Abstract
Introduction
Postsurgical gastric atony occurs infrequently after gastric surgery. However, the symptoms are disabling and refractory to medical management. The only effective treatment is completion gastrectomy. A few studies have examined in detail the long-term results of this radical procedure.
Methods
From 1988 through 2007, 44 patients (84% female, 16% male) underwent near-total or total completion gastrectomies for refractory postsurgical gastric atony. The average age was 52 (range 32–72). Gastric atony was documented using radionuclide solid food emptying studies. Charts were reviewed retrospectively to identify preoperative symptoms and long-term postoperative function, and the patients were contacted by phone to evaluate their current level of function.
Results
Of the original 44 patients, 66% (n = 29) were evaluated postoperatively at a mean of 5.6 + 4.5 years (range 0.5–15.0 years). Fourteen patients (32%) had died, and seven (16%) were lost to follow-up. Most common presenting symptoms were abdominal pain (98%), vomiting (98%), nausea (77%), diet limitation (75%), heartburn (64%), and weight loss (59%, average = 19% of BW). Postoperative complications occurred in 36% (n = 16), most commonly bowel obstruction (11%), anastomotic stricture (9%), and anastomotic leak (7%), and there was one perioperative death. At last follow-up, there were significant improvements in abdominal pain (97% to 59%, p < 0.001), vomiting (97% to 31%, p < 0.001), nausea (86% to 45%, p < 0.001), and diet limited to liquids or nothing at all (57% to 7%, p < 0.001). Some symptoms were more common postoperatively, including early satiety (24% to 89%, p < 0.001), and postprandial fullness (10% to 72%, p < 0.001). Average BMI at the time of surgery and at last follow-up were 23 and 21, respectively. Osteoporosis was diagnosed pre- and postoperatively in 17% and 67% of patients, respectively (p < 0.001). Seventy-eight percent of patients stated that they were in better health after surgery, while 17% were neutral, and 6% stated that they were worse off. Mean satisfaction with surgery was 4.7 (1–5 Likert scale).
Conclusion
Completion gastrectomies in this patient population resulted in significant improvements in abdominal pain, vomiting, nausea, and severe diet limitations. Most patients, however, have significant ongoing gastrointestinal complaints, and the incidence of osteoporosis is high. Patient satisfaction is high; about 78% of patients believed their health status is improved. We believe these data support the selective use of completion gastrectomies in patients with severe postsurgical gastroparesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postgastrectomy syndromes occur commonly after gastric procedures for peptic ulcer or cancer. These syndromes can be divided into two categories: postcibal and nutritional. The nutritional consequences of gastric surgery include malabsorption of calories, iron, and nutrients. Weight loss is common due to fear of eating caused by postcibal symptoms and loss of appetite. The postcibal syndromes are largely due to motility disorders such as rapid gastric emptying or delayed gastric emptying.1 Postsurgical gastric atony (PSGA) or gastroparesis is an uncommon but devastating consequence of gastric surgery, characterized by nausea, vomiting, abdominal pain, early satiety, bloating, and weight loss. In severe cases, patients with PSGA require frequent hospitalizations and become dependent on parenteral nutrition.2
Acute gastroparesis occurs in as many as 50% of patients undergoing gastric resection or other procedures involving the stomach.3 It usually resolves within a few days without intervention. Chronic gastric atony or gastroparesis typically develops years later and is not always preceded by acute gastroparesis. Chronic PSGA is thought to be related to vagal nerve injury as well as the anatomic changes resulting from surgery.2,4 Although patients who develop gastroparesis following truncal vagotomy and drainage may have rapid initial emptying of liquids due to impaired fundic relaxation with loss of reservoir function, they will not empty solids normally due to an absence of the normal lag phase for solids.4,5,6
The incidence of severe PSGA is probably less than 5% in patients after most operations for peptic ulcer.3,7 The incidence is much higher after secondary procedures for postgastrectomy syndromes, such as Roux-en-Y diversion procedures in patients thought to have bile-reflux gastritis.7,8,9,10 Medical therapy for PSGA consists of dietary restrictions and prokinetic medications such as metoclopramide, erythromycin, and domperidone.2,11 However, these medications have significant side effect profiles and usually have little effect on the symptoms. Many patients become addicted to narcotic analgesics which further impair gastric emptying.2
Most reports describing the results of surgery for intractable PSGA are small with incomplete symptom evaluation and short follow-up. One large series concluded that only 43% of patients had good results after gastrectomy for PSGA.12 On the other hand, several smaller series and one other large series reported successful reduction of symptoms in as many as 80% of patients.6,13–20 The goal of this study was to analyze our series of patients who have undergone completion gastrectomies for documented PSGA, by comparing preoperative symptoms to long-term postoperative symptoms and health status, and to evaluate patient satisfaction with the procedure.
Materials and Methods
Study Participants
A search of the medical records by CPT code for all patients who underwent total or near-total gastrectomies for documented postsurgical gastroparesis by the primary gastric surgeon at our institution was performed. Inclusion criteria were all patients that had total or near-total gastrectomies for documented gastric atony after previous gastric surgery. Exclusion criteria were any patients under the age of 18 and any patients that chose to opt out of the study. Between 1988 and 2007, a total of 44 patients underwent total or near-total gastrectomy by a single surgeon at our institution for documented postsurgical gastric atony. No patients were excluded based on age, and none chose to opt out of the study. Gastroparesis was documented in all 44 patients by symptomology and confirmed with radionuclide solid food emptying studies. Only one patient (2%) was unable to tolerate the solid food emptying study, and while they did have a normal liquid emptying study, their symptoms, combined with the fact that they were unable to tolerate the solid food study, were considered sufficient documentation of gastroparesis. Gastric outlet obstruction was ruled out by endoscopic evaluation of the stomach. Symptoms of gastroparesis were considered to be nausea, vomiting, abdominal pain, diet limitation, weight loss, bloating, early satiety, and postprandial fullness. Data was extracted on all of these patients from the medical record, including demographics, symptoms, functional status, health status, previous operations, diagnostic studies, operation performed, postoperative course, morbidity and mortality, and short- and long-term follow-up.
Follow-up
Long-term follow-up was performed by searching the medical record and contacting the patients. The Social Security Death Index was consulted to identify deceased patients, and all other patients were contacted by mail at their last known address to advise them that the study was in progress, and that they would be contacted with a survey about their current health status. Contact was attempted with all living patients using their last known phone number, and when reached, a phone survey was conducted regarding their current symptoms, health status, diet and weight status, and patient satisfaction. All phone interviews were conducted by individuals without prior contact with the patients. In the event that a living patient could not be reached by phone, a paper copy of the same survey was mailed to their last known address with instructions to fill out and return. Patient satisfaction was evaluated both by directly asking patients if they felt better now with respect to their gastroparesis symptoms than they did prior to their operation and by asking them to rate their satisfaction on a 1–5 Likert scale (1 = not satisfied, 2 = somewhat dissatisfied, 3 = neutral, 4 = somewhat satisfied, 5 = very satisfied). Twenty-nine patients (66%) were evaluated postoperatively at a mean (+SD) of 5.6 + 4.5 years (range 0.5–15.0 years). Fourteen patients (32%) were deceased, and seven (16%) were lost to follow-up.
Demographics
Thirty-seven (84%) patients were female and seven (16%) were male, with an average age of 52 (range 32–72). Average BMI prior to operation was 23 kg/m2 (range 15 to 34). Average weight loss was 19% of their total body weight between the onset of symptoms and their completion gastrectomy. Sixty-three percent of patients had a history of chronic narcotic use for pain related to their gastroparesis symptoms. Presenting symptoms are listed in Table 1, with the most common presenting symptoms being abdominal pain (98%), vomiting (98%), nausea (77%), and any diet limitation (75%).
We noticed that the pain reported in these patients was consistent in character and location, particularly those who had had prior antrectomies. Pain typically occurred minutes after eating a meal and was consistently centered at or just above the left costal margin about 4 cm to the left of the midline. Patients consistently cup the fingers of the left hand with the fingers hooking the left costal margin when asked to localize their pain.
Fifty-six percent of patients reported a diet limited to liquids or nothing at all, and 11% reported being unable to tolerate any oral nutrients [i.e., total parenteral nutrition (TPN) or jejunostomy tube dependent]. Other symptoms upon initial presentation were heartburn (64%), diarrhea (34%), bloating (25%), early satiety (23%), and postprandial fullness (11%). Preoperatively, anemia was reported in 58% of patients, osteoporosis in 19%, and diabetes in 12%. The initial operations are shown in Table 2. Our patients had undergone an average of 2.5 previous gastric operations; 61% had had two or more previous gastric operations. Two patients (5%) previously had a gastric electrical stimulator placed. The indications for the original operations were peptic ulcer disease (75%), morbid obesity (11%), GERD (9%), and bile reflux (5%). Most patients, 35 out of 44, had vagotomies performed at their index operations. In the remaining nine patients with complications of bariatric surgery or anti-reflux surgery, it was our opinion that vagal damage was a contributing factor in their gastric atony. Congo red testing was used to confirm abnormal vagal innervation in these patients. Early in our experience, we performed preoperative vagotomy testing (sham feeding and/or Congo red testing) in all patients. In a previous report,21 we concluded that vagotomy testing affected operative planning or intraoperative decision making in many patients, since complete vagotomy is an essential component of any Roux-en-Y gastrojejunostomy. Of patients tested, about 20% had evidence of persistent vagal innervation or incomplete vagotomy. More recently, in patients undergoing total or near-total (less than 20 cc remnant) gastrectomies, we have not routinely performed vagotomy testing
Operation
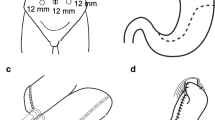
Of the 44 patients, 19 (43%) underwent total completion gastrectomies with esophageal transection and Roux-en-Y esophagojejunostomy reconstruction. Esophagojejunostomy was performed using an end-esophagus to side-jejunum anvil stapler technique. Twenty-five (57%) patients underwent near-total completion gastrectomies with a small cuff of stomach tissue left in place (range, small rim of tissue to 25 cc pouch). The anastomosis in these patients was either a two-layer, hand-sewn end to side anastomosis, or alternatively, a stapled end to side anastomosis. Sixty-seven percent of patients had feeding jejunostomies placed at the time of operation.
Data Analysis
All data presented are reported as the mean (+SD) except where noted. The frequency of preoperative versus postoperative symptoms and health status data was evaluated for statistical significance using Fisher’s exact test. A p value of <0.05 was considered to be statistically significant.
Results
Outcomes
Detailed long-term (> 6 months) follow-up was available in 29 (66%) of the 44 patients at a mean of 5.6 + 4.5 years (range 0.5–15.0 years). Long-term follow-up was unavailable in 15 patients, none of whom were listed in the Social Security Death Index. Symptoms of gastroparesis at follow-up were compared to preoperative presentation in the same patients (paired t test). The records were specifically searched to quantify the frequency of the typical left upper quadrant pain descriptor. The location of the pain, when specifically mentioned in the medical record, was classic in 83% of patients.
Table 1 shows symptoms that were improved at follow-up after completion gastrectomies. Patients had improvement in abdominal pain (97% to 59%, p < 0.001), vomiting (97% to 31%, p < 0.001), nausea (86% to 45%, p < 0.001), and diet limited to liquids or nothing at all (57% to 7%, p < 0.001). Symptoms including any limitation in diet (71% to 46%, p = 0.06) and heartburn (62% to 35%, p = 0.06) trended toward improvement at postoperative follow-up. As shown in Table 1, symptoms at presentation were similar in all patients and in those available for long-term follow-up.
Table 1 shows symptoms that were worsened at follow-up. Significant increases were seen in the percent of patients reporting early satiety (24% to 89%, p < 0.001) and postprandial fullness (10% to 72%, p < 0.001). Increased frequencies of bloating (21% to 39%, p = 0.18) and diarrhea (34% to 41%, p = 0.59) were not statistically significant.
Table 3 shows the effect of operation on several health measures. Average BMI decreased from 23.4 + 4.5, preoperatively, to 20.7 + 4.3 at follow-up. Incidence of osteoporosis was significantly increased postoperatively (17% to 67%, p < 0.001). Incidence of anemia (59% to 67%, p = 0.58) and diabetes (10% to 11%, p = 1) were unchanged. Although patients reported less pain, the frequency of chronic narcotic use was 59% and 62% pre- and postoperatively, respectively.
Patient satisfaction was measured in two ways. Patients were asked whether they were better off with respect to their gastroparesis symptoms at follow-up; 78% reported that they were in better health. Seventeen percent reported that their health status was unchanged, and 6% reported that they were worse. When asked to rate their satisfaction with their surgery on a 1–5 Likert Scale (1 not satisfied, 2 somewhat dissatisfied, 3 neutral, 4 somewhat satisfied, 5 very satisfied), the average response was 4.7 + 0.6 (1 = 0%, 2 = 0% , 3 = 6%, 4 = 18%, and 5 = 76%).
Morbidity and Mortality
There was one in-hospital, postoperative death. This patient had a near-total resection and developed a leak at the gastrojejunostomy leading to sepsis and respiratory failure, and care was withdrawn approximately 3 weeks postoperatively. One or more complications occurred in 16 of 44 patients (36%). Small bowel obstruction was the most common complication, occurring in five patients (11%). Three of the five small bowel obstructions occurred at the site of the feeding jejunostomy. Thus, three out of 26 or 12% of patients having jejunostomies placed had known bowel obstructions related to their feeding catheters. Anastomotic strictures occurred in four patients (9%), all of which had undergone a total gastrectomy with esophagojejunostomy. All were treated successfully with endoscopic dilation. Anastomotic leak occurred in three patients (7%), two of which had undergone near-total gastrectomy and one of which had undergone total gastrectomy. Other reported complications included wound infection (7%), intra-abdominal abscess (5%), pancreatic fistula (2%), jejuno-cutaneous fistula (2%), and bile reflux (2%). There have been no known marginal ulcers. At follow-up, 14 patients (32%) were found to be deceased at an average age of 57 + 10.1 years (range 35 to 74 years of age) and at an average of 3.8 + 2.7 years postoperatively (range, 0.1 to 9.0 years). Causes of death were pneumonia in three patients, multiple organ failure, and cerebral vascular accident, all unrelated to the surgical procedure. The cause of death was unable to be determined from our records in eight of the 14 patients (57%) who were deceased at the time of the study.
Feeding Jejunostomy
Feeding jejunostomy data was available in 39 of the 44 patients. Of these, 26 (67%) had feeding jejunostomies placed during the initial operation. Twenty-three (88%) patients remained on at least supplemental alimentation through their feeding jejunostomies after discharge for a minimum of 2 weeks postoperatively. One or more complications directly related to feeding jejunostomy placement occurred in four of the 26 patients (15%). The median duration of use of the feeding jejunostomy was 27 days (range 5–1,475 days), and one patient (4%) was still on supplemental feeding through their jejunostomy tube at long-term follow-up (1,475 days postoperatively).
Discussion
The present study represents the third largest reported series describing the results of completion gastrectomies in patients with postsurgical gastric atony. These patients tend to be women (85%) and had, on average, 2.5 previous gastric operations. This female to male ratio is the same as that seen in patients with idiopathic gastroparesis and is consistent with the hypothesis that many of these patients had impaired gastric emptying prior to their original gastric operation. In addition, a significant number had ill-advised Roux-en-Y diversions for bile reflux. We believe that the primary pathologic state in most patients with the bile reflux syndrome after gastric operations is impaired emptying and/or clearance of bile, not pathologic reflux. This distinction is critical, since the most commonly performed remedial procedure in these patients, a Roux-en-Y diversion, will exchange one syndrome (bile reflux gastritis) for a worse syndrome (the Roux syndrome). In our opinion, the majority of patients with bile-reflux gastritis who need operations should have total or near-total gastrectomies as their definitive operation.
Completion gastrectomy was successful in approximately 78% of patients, with significant reductions in incidence of abdominal pain, nausea, vomiting, and severe limitations in diet. While several studies have suggested similar success rates,6,13–20 Forstner-Barthell and co-workers from the Mayo Clinic reported a success rate of only 43% based upon classification of patients by Visick grade.12 In the Visick grading system, patients are classified according to the frequency and severity of symptoms. Thus, in the Mayo Clinic series, only 43% of patients were in the favorable Visick grades I–II. Most patients continue to have significant gastrointestinal symptoms after total gastrectomies, thus resulting in frequent Visick III–IV classification. In our opinion, this ignores the clinically and statistically significant improvement in most symptoms. That is, we believe that patients with significant improvements in almost all symptoms and improved health status after surgery should be classified as successes. For example, prior to surgery, 57% of our patients reported that their diet was limited to liquids or nothing at all. At postoperative follow-up, only 7% were limited to liquids or less, a reduction by 88% of patients whose diets were limited in this severe manner. We also found that 39% of patients had complete resolution of their abdominal pain, 68% had complete resolution of their vomiting, and 48% had complete resolution of their nausea. Many of our patients reported modest weight loss after surgery, with an average reduction in BMI of only 2.7 kg/m2. Other gastrointestinal symptoms were still common: 59% of patients still had some abdominal pain (most still used narcotics), 45% still had occasional nausea, 31% still had occasional vomiting, and 46% still had some type of diet limitation. The explanation for the lack of weight gain in many of our patients despite subjective improvement in their symptoms is unclear. Experience in patients with total gastrectomies for gastric cancer (10–20% loss in body weight) suggests that anorexia after gastrectomy is a very important contributor to weight loss. We now realize that pre- and postoperative consultation with dieticians with special interests in postgastrectomy syndromes is an essential part of care.
We noticed that the pain reported in these patients was consistent in character and location, particularly those who had had prior antrectomies. Pain typically occurred minutes after eating a meal and was consistently centered at or just above the left costal margin about 4 cm to the left of the midline. Patients consistently cup the fingers of the left hand with the fingers hooking the left costal margin when asked to localize their pain. PSGA is the only known condition that presents in this manner with chronic postprandial pain centered at or just above the left costal margin about 4 cm to the left of the midline. This sign was seen in 83% of the patients in our study.
Osteoporosis, a well-known complication of total gastrectomy, was common in our patient population. Absorption of calcium has been shown to be normal in most patients after gastric resections.22 Malabsorption of fats and fat-soluble vitamins due to a decrease in pancreatic enzyme secretion and poor mixing likely contributes to osteoporosis. Previous studies of postgastrectomy patients have documented abnormal bone biopsies, elevated serum alkaline phosphatase, and parathyroid hormone levels, and decreased serum 25-hydroxy vitamin D levels.1 These patients are prone to pathologic fractures. Standard of care in these patients involves supplementation of vitamin B12, folate, iron, calcium, and vitamin D. Although there are no studies that have demonstrated convincingly that supplementation in this manner will decrease the likelihood of developing bone disease, we recommend that postgastrectomy patients be monitored closely postoperatively for development of osteoporosis. Many of our patients did not supplement their diets as prescribed.
Feeding jejunostomies were placed in a majority of our patients, and in the last several years have been placed in all of our completion gastrectomy patients. A majority of patients were discharged on at least partial tube feedings with the median duration of use of the feeding jejunostomy being 27 days. Although jejunostomy tubes resulted in small bowel obstructions in about 10% of patients (a frequency similar to that in the trauma literature), we believe the benefits are greater than the risk. Others have utilized tube duodenostomies for temporary alimentation in this patient population. We recommend that all patients undergoing this procedure for PSGA have feeding catheters placed at the time of operation.
A Roux-en-Y gastrojejunostomy is an ulcerogenic operation. Thus, if any stomach is retained, the remnant must be very small (i.e., less than 20 cc pouch). If more stomach is retained, the risk for marginal ulceration is significant, and preoperative vagotomy testing and repeat vagotomy should be strongly considered. Furthermore, we believe the risk for recurrent symptoms of poor emptying is likely if the remnant is larger than 20–30 cc. The size of the gastric remnant must be very small. We have begun to use the near-total gastric resections with Roux-en-Y gastrojejunostomy reconstructions in these patients whenever possible. We feel as though this operation is safer than a total gastrectomy, as esophagojejunostomy with a small cuff of stomach provides a safer anastomotic conduit. Reported leak rates in the literature are between 2% and 9% for esophagojejunostomy after total gastrectomy,23–25 versus the standard leak rate of less than one percent reported in recent bariatric literature after a standard Roux-en-Y gastric bypass.26,27 Furthermore, all four of our patients who had postoperative anastomotic strictures requiring dilatation had undergone a total resection with esophagojejunostomy. In some patients, leaving even a small gastric remnant is impossible, due to the nature of the previous operations, adhesions, and distortion at the gastroesophageal junction. Thus, total gastrectomy with esophagojejunostomy may be necessary. However, we recommend performing near-total resection with gastrojejunostomy in most patients.
The number of patients for whom long-term follow-up was not available, mainly due to the number of deceased patients, is a limitation of this study. Most of these deaths were not directly related to their gastric operation or their gastrointestinal complaints, but rather to more chronic medical conditions. However, a lack of proper nutrition may lead to an increase in chronic medical conditions and may have been an underlying cause of death in some of these patients.28,29 The number of deaths in our study raises the concern that the poor nutritional status of this patient population, both before and after surgery, may adversely affect longevity.
In summary, we found that patients undergoing completion gastrectomies for refractory, documented PSGA have statistically significant reductions in abdominal pain, nausea, vomiting, and severe diet limitations. However, most patients still have significant gastrointestinal complaints, and further weight loss is common. The incidence of osteoporosis is increased and must be monitored closely. Feeding jejunostomies should be placed and used in all patients. Patients are satisfied with the results of their operation. A majority (78%) of our patients stated that they felt better as a result of their surgery, and the overall satisfaction rating was high.
References
Clark CJ, Thirlby RC, Picozzi V Jr, Schembre DB, Cummings FP, Lin E. Current problems in surgery: gastric cancer. Curr Probl Surg 2006;43:566–670. doi:10.1067/j.cpsurg.2006.06.003.
Schirmer BD. Gastric atony and the Roux syndrome. Gastroenterol Clin North Am 1994;23:327–343.
Dong K, Yu XJ, Li B, Wen EG, Xiong W, Guan QL. Advances in mechanisms of postsurgical gastroparesis syndrome and its diagnosis and treatment. Chin J Dig Dis 2006;7:76–82. doi:10.1111/j.1443-9573.2006.00255.x.
Dong K, Li B, Guan QL, Huang T. Analysis of multiple factors of postsurgical gastroparesis syndrome after pancreaticoduodenectomy and cryotherapy for pancreatic cancer. World J Gastroenterol 2004;10:2434–2438.
Azpiroz F, Malagelada JR. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology 1987;92:934–943.
Eckhauser FE, Conrad M, Knol JA, Mulholland MW, Colletti LM. Safety and long-term durability of completion gastrectomy in 81 patients with postsurgical gastroparesis syndrome. Am Surg 1998;64:711–716.
Hirao M, Fujitani K, Tsujinaka T. Delayed gastric emptying after distal gastrectomy for gastric cancer. Hepatogastroenterology 2005;52:305–309.
McAlhany JC Jr, Hanover TM, Taylor SM, Sticca RP, Ashmore JD Jr. Long-term follow-up of patients with Roux-en-Y gastrojejunostomy for gastric disease. Ann Surg 1994;219:451–455. doi:10.1097/00000658-199405000-00002.
Britton JP, Johnston D, Ward DC, Axon AT, Barker MC. Gastric emptying and clinical outcome after Roux-en-Y diversion. Br J Surg 1987;74:900–904. doi:10.1002/bjs.1800741010.
Pellegrini CA, Patti MG, Lewin M, Way LW. Alkaline reflux gastritis and the effect of biliary diversion on gastric emptying of solid food. Am J Surg 1985;150:166–171. doi:10.1016/0002-9610(85)90027-3.
Patterson DJ. Prokinetic agents in postgastrectomy patients. Gastroenterol Clin North Am 1994;23:313–325.
Forstner-Barthell AW, Murr MM, Nitecki S, Camilleri M, Prather CM, Kelly KA, Sarr MG. Near-total completion gastrectomy for severe postvagotomy gastric stasis: analysis of early and long-term results in 62 patients. J Gastrointest Surg 1999;3:15–21. discussion doi:10.1016/S1091-255X(99)80003-1.
Jones MP, Maganti K. A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol 2003;98:2122–2129. doi:10.1111/j.1572-0241.2003.07721.x.
Eckhauser FE, Knol JA, Raper SA, Guice KS. Completion gastrectomy for postsurgical gastroparesis syndrome. Preliminary results with 15 patients. Ann Surg 1988;208:345–353. doi:10.1097/00000658-198809000-00012.
Karlstrom L, Kelly KA. Roux-Y gastrectomy for chronic gastric atony. Am J Surg 1989;157:44–49. doi:10.1016/0002-9610(89)90418-2.
Vogel SB, Woodward ER. The surgical treatment of chronic gastric atony following Roux-Y diversion for alkaline reflux gastritis. Ann Surg 1989;209:756–761. doi:10.1097/00000658-198906000-00013.
McCallum RW, Polepalle SC, Schirmer B. Completion gastrectomy for refractory gastroparesis following surgery for peptic ulcer disease. Long-term follow-up with subjective and objective parameters. Dig Dis Sci 1991;36:1556–1561. doi:10.1007/BF01296397.
Hinder RA, Esser J, DeMeester TR. Management of gastric emptying disorders following the Roux-en-Y procedure. Surgery 1988;104:765–772.
Farahmand M, Sheppard BC, Deveney CW, Deveney KE, Crass RA. Long-term outcome of completion gastrectomy for nonmalignant disease. J Gastrointest Surg 1997;1:182–187. doi:10.1016/S1091-255X(97)80107-2.
Gustavsson S, Kelly KA. Total gastrectomy for benign disease. Surg Clin North Am 1987;67:539–550.
Bradshaw BGG, Thirlby RC. The value of sham-feeding tests in patients with post-gastrectomy syndromes. Arch Surg 1993;128:982–987.
Meyer JH. Nutritional outcomes of gastric operations. Gastroenterol Clin North Am 1994;23:227–260.
Pol B, LeTreut YP, Hardwigsen J, Rosset E, Houvenaeghel G, Delpero JR. Mechanically stapled esophagojejunostomy. Results of a prospective series of 176 cases. Hepatogastroenterology 1997;44:458–466.
Viste A, Eide GE, Soreide O. Stomach cancer: a prospective study of anastomotic failure following total gastrectomy. Acta Chir Scand 1987;153:303–306.
Sannohe Y, Hiratsuka R, Doki K. Single layer suture by manual or mechanical stapling technique in esophagojejunostomy after total gastrectomy. Am J Surg 1981;142:403–406. doi:10.1016/0002-9610(81)90361-5.
Agaba EA, Shamseddeen H, Gentles CV, Sasthakonar V, Gellman L, Gadaleta D. Laparoscopic vs open gastric bypass in the management of morbid obesity: a 7-year retrospective study of 1,364 patients from a single center. Obes Surg 2008;18:1359–1363. doi:10.1007/s11695-008-9455-5.
Jones KB Jr, Afram JD, Benotti PN, Capella RF, Cooper CG, Flanagan L, Hendrick S, Howell LM, Jaroch MT, Kole K, Lirio OC, Sapala JA, Schuhknecht MP, Shapiro RP, Sweet WA, Wood MH. Open versus laparoscopic Roux-en-Y gastric bypass: a comparative study of over 25,000 open cases and the major laparoscopic bariatric reported series. Obes Surg 2006;16:721–727. doi:10.1381/096089206777346628.
Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr 2008;27:5–15. doi:10.1016/j.clnu.2007.10.007.
Ely JJ. Inadequate levels of essential nutrients in developed nations as a risk factor for disease: a review. Rev Environ Health 2003;18:111–129.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Speicher, J.E., Thirlby, R.C., Burggraaf, J. et al. Results of Completion Gastrectomies in 44 Patients with Postsurgical Gastric Atony. J Gastrointest Surg 13, 874–880 (2009). https://doi.org/10.1007/s11605-009-0821-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-0821-y