Abstract
Introduction
Although antegrade en bloc distal pancreatectomy is appropriate for invasive distal pancreatic malignancies, this technique is not easy to perform because the end-point of deep vertical resections cannot be controlled. This report describes the usefulness of the application of hanging maneuver in performing the radical surgery.
Methods
A tape for guidance is passed in a space behind the bundles of the left celiac and mesenteric plexus, followed by sagittal resection of the distal pancreas exposing the root of the celiac artery and superior mesenteric artery. After dividing the pancreas down to the level of the roots of the celiac and superior arteries, the distal pancreas is dissected from the retroperitoneum in medial to lateral fashion.
Results
This technique was applied in six patients with distal pancreas malignancies, without any positive cancer cells at the resected margin. The mean tumor size was 3.0 ± 0.9 cm. The mean duration of surgery and intraoperative blood loss were 258 ± 71 min and 226 ± 240 ml, respectively.
Conclusion
Antegrade en bloc distal pancreatectomy with plexus hanging maneuver is an appropriate technique for treating distal pancreatic malignancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distal pancreatectomy (DP) is the standard treatment for pancreas cancer originating in the body or tail of the pancreas. Such procedures are widely performed using a lateroposterior approach, whereby dissection proceeds from mobilization of the spleen to dissecting the posterior plane from left to right direction.1 However, these conventional approaches have some limitations, particularly during mobilization, including possible spillage of cancer cells by compression and possible exposure of the cancer cells on the retropancreas dissection plane.2 To avoid such risks, Strasberg et al.3 reported a rational anterior approach, called radical antegrade modular pancreatosplenectomy (RAMPS) in 2003. However, en bloc resection of the thick nerve plexus (NP), including lymph nodes around the root of the celiac artery (CeA) or superior mesenteric artery (SMA), is still not easy in RAMPS. Furthermore, the end-point of the vertical resection in radical DP is the roots of CeA and SMA, which are covered by the thick NP. Hanging maneuver was developed by Belghiti et al.4 for liver surgery. In this maneuver for hepatectomy, a tape is passed behind the liver to guide the hepatic resection via the anterior approach. We have since applied the maneuver to outline the proper sagittal dissection plane in performing antegrade en bloc DP.
Methods
Via a bilateral subcostal incision with midline extension, the gastrocolic ligament is opened, followed by the division of the root of the splenic artery. A blunt Pean clamp is passed across the cranial surface of the CeA, followed by division of the cranial side of the celiac NP and exposure of the origin of the CeA. A vessel sealing system (VSS; LigaSure Atlas and V™, Valleylab Inc., Boulder, CO, USA) is used for the division of the thick nerve tissues. Secure ligations or the use of ultrasonic coagulation sheers (SonoSurg®, Olympus Surgical & Industrial Inc., Tokyo, Japan) might be the alternatives. Even under the use of VSS, the distal end of the thick nerve plexus should be ligated off and divided to prevent chylous leakage. All the short gastric vessels are also divided using the VSS.5
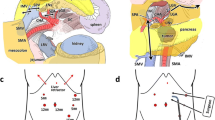
After performing the full right-side visceral rotation, the NP on the dorsal side of the proximal SMA is opened, and the origin of the SMA is exposed and controlled. Then, a blunt Kelly clamp is passed cranially through a space between the root of the SMA and the left NP lateral to the SMA (Fig. 1a), reaching a space between the CeA and left NP lateral to the CeA (Fig. 1b). The anatomical relationship between the SMA, CeA, and the aorta should have been be checked by CT prior to the procedures. Recognition of the clockwise position of the origins of the CeA and the SMA from the aorta may help the secure passing of the clamp along the roots of such major arteries. A 10-Fr plastic tape (ATOM™ tube, ATOM Medical Inc., Tokyo, Japan) is passed through the newly created tunnel, and this tape guides the end-point of the sagittal resection process during the antegrade en bloc DP.
The anterior NP is pulled up and divided, and the splenic vein is preserved (a). The left NP is pulled up by the tape, which was previously passed through a space between the origin of the major arteries and the left NP (b). Black arrows indicate the cranial side. CeA celiac artery, NP nerve, SMA superior mesenteric artery
The common hepatic artery is isolated from the pancreas, and the dissection is continued to expose the caudal surface of the CeA from the NP. The anterior surface of the superior mesenteric vein is identified at the root of the transverse mesocolon. A blunt Pean clamp is passed across the superior mesenteric vein behind the pancreas neck, which is divided by electrocautery. The main pancreatic duct should be securely ligated, and the cut surface of the remnant pancreas is heat-coagulated using soft coagulation system (VIO 300™, ERBE Inc., Marietta, GA, USA).
The pancreatomesocolic ligament is divided to the left. Then, the plane of the dissection proceeds vertically, dividing the Toldt’s fusion fascia, and the left renal vein is exposed as the marker of the caudal border of the en bloc DP. Without dividing the splenic vein to prevent congestive bleeding from the distal pancreas, another plastic tape is passed along the anterior surface of the SMA to the caudal surface of the CeA (Fig. 1a). By pulling up the tape, the thick NP lying anterior to the SMA is safely divided. The left NP is divided at the level of the left renal vein. The plastic tape within the space between the left NP and the origins of the SMA and CeA is hanged to safely divide the left NP (Fig. 1b).
At this point, the dissection plane goes laterally. The division of the left celiac ganglion enables us to identify the anterior surface of the adrenal vein. If the tumor appears to invade the retropancreatic fat tissue, the left adrenal vein should be ligated and the dissection plane goes under the adrenal gland and across the renal capsule. The anchor sutures, which were made on the cut edge of the distal pancreas, need to be pulled up to visualize the retroperitoneal dissecting plane visible. After dissecting the splenorenal ligament, the complete total distal pancreas, including vessels, nodes, NP, and retropancreas tissues, are excised en bloc.
Results
Between April 2009 and May 2010, ADDPH was performed in six patients (four males and two females) with the mean age of 67 years. The indication for DP included invasive ductal carcinoma (n = 4) and invasive mucous carcinoma (n = 2). Two patients had prior acute pancreatitis with pseudocyst formation. The other two patients had received radiation therapy before surgery. The mean tumor size was 3.0 ± 0.9 cm. The mean duration of surgery and blood loss were 258 ± 71 min and 226 ± 240 ml, respectively. For three of the six patients, a deeper posterior plane was needed to remove the left adrenal gland and expose the anterior surface of the left kidney.
Although none of the patients died during surgery, four patients had postoperative complications, including chylous ascites (n = 3) and delayed gastric emptying (n = 1). Tumor invasion was seen in the posterior peripancreatic fat close to the adrenal gland in four (65%) patients. Perineural invasion was present in four (65%) patients, and the tumor invasion was present in the NP around the CeA and SMA in one (17%) patient. Negative surgical margins were obtained in all six patients (100%). Although the observation period was short, all the patients are alive and without recurrence at 2, 5, 8, 11, 12, and 13 months, respectively.
Discussion
The basic principles in surgery for malignancies include no-touch isolation technique, negative cancer cells at the resected margin, and en bloc resection.6–8 In previous reports, the positive resection margin ranged from 25% to 28% for DP performed using conventional lateroposterior approach.9,10 Moreover, in the conventional approach, the thick plate of the NP and celiac ganglion around the CeA, SMA, and aorta block the inward retroperitoneal dissection during mobilization, and it becomes difficult to perform en bloc resection of the structures. Therefore, the anterior approach in DP is a rational technique, as Strasberg et al.3 reported. However, the application of radical anterior approach is potentially difficult because the end-point of the sagittal resection process cannot be controlled in the original approach. The end-point of the vertical resection process in radical DP is the origins of CeA and SMA, which are covered by the thick NP. Thus, a vertical division via the anterior approach down to the level of anterior surface of the aorta may cause serious injury of the vessels or organs. Although previous randomized trials in pancreatoduodenectomy for pancreas head cancers revealed no beneficial impacts of extended para-aortic lymph nodes dissection on survivals,11 it has also been reported that only regional R0 resection can only offer the chance of survivals.7,12 The current technique aims for en bloc R0 resection under no-touch technique.
Antegrade en bloc distal pancreatectomy with plexus hanging maneuver (ADPPH) is a secure technique, offering advantages in DP via the anterior approach over the classical technique. First, the tape positioned at the left side of the roots of the CeA and the SMA helps the surgeon to identify the optimal line for the vertical pancreas resection easily. This concept, using the hanging maneuver, was previously reported for complex hepatic resections.13,14 The most significant role of the hanging maneuver in ADPPH is the tape that elevates the thick nerve plexus on the root of the CeA and SMA and outlines a proper dissection plane from the anterior to posterior. It also protects the structures behind it: CeA, SMA, left renal vein, and aorta. A surgeon can simply follow the tape for deeper dissection, and the division of the tissue anterior to the tape results in the completion of the sagittal resection down to the root of the CeA and SMA. Moreover, by using the hanging maneuver to divide the NP anterior to the SMA, the splenic vein could be kept open until the final stage of the surgery. Occlusion or division of the splenic vein in an early stage of DP sometimes causes intraoperative congestive bleeding because the vein is the only major drainage vein from the distal pancreas. We believe that the small amount of blood loss during radical ADPPH (226 ± 240 ml) may be due to preservation of the splenic vein.
The incidence of chylous ascites was high (50%) ascites in the current technique. However, daily output of the chylous ascites from the abdominal drain was less than 100 ml in all the three patients, and non-per oral for several days cured all the chylous ascites. In ADPPH, the left-side half of the periarterial plexus along the CeA and SMA is completely cleared. Therefore, we speculate that the division of the small lymphatic nests around the proximal SMA resulted in such complications.
In summary, the use of hanging maneuver is useful when performing radical DP via the anterior approach. DPAH is an innovative, safe, and rational technique and is applicable for the resection of distal pancreas malignancies.
Abbreviations
- ADPPH:
-
Antegrade en bloc distal pancreatectomy with plexus hanging maneuver
- CeA:
-
Celiac artery
- DP:
-
Distal pancreatectomy
- NP:
-
Nerve plexus
- RAMPS:
-
Radical antegrade modular pancreatosplenectomy
- SMA:
-
Superior mesenteric artery
References
Fabre JM, Houry S, Manderscheid JC, et al. Surgery for left-sided pancreatic cancer. Br J Surg. 1996;83:1065–70.
Hirota M, Kanemitsu K, Takamori H, et al. Pancreatoduodenectomy using a no-touch isolation technique. Am J Surg. 2010;199:e65–8.
Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133:521–7.
Belghiti J, Guevara OA, Noun R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–11.
Ikegami T, Toshima T, Takeishi K, et al. Bloodless splenectomy during liver transplantation for terminal liver diseases with portal hypertension. J Am Coll Surg. 2009;208:e1–4.
Turnbull RB Jr, Kyle K, Watson FR, et al. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166:420–7.
Fatima J, Schnelldorfer T, Barton J, et al. Pancreatoduodenectomy for ductal adenocarcinoma: implications of positive margin on survival. Arch Surg. 2010;145:167–72.
Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–62.
Shoup M, Conlon KC, Klimstra D, et al. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Gastrointest Surg. 2003;7:946–52
O’Morchoe CC. Lymphatic system of the pancreas. Microsc Res Tech. 1997;37:456–77.
Yokoyama Y, Nimura Y, Nagino M. Advances in the treatment of pancreatic cancer: limitations of surgery and evaluation of new therapeutic strategies. Surg Today. 2009;39:466–75.
Hirano S, Kondo S, Hara T, et al. Distal pancreatectomy with en bloc celiac axis resection for locally advanced pancreatic body cancer: long-term results. Ann Surg. 2007;246:46–51.
Kim SH, Park SJ, Lee SA, et al. Various liver resections using hanging maneuver by three Glisson’s pedicles and three hepatic veins. Ann Surg. 2007;245:201–5.
López-Andújar R, Montalvá E, Bruna M, et al. Step-by-step isolated resection of segment 1 of the liver using the hanging maneuver. Am J Surg. 2009;198:e42–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikegami, T., Maeda, T., Oki, E. et al. Antegrade En Bloc Distal Pancreatectomy with Plexus Hanging Maneuver. J Gastrointest Surg 15, 690–693 (2011). https://doi.org/10.1007/s11605-010-1382-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1382-9