Abstract
Background
Gastric tube reconstruction is a form of esophagogastrostomy performed after laparoscopic proximal gastrectomy (LPG). It is a simple and safe technique, but it may cause reflux esophagitis (RE) and impair postsurgical QOL. For several years, we have developed the gastric tube reconstruction and performed it on more than 100 patients. This study aimed to determine whether gastric tube reconstruction can be a feasible choice after LPG in regard to surgical safety and postoperative nutritional status.
Methods
The subjects consisted of 171 patients who underwent LPG (n = 102) or laparoscopic total gastrectomy (LTG) (n = 69). We compared the two groups in terms of surgical outcomes, incidence rate of RE, and nutritional status including postoperative weight loss and hemoglobin levels.
Results
There were no significant differences with regard to the surgical duration and blood loss between the two groups. The incidence of RE was not significantly higher with LPG than with LTG (16.7% vs. 10.1%, respectively; P = 0.07). Later than 2 years and 6 months after surgery, the body weight percentage of preoperative body weight in the LPG group was significantly higher than that in the LTG group. Hemoglobin and ferritin levels in the LPG group were significantly higher than those in the LTG group, later than one after surgery. The overall survival rates were similar between the two groups (5-year survival rates: 97.1% vs. 94.2% in the LPG and LTG groups, respectively; P = 0.69).
Conclusions
Gastric tube reconstruction after LPG is simple and had better outcomes than LTG in terms of postoperative nutritional status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of upper third gastric cancer has gradually increased [1, 2]. Total gastrectomy (TG) including extensive lymph node dissection is traditional treatment in Japan and considered the standard operation for gastric cancer invading the upper third of the stomach [3]. Proximal gastrectomy (PG) is indicated for early gastric cancer (EGC) in the upper third of the stomach for cases in which at least half of the remnant stomach can be preserved. Although, some reports suggested that PG has advantages in regard to postoperative nutritional status compared with TG [4,5,6], recent studies have indicated that there was no significant difference in terms of hematologic and nutritional outcomes between PG and TG [7, 8]. With the increasingly widespread application of laparoscopic surgery, laparoscopic proximal gastrectomy (LPG) has been adaptable for EGC in the upper third of the stomach. However, LPG destroys anatomic anti-reflux barriers, which is composed of the lower esophageal sphincter (LES) and the angle of His. It can lead up to reflux esophagitis (RE) easily by regurgitation of acid. Esophagogastrostomy, one of the simplest reconstructions after PG, sometimes causes severe RE. We previously reported gastric tube reconstruction as a modified esophagogastrostomy following LPG in 2010 [9]. We have developed it for several years and performed it on more than 100 patients. Furthermore, our group presented that RE after gastric tube reconstruction following LPG gradually improved, as gastrointestinal motility recovered [10]. To prevent RE, several reconstructive procedures after LPG have been reported such as double-flap [11], double-tract [12, 13], and jejunal interposition [14], but these techniques are complicated, time-consuming, and sometimes unsatisfactory. This study aimed to determine whether gastric tube reconstruction can be a feasible choice after LPG in regard to surgical safety and postoperative nutritional status.
Subjects and methods
This retrospective study was approved by the Institutional Review Board and the local ethics committee of the Saitama Medical Center of Saitama Medical University (approval number 2280) and Gunma University and was performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki. Informed consent was obtained from all patients prior to participation for inclusion in the study.
Patients
We retrospectively reviewed a database of 171 patients with EGC in the upper third of the stomach who underwent gastric tube reconstruction following LPG or laparoscopic total gastrectomy (LTG) at Saitama Medical Center of Saitama Medical University or Gunma University Graduate School of Medicine from April 2006 to January 2017. The enrolled patients were diagnosed with clinical stage I gastric cancer in the upper stomach with no lymph node involvement (cT1-2N0M0), based on the Japanese Classification of Gastric Carcinoma [15]. The indications for LPG at our institutes are (1) gastric cancer in the upper third stomach with no lymph node involvement and (2) more than half of the distal stomach can be preserved.
Surgical techniques for gastric tube reconstruction and LPG
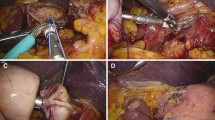
Five ports were introduced as shown in Fig. 1a. Before reconstruction, lymph nodes dissection was completed according to the Japanese guidelines [16]. The esophagus was transected with a Powered ECHELON 45mm stapler (Ethicon endo-surgery, Tokyo, Japan). An incision in the umbilical region was extended, and the stomach was extruded through it. The upper part of the stomach was excised along a dashed line to make a gastric tube (20 cm long, 4 cm wide) (Fig. 1b). An entry hole was made on the posterior side of the esophagus stump and on the anterior wall of the gastric tube 40mm distal from the proximal stump laparoscopically. Then, the linear stapler was applied between the anterior wall of the gastric tube and the posterior wall of the esophagus laparoscopically (Fig. 1c). After anastomosis was performed, the entry hole was closed with barbed suture or the linear stapler. The greater curvature side of the proximal stump of the gastric tube was folded over the anterior aspect of the esophagus. The wall of the gastric tube was secured to the right margin of the esophagus with two or three sutures, so that it was wrapped to the anterior aspect of the esophagus (around 180 degree of circumference of the esophagus) to prevent RE. The gastric tube was anchored with the right and left crus of the diaphragm by one stitch each to prevent hiatus hernia (Fig. 1d). Vagus nerve was not preserved.
Surgical procedure. (a) Port sites for LPG. (b) After the esophagus was transected, the upper part of the stomach was excised along a dashed line to create a gastric tube. (c) Under laparoscopy, the anterior wall of the gastric tube and the posterior wall of the esophagus were anastomosed with a 45-mm linear stapler. (d) After anastomosis was performed, the entry hole was closed with barbed suture or a linear stapler. The greater curvature side of the proximal stump of the gastric tube was folded over the anterior aspect of the esophagus. The wall of the gastric tube was secured to the right margin of the esophagus with two or three sutures, so that it was wrapped to the anterior aspect of the esophagus (around 180 degree of circumference of the esophagus) to prevent RE. The gastric tube was anchored with the right and left crus of the diaphragm by one stitch each to prevent hiatus hernia
Follow-up
Patients’ medical records were reviewed to analyze pathological variables and clinical outcomes including early complications. Pathological variables were classified based on the Japanese classification of gastric carcinoma [15]. Early postoperative complications were defined as any event requiring surgical, endoscopic, or radiological intervention. Patients were followed up every 3 months for 2 years after surgery, 6 months up to 5 years. Patients received laboratory tests, measurement of body weight, and assessment of clinical signs. Endoscopic examinations were performed annually after surgery. The degree of RE was classified according to the Los Angeles classification [17].
Statistical analysis
All statistical analyses were carried out using the JMP® version 13 software for Windows. Student’s t test was used to compare continuous variables, while Chi-square test or Fisher’s exact test was used to compare categorical variables. Kaplan–Meier method and log-rank test were used to compare the survival curves (in months) between the two groups. A P value < 0.05 was considered statistically significant.
Results
Patient clinical characteristics and operative results
The clinical and pathological characteristics were shown in Table 1. With regard to age (P = 0.335), gender (P = 0.986), body mass index (BMI; P = 0.291), and American Society of Anesthesiologists physical status (ASA-PS; P = 0.264), the two groups were comparable. There were no significant differences in terms of tumor histological type (P = 0.132). The number of patients with prior endoscopic submucosal dissection (ESD) in the gastric tube group was significantly larger than that in the LTG group (P = 0.031). The median tumor size in the gastric tube group was significantly smaller than that in the LTG group (29.0 vs. 41.1 mm, respectively; P = 0.01). No significant differences were observed in T factor, N factor, or pathological stage (P = 0.745, 0.121, and 0.543, respectively).
Mean duration of surgery was shorter with the gastric tube group than with the LTG group, but the difference was not significant (199.4 vs. 214.0 min, respectively; P = 0.345). Mean blood loss was less with the gastric tube group than with the LTG group, but the difference was not significant (120.2 vs. 132.3 mL, respectively; P = 0.643) (Table 2).
Postoperative complications
There was no case of mortality among the 171 study participants in the early stage. Three patients (2.9%) in the gastric tube group and one patient (1.4%) in the LTG group developed an anastomotic leakage (P = 0.322), but they were treated conservatively (Table 2).
Ten patients (9.8%) in the gastric tube group and two patients (2.9%) in the LTG group experienced anastomotic stricture (P = 0.08) that was treated by balloon dilatation under endoscopy. Two patients (1.9%) in the gastric tube group suffered from abdominal abscess and were treated conservatively. In the gastric tube group, one patient (0.9%) developed a pulmonary thromboembolism and was treated conservatively.
Seventeen patients (16.7%) in the gastric tube group and seven patients (10.1%) in the LTG group were diagnosed as RE (≥ Los Angeles grade A) at 1 year after surgery (P = 0.07).
Nutritional status
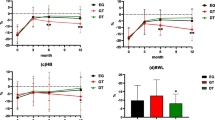
We compared postoperative weight loss and hemoglobin levels between the two groups. To calculate the percentage of preoperative value of each nutritional indicator after surgery, postoperative values were divided by preoperative values. After that, they were multiplied by 100. Figure 2a showed that the percentage of preoperative body weight in both groups declined steadily until 1 year and 6 months after surgery. However, the percentage in the gastric tube group started to increase at 1 year and 6 months after surgery. Later than 2 years and 6 months after surgery, the body weight percentage of preoperative body weight in the gastric tube group was significantly higher than that in the LTG group.
Comparison of the percentage change of the body weight and hemoglobin between the gastric tube group and the LTG group. All postoperative values are represented as mean ± standard deviation (SD). (a) There were significant differences between the two groups later than 2 years and 6 months after surgery. *P < 0.05. (b) There were significant differences between the two groups later than 1 year after surgery. *P < 0.05
Hemoglobin and ferritin levels in the gastric tube group were significantly higher than those in the LTG group later than 1 year after surgery (Figs. 2b and 3a). No significant differences between the two groups were observed in the levels of serum iron, total protein, and serum albumin at any time point (Fig. 3b, c, and d) .
(a) Ferritin levels in the gastric tube group were significantly higher than those in the LTG group later than 1 year after surgery. *P < 0.05. (b) No significant differences between the two groups were observed in the levels of serum iron at any time point. (c) No significant differences between the two groups were observed in the levels of total protein at any time point. (d) No significant differences between the two groups were observed in the levels of serum albumin at any time point. All postoperative values are represented as mean ± standard deviation (SD)
Survival data
One patient (0.9%) in the gastric tube group and two patients (2.9%) in the LTG group experienced recurrence. One patient after LPG died from meningeal dissemination recurrence. Two patients after LTG died from peritoneal dissemination. The overall survival rates were similar between the two groups. The overall 5-year survival rate of the gastric tube group and the LTG group were 97.1% and 94.2%, respectively, and not statistically different (P = 0.69) (Fig. 4).
Discussion
Our study showed that gastric tube reconstruction with LPG has advantages over LTG in regard to less postoperative anemia and less postsurgical weight loss. We believe that the gastric tube reconstruction could become a feasible choice after LPG for proximal EGC based on its safety and simplicity.
The utilization of a gastric tube provides a simple and safe anastomosis for LPG because it is a single anastomosis. Kitano et al. introduced a reconstruction method to use a gastric tube following LPG [18]. They suggested that the technique was simple and less invasive for EGC in the upper third of the stomach. The usefulness of the gastric tube reconstruction was evaluated to prevent RE after open PG [19]. They demonstrated that the gastric tube reconstruction was superior to jejunal interposition in terms of simplicity, safety, and less complexity. In 2017, we reported that the gastric tube reconstruction had advantages compared with jejunal interposition in terms of being less invasive, including shorter duration of surgery and less amount of operative blood loss and maintaining postoperative nutritional status [6]. Thus, we suggested that the gastric tube reconstruction is a feasible method from the perspective of low invasiveness. Although the differences were not significant, the shorter surgical duration and lesser intraoperative blood loss with gastric tube reconstruction were due to the number of anastomoses and the extent of lymph node dissection.
Some previous reports showed different types of complications of LPG. RE and anastomotic stenosis have been known as common complications after PG. In the present study, RE with symptoms was diagnosed in 17 (16.7%) of 102 patients. All of the patients with oral proton pump inhibitors achieved excellent or good response. This result was almost compatible with previous reports [20,21,22,23]. On the other hand, the incidence of anastomotic stenosis was also consistent with that of previous reports [20,21,22]. Balloon dilatation was found to be effective in the treatment of gastroesophageal anastomotic stenosis. Esophagogastrostomy is most convenient and simplest reconstruction, but the rate of RE (≥ Los Angeles grade A) has been reported to be over 30% [24, 25]. Some reports showed that the rate of anastomotic stenosis was around 20% [20, 25, 26]. Shoji et al. reported that the rate of RE (≥ Los Angeles grade B) after double-flap technique was 4.2% [11]. Moreover, several studies indicated that the rate of anastomotic stricture after double-flap technique was 4.7–17.5% [11, 27, 28]. Although the double-flap technique after PG seems an excellent reconstruction method, it was a complicated technique. They reported that the mean operation time of the double-flap technique with PG was over 350 min [11, 27, 28]. Even though the gastric tube reconstruction indicated higher rates of anastomotic stricture and RE compared with double-flap technique, we consider that the gastric tube reconstruction could be an acceptable method after LPG, because the procedure is simple.
We demonstrated that the hemoglobin and ferritin levels in the gastric tube group were significantly higher than those in the LTG group later than 1 year after surgery. This result was compatible with the previous reports [27, 29]. Some studies have reported that PG with double-tract reconstruction does not have any advantages postoperative anemia [7, 8, 30]. We think that food passage through the duodenum may be important to absorb dietary iron, because the duodenal mucosa primary plays a significant role to absorb dietary iron in mammals. Our data showed no significant difference in total protein and serum albumin. This result was consistent with the previous reports [25, 29, 31].
In our study, the body weight loss within 1.5 year after surgery is more obvious in the gastric tube group. Aoyama et al. reported that bodyweight loss can lead to discontinuous of S-1 adjuvant chemotherapy [32]. The rapid weight loss after surgery could be weakness in the patients who needs S-1 adjuvant chemotherapy. However, the body weight percentage of preoperative body weight in the gastric tube group started to increase at 1 year and 6 months after surgery, and it was significantly higher than that in the LTG group later than 2 years and 6 months after surgery. Our data is aligned with several previous studies [26, 29]. We investigated survival rates as oncological outcomes. The overall survival rate of the gastric tube group was similar to that of the LTG group. This result was compatible with the previous reports [4, 5, 29].
Our study has several limitations. First, the present study was a retrospective study with a small sample size. Second, we did not use a validated questionnaire to evaluate quality of life. Third, the comparison of outcomes did not include other reconstructions, such as the double-flap technique and double-tract reconstruction after LPG. However, we consider it is important for food to pass through the remnant stomach and the duodenum in order to preserve postoperative nutritional status after PG. We believe that esophagogastrostomy is a feasible reconstruction in terms of simple procedure and reasonable method from the viewpoint of physiology. Several reports showed that simple esophagogastrostomy led to higher rates of RE and anastomotic stenosis [20, 24,25,26]. Two reports showed that esophagogastrostomy plus fundoplication indicated higher incidence of RE (over 20%) despite time-consuming procedure [33, 34]. Yamashita et al. reported that side overlap esophagogastrostomy was a simple method and led to lower rate of RE [35], but long-term outcomes have not been reported yet. Although the gastric tube reconstruction showed higher rates of RE and anastomotic stenosis than double-flap technique, it could be an acceptable method because of simplicity. For better clinical outcomes of gastric tube reconstruction, further ingenuity and investigation will be needed.
In conclusion, we found that gastric tube reconstruction after LPG is simple and had better outcomes than LTG in regard to postoperative nutritional status. We suggest that gastric tube reconstruction might be a feasible choice following LPG for proximal EGC based on its safety and simplicity.
References
Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH, Kim WH, Lee KU, Yang HK (2011) Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg 98:255–260
Okabatashi T, Gotoda T, Kondo H, Inui T, Ono H, Saito D, Yoshida S, Sasako M, Shimoda T (2000) Early carcinoma of the gastric cardia in Japan: is it different from that in the West? Cancer (Phila) 89:2555–2559
Adachi Y, Kitano S, Sugimachi K (2001) Surgery for gastric cancer: 10-year experience worldwide. Gastric cancer 4:166–174
An JY, Youn HG, Choi MG, Noh JH, Sohn TS, Kim S (2008) The difficult choice between total and proximal gastrectomy in proximal early gastric cancer. Am J Surg 196:587–591
Masuzawa T, Takiguchi S, Hirao M, Imamura T, Kimura Y, Fujita J, Miyashiro I, Tamura S, Hiratsuka M, Kobayashi K, Fujiwara Y, Mori M, Doki Y (2014) Comparison of perioperative and long-term outcomes of total and proximal gastrectomy for early gastric cancer: a multi-institutional restrospective study. World J Surg 38:1100–1106
Toyomasu Y, Ogata K, Suzuki M, Yanoma T, Kimura A, Kogure N, Yanai M, Ohno T, Mochiki E, Kuwano H (2017) Restoration of gastrointestinal motility ameliorates nutritional deficiencies and body weight loss of patients who undergo laparoscopy-assisted proximal gastrectomy. Surg Endosc 31:1393–1401
Cho M, Son T, Kim HI, Noh SH, Choi S, Seo WJ, Roh CK, Hyung WJ (2019) Similar hematologic and nutritional outcomes after proximal gastrectomy with double-tract reconstruction in comparison to total gastrectomy for early upper gastric cancer. Surg Endosc 33:1757–1768
Park JY, Park KB, Kwon OK, Yu W (2018) Comparison of laparoscopic proximal gastrectomy with double-tract reconstruction and laparoscopic total gastrectomy in terms of nutritional status or quality of life in early gastric cancer patients. Eur J Surg Oncol 44:1963–1970
Aihara R, Mochiki E, Ohno T, Yanai M, Toyomasu Y, Ogata K, Ando H, Asao T, Kuwano H (2014) Laparoscopy-assisted proximal gastrectomy with gastric tube reconstruction for early gastric cancer. Surg Endosc 24:2343–2348
Mochiki E, Fukuchi M, Ogata K, Ohno T, Ishida H, Kuwano H (2014) Postoperative functional evaluation of gastric tube after laparoscopic proximal gastrectomy for gastric cancer. Anticancer Res 34:4293–4298
Shoji Y, Nunobe S, Ida S, Kumagai K, Ohashi M, Sano T, Hiki N (2019) Surgical outcomes and risk assessment for anastomotic complications after laparoscopic proximal gastrectomy with double flap technique for upper-third gastric cancer. Gastric Cancer 22(5):1036–1043
Aburatani T, Kojima K, Otsuki S, Murase H, Okuno K, Gokita K, Tomii C, Tanioka T, Inokuchi M (2017) Double-tract reconstruction after laparoscopic proximal gastrectomy using detachable ENDO-PSD. Surg Endosc 31(11):4848–4856
Sato R, Kinoshita T, Akimoto E, Yoshida M, Nishiguchi Y, Harada J (2021) Feasibility and quality of life assessment of laparoscopic proximal gastrectomy using double-tract reconstruction. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-020-02076-7 Online ahead of print
Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T (2013) Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc 27(1):146–153
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14(2):113–123
Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, Tytgat GN, Wallin L (1996) The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 111(1):85–92
Kitano S, Adachi Y, Shiraishi N, Suematsu T, Bando T (1999) Laparoscopic-assisted proximal gastrectomy for early gastric carcinomas. Jpn J Surg 29:389–391
Adachi Y, Inoue T, Hagino Y, Shiraishi N, Shimoda K, Kitano S (1999) Surgical results of proximal gastrectomy for early-stage gastric cancer. jejunal interposition and gastric tube reconstruction. Gastric cancer 2:40–50
Chen XF, Zhang B, Chen ZX, Hu JK, Dai B, Wang F, Yang HX, Chen JP (2012) Gastric tube reconstruction reduces postoperative gastroesophageal reflux in adenocarcinoma of esophagogastric junction. Dig Dis Sci 57:738–745
Hosogi H, Yoshimura F, Yamaura T, Satoh S, Uyama I, Kanaya S (2014) Esophagogastric tube reconstruction with stapled pseudo-fornix in laparoscopic proximal gastrectomy: a novel technique proposed for siewert type II tumor. Langenbecks Arch Surg 399:517–523
Yasuda A, Yasuda T, Imamoto H, Kato H, Nishiki K, Iwama M, Makino T, Shiraishi O, Shinkai M, Imano M, Furukawa H, Okuno K, Shiozaki H (2015) A newly modified esophagogastrostomy with a reliable angle of His by placing a gastric tube in the lower mediastinum in laparoscopy-assisted proximal gastrectomy. Gastric Cancer 18:850–858
Ueda Y, Shiraishi N, Toujigamori M, Shiroshita H, Etoh T, Inomata M (2016) Laparoscopic proximal gastrectomy with gastric tube reconstruction. JSLS 20(3):1–5
Tokunaga M, Ohyama S, Hiki N, Hoshino E, Nunobe S, Fukunaga T, Seto Y, Yamaguchi T (2008) Endoscopic evaluation of reflux esophagitis after proximal gastrectomy: comparison between esophagogastric anastomosis and jejunal interposition. World J Surg 32:1473–1477
Chen S, Li J, Liu H, Zeng J, Yang G, Wang J, Lu W, Yu N, Huang Z, Xu H, Zeng X (2014) Esophagogastrostomy plus gastrojejunostomy: a novel reconstruction procedure after curative resection for proximal gastric cancer. J Gastrointest Surg 18:497–504
Nakamura M, Nakamori M, Ojima T, Katsuda M, Iida T, Hayata K, Matsumura S, Kato T, Kitadani J, Iwahashi M, Yamaue H (2014) Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery 156:57–63
Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, Ida S, Watanabe M, Sano T, Yamaguchi T (2017) Clinical outcomes and evaluation of laparoscopic proximal gastrectomy with double-flap technique for early gastric cancer in the upper third of the stomach. Ann Surg Oncol 24:1635–1642
Hosoda K, Wahio M, Mieno H, Moriya H, Ema A, Ushiku H, Watanabe M, Yamashita K (2019) Comparison of double-flap and Orvil technique of laparoscopy-assisted proximal gastrectomy in preventing gastroesophageal reflux: a retrospective cohort study. Langenbeck's Arch Surg 404:81–91
Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, Fujiwara H, Otsuji E (2014) Long-term outcomes of patients who underwent limited proximal gastrectomy. Gastric Cancer 17:141–145
Sugiyama M, Oki E, Ando K, Nakashima Y, Saeki H, Maehara Y (2018) Laparoscopic proximal gastrectomy maintains body weight and skeletal muscle better than total gastrectimy. World J Surg 42:3270–3276
Ahn SH, Lee JH, Park DJ, Kim HH (2013) Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric cancer 16:282–289
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, Rino Y, Masuda M, Tsuburaya A (2013) Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 20:2000–2006
Sakuramoto S, Yamashita K, Kikucchi S, Futawatari N, Katada N, Moriya H, Hirai K, Watanabe M (2009) Clinical experience of laparoscopy-assisted proximal gastrectomy with toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 209:344–351
Nishigori T, Okabe H, Tsunoda S, Shinohara H, Obama K, Hosogi H, Hisamori S, Miyazaki K, Nakayama T, Sakai Y (2017) Superiority of laparoscopic proximal gastrectomy with hand-sewn esophagogastrostomy over total gastrectomy in improving postoperative body weight loss and quality of life. Surg Endosc 31:3664–3672
Yamashita Y, Yamamoto A, TamamoriY YM, Nishiguchi Y (2017) Side overlap esophagogastrostomy to prevent reflux after proximal gastrectomy. Gastric cancer 20:728–735
Author information
Authors and Affiliations
Contributions
Study conception and design: Toyomasu Y and Mochiki E. Acquisition of data: Toyomasu Y, Ishiguro T, Ito T, Suzuki O, Ogata K, and Mochiki E. Analysis and interpretation of data: Toyomasu Y, Kumagai Y, Ishibashi K, and Saeki H. Drafting of manuscript: Toyomasu Y and Ishida H. Critical revision: Shirabe K and Ishida H
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toyomasu, Y., Mochiki, E., Ishiguro, T. et al. Clinical outcomes of gastric tube reconstruction following laparoscopic proximal gastrectomy for early gastric cancer in the upper third of the stomach: experience with 100 consecutive cases. Langenbecks Arch Surg 406, 659–666 (2021). https://doi.org/10.1007/s00423-021-02132-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02132-w