Abstract
Introduction
Fibroinflammatory biliary stricture (FIBS) is a rare benign tumor-like process of the extrahepatic bile duct that masquerades as cholangiocarcinoma.
Methods
In order to distinguish this unusual entity from cancer, we performed a systematic analysis of 11 patients with FIBS. All patients presented with jaundice; six patients had coexisting autoimmune disease. Preoperative evaluation included computed tomography scan and endoscopic retrograde cholangiopancreatography with benign brush cytology. Surgical treatment included nine bile duct resections with five concurrent liver resections and two incisional biopsies. Light microscopy demonstrated fibrous lesions admixed with chronic inflammation.
Results and discussion
Immunohistochemistry demonstrated smooth muscle actin expression in all lesions except one; five tumors exhibited IgG4 positive plasma cells. The lesions were negative for cytokeratin, ALK1, CD21, S100, Ki67, and p53. Six patients received postoperative immunosuppression. At 41 month median follow-up (range 15–58 months), there was no evidence of recurrent FIBS in ten patients, while one was lost to follow-up.
Conclusion
FIBS is a rare myofibroblastic lesion with an immunohistochemical profile distinct from other epithelial and stromal neoplasms of the extrahepatic bile duct. A subset of these cases appear to represent IgG4-related sclerosing cholangitis. Because preoperative cytology is not diagnostic of FIBS, surgical resection remains the mainstay of diagnosis and treatment, while immunosuppression may reduce the risk of recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroinflammatory biliary strictures (FIBS) are rare benign lesions of the extrahepatic bile duct that can masquerade as cholangiocarcinoma. Because histopathologic examination is currently required to distinguish between benign and malignant lesions of the bile duct, resection has become the method for diagnosis as well as treatment of extrahepatic biliary strictures. Emerging technologies like endoscopically directed forceps biopsy (Spyglass™)1, 2-fluoro-2-deoxy-d-glucose-positron emission tomography,2 contrast-enhanced MRI,3 and molecular analysis of cytologic specimens4 may soon have sufficient sensitivity and specificity to identify benign bile duct lesions nonoperatively. Clinical application of these technologies requires uniform classification of benign lesions of the bile duct as well as an understanding of their natural history, unique pathology, and response to treatment.
The term “malignant masquerade” was first used by Hadjis in 1985 to describe the clinical and radiographic similarities between benign and malignant lesions of the bile duct.5 Although once thought to be rare, recent data suggest that benign tumors of the extrahepatic bile duct cause 8–13.4% of all biliary strictures.6 Verbeek and Corvera reported unsuspected focal fibrotic strictures instead of cholangiocarcinoma in 11 of 82 (13.4%) and 22 of 275 (8%) patients, respectively.6,7 Similarly, Wetter reported eight patients (8%) with benign strictures in a series of 98 cases of suspected bile duct cancer, including three cases labeled “idiopathic benign focal stenosis.”8 Published descriptions of benign bile duct tumors6,8,9 include disparate diseases like lymphoplasmacytic sclerosing cholangitis associated with autoimmune pancreatitis,10 idiopathic benign focal stenosis,8 periductal fibrosis,11 cholangitis glandularis proliferans,12,13 and inflammatory pseudotumor.14 Although these reports do not provide a uniform classification of the underlying bile duct pathology, the bile duct lesions consistently demonstrate an infiltrating, non-neoplastic mass associated with chronic inflammation and fibrosis.
The term “inflammatory pseudotumor” loosely describes a diverse group of fibroinflammatory diseases characterized by the growth of an inflammatory mass which displaces surrounding structures and causes organ dysfunction related to compression. These inflammatory lesions were first described in the lung but also have been reported in the spleen, liver, lymph nodes, and common bile duct.15,16 The descriptive name arises from the discrepancy between the macroscopic appearance of the biliary lesion, suggesting a mass lesion infiltrating the bile duct, and its histological appearance of inflammation and fibrosis. Fibroinflammatory disorders are a heterogeneous group of clinical conditions of unclear etiology including retroperitoneal fibrosis, sclerosing cholangitis, sclerosing mesenteritis, and Reidel’s thyroiditis. The pathogenesis of these lesions has recently been linked to autoimmune diseases like collagen vascular disease and IgG4- related sclerosing diseases.17
We selected patients undergoing surgery for suspected bile duct cancer at the University of Pittsburgh Medical Center and excluded those with confirmed cancer, classic intrahepatic primary sclerosing cholangitis, autoimmune pancreatitis, anastomotic strictures from prior biliary surgery, and choledocholithiasis. We chose the term FIBS to encompass the remaining group of benign biliary lesions of the extrapancreatic bile duct, and we summarized the clinical, radiographic, pathologic, and immunohistochemical features of this rare entity in 11 patients.
Materials and Methods
Study procedures were approved by the Institutional Review Board of the University of Pittsburgh. De-identified medical records were reviewed for demographics, symptoms, and coexisting medical conditions, results of radiographic and laboratory evaluations, operative findings, pathologic diagnosis, medical management, and postoperative follow-up. Potential cases of FIBS were screened by two pathologists (AK and JD) using hematoxylin and eosin-stained tissue sections. Classic intrahepatic primary sclerosing cholangitis, preexisting bile duct injury or surgery, intrapancreatic duct involvement by autoimmune pancreatitis, choledocholithiasis, or the unexpected finding of cholangiocarcinoma precluded a diagnosis of FIBS. The radiographs and endoscopic retrograde cholangiograms (ERC) of patients with confirmed FIBS were reviewed (M.E.T.) for common diagnostic features. Operative reports and frozen section results were assessed to identify findings unique to this type of bile duct lesion.
Archived pathology specimens were retrieved to perform immunohistochemical analysis. Additional formalin-fixed, paraffin-embedded 4-μm thick sections were cut from available tissue blocks and processed using the Ventana Benchmark XT automated platform (Ventana Medical Systems, Inc., Tucson, AZ, USA) with established positive and negative controls. Sections were incubated for 28 min at 37°C with a panel of primary antibodies (Table 1). Antibody binding was visualized using the Ventana iVIEW™ detection system (Ventana Medical Systems, Inc.). Because the frequency of IgG4-positive cells is not firmly established for IgG4-related biliary tract disease, we selected a minimum of ten IgG4-positive plasma cells per hpf consistent with diagnostic criteria for autoimmune pancreatitis.18 Serum IgG levels were not measured in our series.
Results
Clinical and Radiographic Features
Eleven patients were treated for FIBS of the extrahepatic bile duct between 1998 and 2007. The median age of the patients was 53 years (range 29–68) with seven females and four males (Table 2). All patients presented with vague abdominal pain, jaundice, light-colored stools, and dark urine. Four patients had coexisting autoimmune diseases at the time of diagnosis, while two patients were diagnosed with an autoimmune disease postoperatively. The presenting serum CEA values were all within normal limits (0.4–2.0 ng/ml), whereas Ca 19-9 was elevated (>37 U/ml) in three patients and ranged from 6 to 1,085 (median = 33 U/ml).
Computed tomography (CT) was performed in all patients; radiographic features of bile duct cancer were seen in eight patients (Table 3, Fig. 1). Four patients had a definable mass associated with the extrapancreatic bile duct, while four patients had soft tissue infiltrating the porta hepatis, five had abnormal-appearing hilar lymph nodes, and two had vascular encasement or occlusion of the hepatic artery and/or portal vein. Only one patient had gallstones; however, five patients had undergone prior cholecystectomy. Among the five patients with prior cholecystectomy, no relationship was established with timing of presentation or location of hemoclips. No additional clips were noted at surgical exploration which might have indicated an anatomically challenging prior procedure nor were any clips noted to be in close proximity to the presenting stricture. The preoperative CT scans showed residual biliary ductal dilatation following stenting in eight patients. The sensitivity and specificity of these CT findings may have been compromised by prior endoscopic manipulation of the biliary tree and biliary stenting. Endoscopic retrograde cholangiography (ERC) revealed a dominant stricture of the extrapancreatic bile duct in all patients, and endoscopic biliary stents were inserted to relieve obstructive jaundice in all patients (Fig. 2). Brush cytology was performed in ten of 11 patients, and all were negative for malignancy. Five specimens showed atypical epithelial cells, while five had reactive or degenerated epithelial cells (two with scant acute and chronic inflammation).
Operative Management and Follow-up
All patients with FIBS underwent surgical exploration with the intention of resecting bile duct cancer (Table 2). Operative findings were generally consistent with the preoperative suspicion of cholangiocarcinoma. As a result, nine patients underwent resection of the extrahepatic bile duct, including five concurrent major hepatic resections for definitive tumor clearance. Two patients were found to have unresectable involvement of the porta hepatis and underwent biopsy of the bile duct to establish a tissue diagnosis. No discrete pancreatic masses were identified. Frozen section examination was suspicious for malignancy in only one case. The remaining cases were classified intraoperatively as benign and/or inflammatory. Operative exploration of patients with FIBS demonstrated the following common features: the bile duct was firm in all cases; the infiltrative process originated in the mid common bile duct in six patients and the hilum in five. Eight patients had identifiable masses associated with the bile duct, and three were suspected of having malignant lymphadenopathy. The mass infiltrated the transverse mesocolon in one case and appeared to encase the portal vasculature in two cases.
Postoperatively, six patients received immunosuppression with azathioprine and/or tapered dose prednisone based on the final pathologic impression of an inflammatory process/autoimmune disease. After 41 month median follow-up (range 15–58 months), ten patients were free of recurrent FIBS, and one was lost to follow-up. Two patients died without evidence of malignancy. A third patient died 12 months after bile duct resection due to bilobar hepatic metastases caused by adenocarcinoma of unknown primary. Re-review of the original cases’ pathologic material did not identify any evidence of malignancy in the resected bile duct. It is possible that the malignant biliary stricture was missed on the initial bile duct resection; however, the presenting clinical picture of jaundice resolved for 1 year postoperatively, and the bile duct resection specimen did contain a dominant stricture.
Pathologic and Immunohistochemical Characterization
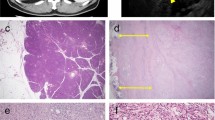
Routine histology of the bile duct lesions exhibited varying degrees of fibrosis in all cases with a mild to marked inflammatory infiltrate (Table 4). Five lesions (45%) demonstrated a lymphocytic and plasma cell infiltrate centered around the bile duct (Fig. 3a, b). Four additional lesions (36%) showed an inflammatory cell infiltrate containing either scattered eosinophils or prominent lymphoid follicles with germinal centers. The mixed fibroinflammatory process spread beyond the porta hepatis in four cases (36%) and involved the peripancreatic fat, mesentery, liver, and colonic serosa (Fig. 3c).
Pathologic findings. In five of the cases, the chronic inflammatory infiltrate was centered around the bile duct and its branches. a In this example, there is a moderate amount of chronic inflammation present beneath the bile duct epithelium and increased periductal fibrosis (×40); b At higher magnification, numerous plasma cells are present within the inflammatory infiltrate (×400). In two cases, the fibroinflammtory process was predominantly fibrotic, more diffuse, and involved periductal soft tissue and fat, as well as other organs, consistent with sclerosing mesenteritis. c In this example, dense fibrosis and short fibrotic bands are admixed with chronic inflammatory cells and the fibroinflammatory process infiltrates adipose tissue (the bile duct is not present in this field) (×40). d Representative example of an IgG4-postive case showing numerous IgG4-positive cells beneath the ductal epithelium and within the wall of the bile duct (IgG4 immunohistochemical stain), consistent with IgG4-related sclerosing cholangitis (×400).
To investigate the biology of these lesions, immunohistochemistry was used to probe for markers of cell proliferation, neoplastic transformation, and differentiation including Ki67, pancytokeratin, p53, smooth muscle actin, CD34, ALK1, CD21, S100, and IgG4 (Table 4). All lesions except one expressed smooth muscle actin, consistent with smooth muscle or myoepithelial (myofibroblastic) origin. Rare ki67 positivity among the stromal cells indicated a low proliferative index and did not support neoplastic transformation of a mesenchymal tumor. The focal CD34 labeling was interpreted as nonspecific antibody binding rather than evidence for a solitary fibrous tumor or stromal tumor. Conversely, none of the lesions expressed cytokeratins or p53 to indicate an epithelial-derived carcinoma like bile duct cancer. The absence of ALK1, CD21, and S100 expression excluded the remaining tumors in the differential diagnosis of neoplastic bile duct stricture, which includes spindle cell carcinoma, dendritic cell sarcoma, and neural tumors arising from the bile duct.
Since fibroinflammatory processes can be caused by a systemic IgG4-related sclerosing disease, we probed the available tissue blocks for evidence of IgG4-secreting plasma cells.12,13 Five tumors had >10 IgG4-positive cells per hpf (Fig. 3d), all of which had ductocentric lymphoplasmacytic inflammation indicating IgG4-related sclerosing cholangitis (FIBS + IgG4). The remaining six cases did not contain IgG4-positive plasma cells but possessed a similar pattern of inflammation and fibrosis (FIBS-IgG4).
Discussion
This report is the first detailed characterization of extrapancreatic bile duct stricture caused by a benign lesion that we designated “fibroinflammatory biliary stricture.” FIBS patients presented with signs and symptoms typical of bile duct cancer including vague abdominal pain and obstructive jaundice. The median age at the time of diagnosis was 53 years with a 2:1 female preponderance. Six of eleven patients had coexisting autoimmune diseases. Serum CEA levels were normal in all patients, while Ca19-9 exceeded 100 U/ml in only one patient (11%). ERC demonstrated a dominant stricture of the extrapancreatic bile duct in all patients, while brush cytologies were benign or inconclusive. Operative findings included a thickened bile duct with an infiltrating mass, the suspicion of malignant adenopathy, encasement or occlusion of the portal vasculature, and involvement of adjacent organs. Although these features are characteristic of cholangiocarcinoma, malignancy was not identified on any intraoperative frozen sections with the exception of one sample regarded as suspicious. The final pathologic diagnosis of FIBS required immunohistochemical analysis of resected tissue. Six patients received postoperative immunosuppression, four on the basis of IgG4-positive plasma cells suggesting a component of IgG4-related sclerosing cholangitis. After 41 months median follow-up, all patients remained free of disease. Three died of unrelated causes of which one died of bilobar hepatic metastases from adenocarcinoma of unknown primary.
We designated these diverse fibroinflammatory lesions “FIBS” on the basis of a non-neoplastic pattern of tumor marker expression and low proliferative index. By definition, FIBS is a subset of benign bile duct tumors unrelated to autoimmune pancreatitis, primary sclerosing cholangitis, cholangiocarcinoma, prior bile duct injury or repair, and choledocholithiasis. Histologically, the FIBS lesion is composed of spindle cells, fibroblasts, and myoblasts with varying degrees of fibrosis and lymphoplasmacytic inflammatory infiltrate.19 If a defined mass was present, it was firm and lacked a capsule. By comparison with patients having bile duct cancer, FIBS patients presented at a significantly younger age, 53 vs. 69 years old, were more likely to be female, and had a high incidence of coexisting autoimmune diseases (54%).20 No FIBS patients had a serum CEA level >2.2 ng/ml unlike a recently published cohort of cholangiocarcinoma patients, 69% of whom had serum CEA >2.2 ng/ml.21 Only one FIBS patient (11%) had a serum Ca 19-9 level exceeding 100 U/ml, a diagnostic threshold for cholangiocarcinoma which has 68% positive predictive value. Finally, none of the FIBS patients had positive endoscopic retrograde cholangiopancreatography (ERCP) brush cytology unlike a cohort of patients with surgically confirmed cholangiocarcinoma, 31% of whom had positive cytology specimens.22
Inflammatory pseudotumors were originally described in the lung but have also been reported in a variety of extrapulmonary sites including the liver, omentum, ureters, lymph nodes.23 The varying degrees of fibrosis and inflammatory infiltration associated with FIBS is responsible for confusing nomenclature that includes inflammatory pseudotumor, inflammatory myofibroblastic tumor (IMFT), postinflammatory tumor, idiopathic benign focal stenosis, and nonspecific inflammatory process.6,24–28 The term “inflammatory pseudotumor” has been used to describe nonneoplastic lesions of the viscera and soft tissue, and unfortunately, the same term has been applied to some true inflammatory neoplasms like dendritic cell tumors29 and myofibroblastic tumors30 including inflammatory fibrosarcoma.31 Given such diverse histologies, the biological behavior of inflammatory pseudotumor is actively debated in the literature,31,32 although the term is now most commonly used to describe a “benign nonmetastasizing proliferation of myofibroblasts with potential for recurrence and persistent local growth.”32 Long-term follow-up of 38 patients undergoing surgical resection for histopathologically similar inflammatory tumors of the retroperitoneum and mesentery demonstrated a 37% local recurrence rate as well as an 11% rate of distant metastasis. The term “inflammatory fibrosarcoma” was applied to these lesions because of their aggressive behavior.31 Cytogenetic abnormalities discovered in inflammatory lesions of the mesentery, liver, lung, and soft tissue lesion have been cited as potential evidence of malignant behavior.33–36 Conversely, a series of 84 extrapulmonary inflammatory tumors has been reported with a 15% rate of intra-abdominal recurrence but no distant metastases.
The malignant potential of FIBS in the extrapancreatic bile duct remains unknown due to the rarity of this disease. These lesions can masquerade as cholangiocarcinoma due to reactive epithelial hyperplasia, perineural extension, and involvement of vascular structures and adjacent organs. These features can be particularly misleading when examining intraoperative frozen sections due to the limitations of sample processing and the time constraints of immunohistochemistry. Nonetheless, the absence of cytokeratin, CD34, CD21, p53, and Ki67 expression among FIBS patients significantly reduced the likelihood of a neoplastic diagnosis. Although the development of liver metastases in one of our patients raised the possibility of missed cholangiocarcinoma, metastatic adenocarcinoma is not consistent with malignant degeneration of a benign fibrous inflammatory tumor because of published data indicating fibrosarcoma histology in such metastatic deposits.31
The pathological characteristics of FIBS resemble inflammatory pseudotumors and are plasma-cell predominant. Immunohistochemistry may demonstrate muscle-specific actin and smooth muscle actin expression consistent with myofibroblasts as well as positivity for anaplastic lymphoma kinase (ALK), an oncogenic tyrosine kinase.37 Though IgG4-positive infiltrates have been identified in the IgG4-related sclerosing diseases of autoimmune sclerosing cholangitis and autoimmune pancreatitis,38 there is little published data linking an IgG4-positive sclerosing process with fibrous inflammatory strictures/tumors of the extrahepatic bile duct.
We subclassified FIBS on the basis of the IgG4 status of the dense lymphoplasmacytic infiltrate as strictures with autoimmune cholangitis (FIBS + AC).39,40 Although primary sclerosing cholangitis is also associated with autoimmune diseases, the clinical features of FIBS are distinct from PSC. No patient in this series exhibited either the characteristic onion-skinning appearance of PSC or biliary cirrhosis on final pathology. PSC patients show a slow progression of disease over a 5- to 10-year period compared to those with inflammatory myofibroblastic tumors who have a more aggressive natural history and a focal stricture which generally regresses following immunosuppression.41–43,49
Although the etiology of FIBS is unclear, recent data suggest that IgG4-related sclerosing pancreatititis and cholangitis are inflammatory disorders caused by activation of T helper 2 (Th2) cells and T regulatory cells. Real-time PCR and immunohistochemistry of human tissues demonstrate overproduction of Th2 cells and regulatory cytokines, such as interleukin-10, interferon-γ, and TGF-β, which precede IgG4 class switching and fibroplasia in autoimmune pancreatitis.44 Additional stimuli for cytokine overproduction may include biliary tract infection. Case reports of hepatic inflammatory tumors demonstrate parasitic fragments and bacteria, and there is speculation that Ebstein–Barr virus (EBV) or dendritic cells may be responsible for FIBS.45,46 Recent data suggest a link between hepatic stellate cells and the formation of an inflammatory stricture. Cytokines cause hepatic stellate cells to acquire myofibroblast-like features and produce extracellular matrix during liver fibrogenesis.47 We speculate that the myofibroblasts observed in FIBS are derived from activated stellate cells adjacent to the bile ducts which cause fibrogenesis and extracellular matrix production resulting in stricture formation. By inhibiting lymphocyte activation and cytokine production, immunosuppression may prevent the myofibroblast transformation of stellate cells and resulting fibrogenesis. Because platelet-derived growth factor mediates cytokine-induced signaling and proliferation of stellate cells, anti-PDGF therapy may offer a targeted approach to FIBS which will ameliorate the phenotype of this disease and prevent recurrence after resection.47
Given the potential morbidity of hepatobiliary resection for suspected bile duct cancer, FIBS should be entertained in the differential diagnosis of bile duct stricture in relatively younger patients with coexisting autoimmune diseases and normal serum tumor marker levels. Negative or atypical preoperative brush cytology has insufficient specificity to exclude cholangiocarcinoma in the presence of a biliary stent, and intraoperative findings may suggest locally advanced cancer, for which nonsurgical palliation has a dismal outcome. Until new methods of detecting cholangiocarcinoma are developed, surgical resection will remain the mainstay of diagnosis and treatment for presumed malignant biliary strictures. Recent data indicate that major postoperative complications develop in 32% of patients treated for benign biliary stricture with long-term sequelae of surgery developing in a further 36% of surgical patients.6 The clinical suspicion of FIBS may significantly alter intraoperative decision-making if frozen sections are negative for malignancy and a conservative surgical approach is warranted to permit a short-course of postoperative corticosteroid treatment.48,49 The importance of ancillary studies and extensive pathologic evaluation remains paramount to rule out true biliary malignancies. The combination of molecular pathology, including loss of heterozygosity analysis and gene sequencing for k-ras mutations, and endoscopically directed forceps biopsy of the bile duct lesion (Spyglass™) are promising techniques which may be applied to the preoperative diagnosis of FIBS.1,4 The testing for elevated IgG4 in the serum of patients and increased numbers of IgG4+ cells in preoperative tissue biopsies hold the potential to render the diagnosis preoperatively in the future. Given the rare nature of this lesion, the long-term prognosis and risk of recurrence following surgical treatment for FIBS remains a subject of active scrutiny.
We acknowledge that this is a rare disorder, and current management remains surgical resection given that most cases will represent cholangiocarcinoma. Biliary brushings and tumor markers are important elements for consideration in all biliary stricture cases. Currently, we continue to address resectable lesions operatively and have found that this diagnosis should be considered with a patient with normal tumor markers, autoimmune disease, young in age, and perhaps female presents with a dominant stricture. The inflammatory elements of this stricture and its common occurrence with autoimmune disease support the logic of immunosuppression, and patients are typically followed with yearly contrast imaging (CT/MRI).
References
Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc 2007;65(6):832–841. doi:10.1016/j.gie.2007.01.025.
Nishiyama Y, Yamamoto Y et al. Comparison of early and delayed FDG PET for evaluation of biliary stricture. Nucl Med Commun 2007;28(12):914–919.
Kim JY, Lee JM et al. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: differentiation of malignant from benign bile duct strictures. J Magn Reson Imaging 2007;26(2):304–312.
Khalid A, Pal R, Sasatomi E, Swalsky P, Slivka A, Whitcomb D, Finkelstein S. Use of microsatellite marker loss of heterozygosity in accurate diagnosis of pancreaticobiliary malignancy from brush cytology samples. GUT 2004;53:1860–1865.
Hadjis NS, Collier NA, Blumgart LH. Malignant masquerade at the hilum of the liver. Br J Surg 1985;72(8):659–661. Aug.
Corvera CU, Blumgart LH, Darvishian F, Klimstra DS, DeMatteo R, Fong Y, D’Angelica M, Jarnagin WR. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg 2005;201(6):862–869. Epub 2005 Oct 13, Dec.
Verbeek PC, van Leeuwen DJ, de Wit LT, Reeders JWAJ, Smits NJ, Bosma A, Huibregtse K, van der Heyde MN. Benign fibrosing disease at the hepatic confluence mimicking Klatskin tumors. Surgery 1992;112:866–870.
Wetter LA, Ring EJ, Pellegrini CA, Way LW. Differential diagnosis of Sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg 1991;161:57–63.
Standfield NJ, Salisbury JR, Howard ER. Benign non-traumatic inflammatory strictures of the extrahepatic biliary system. Br J Surg 1989;76:849–852.
Kram MT, May LD, Cooperman A et al. Lymphoplasmacytic sclerosing pancreatitis and cholangitis. Gastrointest Endosc 2002;55:588–590.
Hawegawa K, Kubota K, Komatsu Y et al. Mass-forming inflammatory periductal fibrosis mimicking hilar bile duct carcinoma. Hepatogastroenterology 2000;47:1230–1233.
Graham SM, Barwick K, Cahow CE, Baker CC. Cholangitis glandularis proliferans. A histologic variant of primary sclerosing cholangitis with distinctive clinical and pathological features. J Clin Gastroenterol 1988;10:579–583.
Krukowski ZH, McPhie JL et al. Proliferative cholangitis (cholangitis glandularis proliferans). Br J Surg 1983;70(3):166–171.
Ozeki Y, Matsubara N et al. [Inflammatory tumor of the hepatic hilus mimicking bile duct cancer–report of a case]. Nippon Geka Gakkai Zasshi 1993;94(9):1064–1067.
Hamdi I, Marzouk I et al. [Inflammatory pseudotumor of the spleen and radiopathologic correlation]. J Radiol 2006;87(12 Pt 1):1894–1896.
Locke JE, Choti MA et al. Inflammatory pseudotumor of the liver. J Hepatobiliary Pancreat Surg 2005;12(4):314–316.
Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: the inflammatory pseudotumor as another expression of the fibrohistiocytic complex (Abstract). Lab Invest 1986;54:15. [typically, abstracts are not cited] [a better older reference might be: Dehner LP.
Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol 2006, Sept 15.
Palazzo JP, Chang CD. Inflammatory pseudotumor of the pancreas. Histopathology 1993;23:86–91.
Jepsen P, Vilstrup H et al. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst 2007;99(11):895–897.
Qin XL, Wang ZR et al. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol 2004;10(3):427–432.
Are C, Gonen M et al. Differential diagnosis of proximal biliary obstruction. Surgery 2006;140(5):756–763.
Nonmura A, Minato H, Shimizu K, Kadoya M, Matsui O. Hepatic hilar inflammatory pseudotumor mimicking cholangiocarcinoma with cholangitis and phlebitis: a variant of primary sclerosing cholangitis? Pathol Res Pract 1997;193:519–525.
Bahadori M, Liebow AA. Plasma cell granuloma of the lung. Cancer 1973;31:191–208.
Gonzalez-Crussi F, deMello DE, Sotelo-Avilla C. Omental mesenteric myxoid hamartomas: infantile lesions simulating malignant tumors. Am J Surg Pathol 1983;7(6):567–578.
Pisciotto PT, Gray GF, Miller DR. Abdominal plasma cell pseudotumor. J Pediatr 1978;93:628–630.
Tang TT, Segura AD, Oechler MD et al. Inflammatory myofibrohistiocytic proliferation simulating sarcoma in children. Cancer 1990;65:1626–1634.
Umiker WO, Iverson L. Post inflammatory tumors of the lung: report of four cases simulating xanthoma, fibroma or plasma cell tumor. J Thorac Surg 1954;28:55–62.
Shek TWH, Ho FCS, Ng IOC et al. Follicular dendritic cell tumor of the liver. Evidence for an Ebstein-Barr virus related proliferation of follicular dendritic cells. Am J Surg Pathol 1996;20(3):313–324.
Fukushima N, Suzuki M et al. A case of inflammatory pseudotumour of the common bile duct. Virchows Arch 1997;431(3):219–224.
Meis JM, Enzinger FM. Inflammatory fibrosarcoma of the mesentery and retroperitoneum. A tumor closely simulating inflammatory pseudotumor. Am J Surg Pathol 1991;15:1146–1156.
Coffin Cm, Watterson J, Priest JR et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathological and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19(8):859–872.
Donner L, Tompler R, White R. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol 1996;27:1096–1098.
Spencer H. The pulmonary plasma cell/histiocytoma complex. Histopathology 1984;8:90316.
Treissman SP, Gillis DA, Lee CLY et al. Omental–mesenteric inflammatory pseudotumor. Cytogenetic demonstration of genetic changes and monclonality in one tumor. Cancer 1994;73:1433–1437.
Zavaglia C, Barberis M, Gelosa F. Inflammatory pseudotumor of the liver with malignant transformation. Report of two cases. Ital J Gastroenterol 1996;28(3):152–159.
Chiarle R, Voena C et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008;8(1):11–23.
Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis. Am J Surg Pathol 2004;28(9):1193–1203. Sep.
Hamano H, Kawa S, Uehara T, Ochi Y, Takayama M, Komatsu K, Muraki T, Umino J, Kiyosawa K, Miyagawa S. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: part of a spectrum of autoimmune pancreatitis. Gastrointest Endosc 2005;62(1):152–157. Jul.
Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology 2006;6(1–2):132–137.
Stathopoulos G, Nourmand AD, Blackstone M, Anderson D, Baker AL. Rapidly progressive sclerosing cholangitis following surgical treatment of pancreatic pseudotumor. J Clin Gastroenterol 1995;21(2):143–148.
Weisner RH, Grambsch PM, Dickson ER et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology 1989;10:430–436.
Knox T, Kaplan M. A double-blind controlled trial of oral-pulse methotrexate therapy in the treatment of primary sclerosing cholangitis. Gastroenterology 1994;106:494–499.
Zen Y, Fujii T et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007;45(6):1538–1546.
Arber A, Kamel OW, Van de Rijn M et al. Frequent presence of Ebstein–Barr virus in inflammatory pseudotumor. Hum Pathol 1995;26:1093–1098.
Selves J, Meggetto F, Bousset P et al. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein–Barr virus. Am J Surg Pathol 1996;20:747–753.
Borkham-Kamphorst E, Stoll D et al. Inhibitory effect of soluble PDGF-beta receptor in culture-activated hepatic stellate cells. Biochem Biophys Res Commun 2004;317(2):451–462.
Wakabayashi T, Kawaura Y et al. Long-term prognosis of duct-narrowing chronic pancreatitis: strategy for steroid treatment. Pancreas 2005;30(1):31–39.
Nishino T, Toki F et al. Long-term outcome of autoimmune pancreatitis after oral prednisolone therapy. Intern Med 2006;45(8):497–501.
Acknowledgment
Grant support is from N.I.H. K12 HD 049109 (T.C.G.); N.I.H. DK-51485 and the Competitive Medical Research Foundation (A.J.M.)
Author information
Authors and Affiliations
Corresponding author
Additional information
Poster Presentation at Digestive Disease Week, May 2006, Los Angeles CA, USA.
Rights and permissions
About this article
Cite this article
Gamblin, T.C., Krasinskas, A.M., Slivka, A.S. et al. Fibroinflammatory Biliary Stricture: A Rare Bile Duct Lesion Masquerading as Cholangiocarcinoma. J Gastrointest Surg 13, 713–721 (2009). https://doi.org/10.1007/s11605-008-0750-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0750-1