echogenicity of colorectal liver metastases on intraoperative ultrasonography. Arch Surg 2010; 147:661–7.
Torzilli G, Del Fabbro D, Palmisano A et al. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg 2005; 9:1148–53
Leen E, Ceccotti P, Moug SJ et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg 2006; 243:236–40.
Torzilli G, Botea F, Procopio F et al. Use of contrast-enhanced intraoperative ultrasonography during liver surgery for colorectal cancer liver metastases—its impact on operative outcome. Analysis of a prospective cohort study. Eur J Cancer 2008; 6:16–23
Kishi Y, Zorzi D, Contreras CM, et. al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010; 17:2870–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Detection of disappearing colorectal liver metastases and residual disease.
Residual disease detection
The authors have no commercial interest and sources of funding for research and/or publication.
Synopsis
IOUS increases intraoperative residual disease detection of disappearing colorectal liver metastases after chemotherapy.
Rights and permissions
About this article
Cite this article
Ferrero, A., Langella, S., Russolillo, N. et al. Intraoperative Detection of Disappearing Colorectal Liver Metastases as a Predictor of Residual Disease. J Gastrointest Surg 16, 806–814 (2012). https://doi.org/10.1007/s11605-011-1810-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1810-5