Abstract
Biliary epithelial cells preferentially respond to various insults under chronic pathological conditions leading to reactively atypical changes, hyperplasia, or the development of biliary neoplasms (such as biliary intraepithelial neoplasia, intraductal papillary neoplasm of the bile duct, and cholangiocarcinoma). Moreover, benign biliary strictures can be caused by a variety of disorders (such as IgG4-related sclerosing cholangitis, eosinophilic cholangitis, and follicular cholangitis) and often mimic malignancies, despite their benign nature. In addition, primary sclerosing cholangitis is a well-characterized precursor lesion of cholangiocarcinoma and many other chronic inflammatory disorders increase the risk of malignancies. Because of these factors and the changes in biliary epithelial cells, biliary strictures frequently pose a diagnostic challenge. Although the ability to differentiate neoplastic from non-neoplastic biliary strictures has markedly progressed with the advance in radiological modalities, brush cytology and bile duct biopsy examination remains effective. However, no single modality is adequate to diagnose benign biliary strictures because of the low sensitivity. Therefore, understanding the underlying causes by compiling the entire clinical, laboratory, and imaging data; considering the under-recognized causes; and collaborating between experts in various fields including cytopathologists with multiple approaches is necessary to achieve an accurate diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary epithelial cells lining the biliary tree preferably exhibit reactive changes (such as mild papillary hyperplasia and nuclear condensation) and occasionally develop biliary neoplasia [such as biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasms of bile duct (IPNB)] under the pathological conditions of chronic inflammatory biliary diseases and cholangiocarcinoma. Although the radiological improvements for the differentiation between neoplastic and non-neoplastic biliary strictures have markedly progressed using several recent modalities, in patients exhibiting biliary strictures, neoplastic lesions are difficult to differentiate from non-neoplastic lesions radiologically. Despite the rarity of these clinical cases, pathological examination is still required to distinguish these cases and a bile duct biopsy is effective for differentiating benign, inflammatory from malignant or neoplastic lesions. The specimens obtained by the choledochoscope are typically small fragments that are comparatively crushed, and biliary exfoliative cytology reveals various cellular features irrespective of the tumorous or non-neoplastic biliary cells. In this paper, the most recent advances in benign biliary strictures and the caution required for diagnosing this condition are reviewed.
Etiology and classification of benign biliary strictures
A variety of causes can lead to biliary strictures, each with different natural histories and responses to therapy. Therefore, determining the underlying etiologies is important to provide appropriate treatment. The majority of biliary strictures are malignant and mainly a result of pancreatic adenocarcinoma and cholangiocarcinoma, with up to 30 % of these strictures found to be benign [1]. The causes of benign biliary strictures are listed in Table 1. Although the actual incidence remains unknown and varies among hospital populations, iatrogenic and secondary to biliary injury after cholecystectomy or liver transplantation are the most frequent causes [1]. Prior hepatobiliary surgery is responsible for 80–90 % of benign biliary strictures [2], and chronic pancreatitis is reported to account for 8.2–13.9 %, typically with late or end-stage chronic pancreatitis [1]. Several immune-mediated inflammatory conditions can lead to biliary strictures, most notably primary sclerosing cholangitis, as well as an increasingly recognized condition, IgG4-related sclerosing cholangitis (IgG4-SC). Recently, sclerosing cholangitis in critically ill patients is believed to have a vascular basis and has also been described [3]. Other causes, such as eosinophilic cholangitis, follicular cholangitis, although rare, typically mimic hilar cholangiocarcinoma and should also be taken into account when evaluating biliary stenoses [4, 5].
There are two established classification systems used to evaluate biliary duct strictures: (1) the Bismuth classification based on the location of the stricture; and (2) the Strasberg classification system that uses stricture location, size, and bile leakage [6]. Recently, Kaffes have proposed a novel classification system with an intention to guide the assessment and endoscopic treatment, as well as the site and common etiology of benign strictures as shown in Table 2 [7].
Cytology and intraductal biopsy for the differentiation of benign from malignant biliary strictures
In presurgical evaluations, testing modalities, including circulating markers, imaging tests, and endoscopic tests with tissue sampling are of particular importance and are widely used. However, brush cytology and intraductal biopsies obtained via endoscopic retrograde cholangiopancreatography (ERCP) have a low sensitivity for the diagnosis of cholangiocarcinoma, despite a high specificity. In a meta-analysis published in 2015, the pooled sensitivity and specificity of the brushings were 45 % (95 % confidence interval (CI) 40–50 %) and 99 % (95 % CI 98–100 %), respectively. The values for the intraductal biopsies were 48.1 % (95 % CI 42.8–53.4 %) and 99.2 % (95 % CI 97.6–99.8 %), respectively. A combination of both modalities only modestly increased the sensitivity (59.4 %; 95 % CI 53.7–64.8 %) with a specificity of 100 % (95 % CI 98.8–100 %) [8]. Combining positive or atypical cytology with fluorescence in situ hybridization (FISH) achieves a higher sensitivity of >70 %, but a lower specificity at 89 % [1]. A triple modality approach, consisting of brush cytology, intraductal biopsy, and FISH results in an overall sensitivity of 82 %, specificity of 100 %, positive predictive value of 100 %, and negative predictive value of 87 % [9]. Sampling lesions via endoscopic ultrasonography-guided fine-needle aspiration has also been reported to increase the diagnostic yield with the pooled sensitivity and specificity for the diagnosis of malignant biliary strictures of 80 % (95 % CI 74–86 %), and 97 % (95 % CI 94–99 %), respectively [10]. In addition, targeted biopsies by cholangioscopy yielded a pooled sensitivity and specificity to detect cholangiocarcinoma of 66.2 % (95 % CI 59.7–72.3 %) and 97.0 % (95 % CI 94.0–99.0 %), respectively, in a systematic review published in 2015 [11]. Despite advanced numerous testing modalities and extensive evaluations, the underlying etiology of up to 20 % of biliary strictures remains unable to be determined [1]. A multimodality approach to carefully consider the clinical, laboratory, and imaging data is needed to optimize the diagnosis of biliary strictures.
Benign biliary stricture in surgically resected cases
In routine works of pathological diagnosis and diagnosing suspicious malignancies in bile duct biopsies and/or bile cytological specimens, cases without malignant lesions in surgically resected biliary specimens are sometimes encountered. According to Fujita et al. [12] in 2010, 2.8 % (5 of 176 cases) of surgically resected biliary specimens have been reported as benign sclerosing biliary diseases, consisting primarily of cholangitis in their facility in Japan. Moreover, a meta-analysis using previous reports from the Japanese and English literature also revealed a relatively high frequency of 5 to 24.5 % [12, 13]. In addition, in the panel discussion of the 49th Annual Meeting of the Japan Biliary Association hold in 2013, the results of several Japanese facilities revealed that approximately 3 % cases are benign biliary strictures differing from preoperative diagnosis.
As the pathological diagnosis occurs from resected specimens following an operation, biliary diseases consist of established causes, including primary sclerosing cholangitis (PSC), IgG4-SC, and hepatolithiasis. Additionally, there are also unclassified and nonspecific cholangitis, including the recently reported follicular cholangitis described in some case reports. In an actual clinicopathological diagnosis, since secondary sclerosing cholangitis caused by extrabiliary and iatrogenic diseases, as well as the biliary manifestation of systemic diseases should also be considered. Additionally, the information regarding the history of biliary operation and infection are required for a pathological diagnosis. The histological manifestation consists of biliary stenosis or an obstruction accompanied by inflammation and periductal fibrosis. Furthermore, in some diseases, progressive and destructive bile duct lesions and biliary cirrhosis are also found. In IgG4-SC, the increased level of serum IgG4 is a valuable indicator, but there are no established clinical markers that reflect the disease and the pathological condition of other forms of sclerosing cholangitis.
Primary sclerosing cholangitis
The basic pathology of PSC consists of sclerosing cholangitis in the intrahepatic large bile duct, hepatic hilus, and extrahepatic bile ducts. However, these biliary lesions are also found in the secondary sclerosing cholangitis caused by a biliary operation or biliary stone formation. The diagnosis of PSC is relatively straightforward when inflammatory bowel diseases (IBD), such as ulcerative colitis are present, but exclusive diagnoses are also important. According to reports from Europe and North America, IBD is complicated in PSC at a rate of 46.5–100 % [14–17] with ulcerative colitis being the most common type (47–97.6 %) in PSC-IBD [14, 18]. Data from the International PSC Study group revealed that 65.2 % of patients with PSC are male, the mean age at diagnosis is 40.5 years, and IBD is present in 69 % of PSC patients (79.5 % ulcer colitis) [19]. In Japan, the IBD complication rate is relatively low at 34 %, based on the national survey of PSC in 2012 [20]. However, the figure for young individuals is higher, at 57 % (compared to 12 % in the elderly) [21] and similar to that in Europe and the United States. Patients with PSC have been distributed into two peak populations in Japan. However, the disease manifestation of IgG4-SC has been broadly recognized, and the contamination of IgG4-SC cases has been speculated for some of the older PSC cases. According to the recent Japanese national survey of PSC, the population of PSC without IgG4-SC cases also exhibited the two peak distributions for the age ranges of 35–40 and 65–70 years [20]. These findings suggest that there is a difference in pathogenesis of PSC between young and older individuals. The level of genetic diversity among races may also affect the prevalence rate, age at risk for development of PSC, as well as the difference in the presence of IBD. Moreover, diffident phenotypes of PSC may also exist among various populations.
Several special PSC populations with differences in clinical features have been described [22–27] including small duct PSC, PSC with features of autoimmune hepatitis (AIH) or PSC-AIH overlap syndrome, and PSC in children. Patients with small duct disease and features of AIH have a significantly (p < 0.001) better transplant-free survival rate and a lower rate of hepatobiliary malignancy [19]. Recently, Sarkar et al. [28] postulated that although the actual number may be far smaller, there are more than 3,000 potential PSC phenotypes based on the age of diagnosis, presence or absence of IBD, small duct involvement, IgG4 levels, dominant strictures, and race. For example, classic PSC, non-IBD large duct PSC, and high IgG4 PSC [28]. An association between high serum IgG4 levels and specific human leukocyte antigen (HLA) haplotypes has also been reported, suggesting a distinct PSC phenotype [29], in which patients may benefit from corticosteroid treatment [30]. However, there have been no confirmed and validated diagnostic criteria established to classify PSC phenotypes.
Current evidence suggests that PSC is an immune-mediated disease, but the precise etiopathogenesis of PSC remains unclear. The etiology of PSC is thought to be multifactorial, including both environmental and genetic causes with 16 genetic risk loci identified to date [24, 31, 32]. An autoimmune cause is strongly supported by several factors: (1) strong genetic links with HLA; (2) tissue infiltration with immune cells; (3) the presence of high circulating autoantibody titers; and (4) a clearly increased frequency of concomitant autoimmune disease in affected individuals, as well as associated family members [33]. Other putative causes for PSC have been suggested, such as mutations in the gene encoding the cystic fibrosis transmembrane receptor, recurrent bacterial infections, toxic biliary damage, and vascular insults [23, 24].
In the diagnosis of PSC, an exclusive diagnosis remains important and a comprehensive review of relevant clinical information, cholangiogram, and histological findings are required. Representative features of PSC are shown in Fig. 1. Histologically, characteristic periductal fibrosis (known as onion skin-like fibrosis) is noted in the large intrahepatic and extrahepatic bile ducts, and the septal and large bile ducts are selectively absent due to the presence of fibrous scars. However, similar periductal fibrosis is found in the intrahepatic small bile ducts in PSC, and similar findings for the small bile ducts have also been found in other biliary diseases that involve both intrahepatic large and extrahepatic bile ducts, including IgG4-SC and PSC [34]. Therefore, the presence or absence of periductal fibrosis in the small bile ducts, especially the interlobular and septal bile ducts are unable to differentiate PSC from IgG4-SC [35]. In contrast, a biliary biopsy is useful to obtain pathological information concerning malignancy and IgG4-positive cells.
Histological features of primary sclerosing cholangitis. a An extrahepatic bile duct with primary sclerosing cholangitis demonstrates that the mucosal layer is mainly affected (arrow). b The biliary epithelial layer is distorted (arrow) associated with periductal edematous and fibrous thickening (asterisks). c Characteristic periductal fibrosis (onion skin-like fibrosis). d Bile loss replaced by a fibrous scar (box). e Higher magnification of the fibrous scar
There is a need to accurately distinguish PSC from cholangiocarcinoma or IgG4-SC. In general, PSC is well known as an important precursor lesion of cholangiocarcinoma. In a 2012 Japanese nationwide survey, cholangiocarcinoma was found in 14/197 (7.3 %) of PSC cases [20]. The frequencies of cholangiocarcinoma in PSC patients have been reported to range between 2.8 and 19.9 % worldwide; with the reported lifetime risk for cholangiocarcinoma ranging from 8 to 36 % and the 10-year cumulative incidence from 11 to 31 % [36]. Moreover, PSC often accompanies dysplastic lesions (e.g., BilIN), and thus, PSC cases can be suspected of cholangiocarcinoma or misdiagnosed as malignant by the biopsy and cytology of the bile ducts and its contents. It is important to note that the pooled sensitivity and specificity of the bile duct brushings for a diagnosis of cholangiocarcinoma in patients with PSC were 43 and 97 %, respectively [37]. This was achieved with the aid of FISH, whereby the pooled sensitivity improved (68 %) but with a lower specificity (70 %) [38]. In addition, elevated serum IgG4 levels have been found in 6–22 % PSC patients [20, 30, 39–42] and a high level of IgG4-positive plasma infiltration in 23–47.5 % [>10/high power field (HPF)] or in 5–15.6 % (>50/HPF) [41, 43, 44]. This may pose difficulty in the differentiation between PSC and IgG4-SC. However, IgG4-positive plasma cells are not diffusely present in PSC, and besides IgG4-positive plasma cell infiltration, the other typical features of IgG4-SC are not observed [43, 44].
IgG4-related sclerosing cholangitis
IgG4-SC is an IgG4-related systemic condition and a manifestation of biliary disease characterized by increased levels of serum IgG4 (>135 mg/dL), marked infiltration of IgG4-positive plasma cells, and good steroid efficiency. It is similar to other organ involving IgG4-related diseases, and histologically, sclerosing fibrosis is observed as an organ-specific feature [45–47]. In general, IgG4-SC is diagnosed in 13–19.5 % of patients with IgG4-related systemic disease [48, 49]. IgG4-SC cases without the involvement of other organs are rare, and most cases (83–95.7 %) are accompanied by type 1 autoimmune pancreatitis (AIP); the prevalence and incidence of IgG4-SC in Japan are approximately 1.0 and 0.3 per 100,000 populations, respectively [48, 50–54]. In addition, about 80 % of patients with AIP suffer complications with stenosis of the distal common bile duct [55]. Moreover, IgG4-SC is preferably found in older males (male:female = 4:1; mean 67 years), characterized by more than 90 % of patients with IgG4-SC aged 50 or older [49]. Although the disease etiology remains poorly understood, allergic and autoimmune reactions against extrinsic or auto-antigens in genetically susceptible individuals have been suggested. This hypothesis is based on the increased number of eosinophils, the presence of autoantibodies, and the effectiveness of immunosuppressive treatments, including steroid therapy [50, 56].
The diagnosis of IgG4-SC is made through a combination of features, such as imaging, serology, histology, the involvement of other organs, and responses to steroid therapy [45, 57]. Elevated IgG4 concentrations in the serum play a significant role in the diagnosis of IgG4-SC. Serum IgG4 levels are elevated (>135 mg/dL) in 80–88 % of patients with IgG4-SC, and in 89.5 % patients with IgG4-SC without autoimmune pancreatitis (AIP) [20, 49, 50]. However, the range varies widely among studies, and the specificity is limited [53, 58–61]. Elevation of serum IgG4 levels is important, but is not necessarily specific to IgG4-SC as the levels may be elevated in patients with PSC, cholangiocarcinoma (~15 %), atopic dermatitis, pemphigus, asthma, and even a non-selected cohort of patients (approximately 7 %) that visited hospitals for various reasons [50, 55, 62]. Besides, nearly 50 % of patients with active IgG4-related diseases had normal serum IgG4 concentrations in a report from 2015 [63]. It is worth noting that some patients with markedly elevated serum IgG4 levels can have false negative levels due to the prozone (or hook) effect [64]. Markedly increased IgG4 levels greater than 270 or 208 mg/dL [twice the upper limit of normal (2 × ULN)] suggestive of IgG4-SC with a specificity and sensitivity of 90 and 50 %, respectively. However, levels greater than 540 or 560 mg/dL (4 × ULN) have been reported to be 100 % specific for distinguishing IgG4-SC from cholangiocarcinoma [53, 58]. In patients with serum IgG4 levels between 1 and 2 × ULN, the ratio of serum IgG4/IgG > 0.10 or IgG4/IgG1 > 0.24 is useful for discriminating IgG4-SC from other neoplastic and non-neoplastic biliary diseases [65]. Blood plasmablasts, particularly IgG4-positive plasmablasts counts decrease substantially after rituximab-mediated B-cell depletion therapy and are identified via flow cytometry. Moreover, plasmablast counts could be a potentially useful biomarker for diagnosis, assessing response to treatment, and determining the appropriate time for retreatment, as a more sensitive analysis than IgG4 concentrations [66, 67]. Furthermore, analyzing the IgG4/IgG RNA-ratio in the blood by qPCR is also potentially helpful [68].
Representative features of IgG4-SC are shown in Fig. 2. Pathological characteristics of IgG4-SC primarily consist of obliterative phlebitis and storiform fibrosis, as well as a marked infiltration of IgG4-positive plasma cells (mostly plasmablasts) in the affected bile ducts, which are included in the diagnostic criteria of IgG4-SC [45]. Inflammation consists of chronic inflammatory cells (e.g., plasma cells and lymphocytes), lymph follicles, and occasionally eosinophils. Differing from PSC, the inflammation is prominently found in the middle layer of the bile duct wall and existing peribiliary glands, rather than in the mucosal layer of the bile ducts. Therefore, biliary epithelial cells lining the bile ducts are usually well preserved, and the erosive change and neutrophil infiltration are rare in the affected bile ducts. These findings are useful in the pathological diagnosis and exfoliative cytodiagnosis of the affected bile ducts [69, 70]. Immunostaining for IgG4 displays the marked and typically diffuse infiltration of IgG4-positive plasma cells. The proposed cut-off values for IgG4 plasma cells for IgG4-SC are >10 cells/HPF for biopsy samples and >50 cells/HPF for surgical specimens. The ratio of IgG4-positive to total IgG4-positive plasma cells is greater than 40 % [46]. However, the presence of IgG4-positive cells is not the histological hallmark of IgG4-related diseases (including IgG4-SC) because the marked infiltration of IgG4-positive cells, while sometimes prominent, are also found in cases of PSC and cholangiocarcinoma (43 % of cases exhibited >10 IgG4-positive plasma cells/HPF) [41, 43, 44, 62, 71]. There have also been a few reported cases of IgG4-SC accompanied by cholangiocarcinoma or BilIN lesions, although rare [72–75]. Moreover, cases of IgG4-related diseases without the increased level of serum IgG4 or IgG4-positive cells in the affected organs have also been reported [76]. These atypical or similar diseases of IgG4-related diseases should be recognized, and its pathogenesis is needed for clarification in the future.
Pathology of IgG4-related sclerosing cholangitis. a Full thickening of the bile duct. b Transmural fibroinflammatory lesion. c High power of b shows lymphoplasmacytic infiltration with sparse eosinophils; the biliary epithelial layer is preserved. d Storiform fibrosis. e Elastic Van Gieson stain reveals obliterative phlebitis (arrows). f IgG4 immunostaining demonstrates numerous IgG4-positive plasma cells, distributing diffusely
Important differential diagnoses of IgG4-SC include cholangiocarcinoma, PSC, and pancreatic carcinoma. Indeed, many earlier cases of IgG4-SC have undergone surgery for suspected malignancies. Differentiating IgG4-SC cases without pancreatic and other organ involvement from PSC or cholangiocarcinoma is challenging [77, 78]. Elevated serum IgG4 concentrations are not always helpful for achieving a differential diagnosis, as previously mentioned. If imaging is not diagnostic, bile duct biopsy and cytological examination are particularly important to exclude malignancies and establish a diagnosis. However, a potential inflammatory reaction with a large number of IgG4-positive plasma cells within or around tumors in cholangiocarcinoma or BilIN lesions associated with IgG4-SC should be taken into consideration when diagnosing such cases [71]. Other histological features of IgG4-SC (e.g., storiform fibrosis and obliterative phlebitis), which can be differentiated from cholangiocarcinoma and PSC, are not located on the superficial biliary mucosa. Therefore, it is difficult to identify the histological findings from small biopsies of the superficial bile duct mucosa, and is impossible to completely exclude cholangiocarcinoma during the diagnosis of IgG4-SC by biopsy and cytology. Multiple biopsies and specimens from the same site may be needed to identify the cancerous or atypical cells [79]. Moreover, biopsies from the papilla of Vater [80] and liver [81] can be useful for IgG4-SC diagnosis.
Secondary sclerosing cholangitis
Bile duct stones, inflammatory polyp, biliary tumor, pancreatitis, aneurysm, and iatrogenic procedures (e.g., biliary operation) could cause persistent biliary obliteration to develop secondary sclerosing cholangitis due to cholestasis and cholangitis. Some cases are accompanied by recurrent pyogenic (ascending) cholangitis. Histologically, erosive changes with chronic and suppurative inflammation are found in the large bile ducts, and the residual biliary epithelium exhibits various reactive and neoplastic changes (e.g., hyperplasia and BilIN) to various degrees of severity. However, rare conditions (e.g., eosinophilic cholangitis and follicular cholangitis) can present a diagnostic dilemma by mimicking cholangiocarcinoma and other benign conditions (including IgG4-SC) due to similarities in some clinical, imaging, and histopathological features. Despite this issue, they typically attract less notice due to the rarity and under-recognition of these conditions, leading to misdiagnosis in clinical practice. In the following section, we selectively pay particular attention to these two diseases.
Eosinophilic cholangitis
Eosinophilic cholangitis is a rare, benign, inflammatory condition characterized by a dense transmural eosinophilic infiltration of the biliary tract that causes fibrosis, stricturing, and obstruction. It is often, but not necessarily associated with peripheral eosinophilia, and responds well to oral steroid therapy [4, 82, 83]. This disease was first reported by Leegaard in 1980 in relation to cholecystitis with obstructive jaundice [84]. In 1985, Butler et al. reported a case of eosinophilic cholangitis with gallbladder wall thickening and focal stricture in the left hepatic duct accompanied by eosinophilic infiltration of the cystic duct, gallbladder, lymph nodes, and bone marrow [85]. To date, only 38 cases have been documented [82–84, 86–89]. Eosinophilic cholangitis can affect a particular region or the entire biliary tract; it is described as localized when it involves only a specific portion of the extrahepatic biliary tree and diffused when it affects the extrahepatic biliary tree including the gallbladder [83]. Approximately 10 % of eosinophilic cholangitis cases involve both the bile duct and gallbladder [82].
Similar to IgG4-SC, eosinophilic cholangitis is believed to be part of a larger spectrum of disorders characterized by eosinophilic infiltration of tissues and organ systems, with or without concomitant peripheral eosinophilia [4]. All patients with this condition have unexplained eosinophilic proliferation. Indeed, multi-organ involvement, including the stomach [4, 86], ureters [90], kidneys [91], pancreas [92], lymph nodes, and bone marrow [85] has been described in the majority of eosinophilic cholangitis patients. However, there is no clear relationship between eosinophilic cholangitis and hypereosinophilic syndrome (HES) described in the literature. Most of the reported eosinophilic cholangitis cases do not appear to have met all of the criteria for HES [83] characterized by: (1) persistent eosinophilia (1500 eosinophils/mm3) for at least 6 months, or death before 6 months with signs and symptoms of HES disease; (2) exclusion of other recognized causes of eosinophilia; and (3) organ system involvement or dysfunction attributable to eosinophil infiltration or not otherwise explained [85].
Although the exact cause of eosinophilic cholangitis remains poorly understood, there have been previously reported associations with cholelithiasis [93], parasitic infection [94], Enterobacter aerogenes infection [95], or antibiotic treatment [89, 96]. A relationship between Helicobacter pylori infection and eosinophilic diseases has also been documented [82]. However, despite these reports, the pathogenesis of eosinophilic cholangitis remains unclear. An allergic mechanism is thought to play a key role in the development of this condition. Elevated levels of serum IgE, interleukin (IL)-5, and eosinophil cationic protein levels have been reported in the majority of cases [83], with about 70 % of patients with eosinophilic cholangitis exhibiting peripheral eosinophilia [82].
The diagnosis of eosinophilic cholangitis is based primarily on the histological findings. Matsumoto et al. have proposed the following diagnostic criteria: (1) wall thickening or stenosis of the biliary system; (2) histopathological findings of eosinophilic infiltration; and (3) reversibility of biliary abnormalities without treatment, or following steroid treatment [96]. Peripheral eosinophilia is one component for the diagnosis of eosinophilic cholangitis, but it is neither sensitive nor specific to evaluate the dense eosinophilic infiltration of the bile duct [4, 93]. Moreover, peripheral eosinophilia has also been observed in primary biliary cirrhosis (cholangitis), primary sclerosing cholangitis, eosinophilic esophagitis, eosinophilic gastroenteritis, and IgG4-SC. Histopathologically, the thickening of the bile duct wall [93], narrow stenosis, and dilation of the bile duct [97] (revealing diffuse or segmental strictures via imaging), have been observed in most cases [82]. Pseudotumors presenting with multilobulated masses encasing and obstructing the common hepatic duct accompanied by similar masses in the wall of the gallbladder, or a mass (up to 5 cm in diameter) encasing the porta hepatis accompanied by a narrowing of the common bile duct has also been noted [98]. The microscopic appearances of eosinophilic cholangitis are characterized by periductal, periglandular fibrosis, consisting of a pronounced transmural infiltration of inflammatory cells in the affected bile duct comprised prominently of eosinophils that distribute diffusely or form clusters and eosinophilic abscesses [4]. Other inflammatory cells, including lymphocytes, plasma cells, histiocytes, and lymphoid follicles may also be observed [98]. The bile duct epithelium is intact or displays variable changes with regards to the level of eosinophil infiltrate, degeneration, ulceration, hyperplasia, and inflammatory/regenerative atypia. The eosinophilic infiltration may involve other organs apart from the bile duct, such as the liver, gallbladder, stomach, and pancreas.
Differentiating eosinophilic cholangitis from cholangiocarcinoma is challenging because it can mimic cholangiocarcinoma both in clinical presentation and in the radiological characteristics [93, 99]. Indeed, most cases of eosinophilic cholangitis reported in the literature were diagnosed with suspected cancer of the bile tract and received a retrograde diagnosis following surgery. From imaging, eosinophilic cholangitis commonly displays a thickening of the bile duct wall (with or without biliary dilatation), and an irregular narrowing appearance has also been noted [4]. However, these findings are nonspecific and are also seen in malignant diseases [2]. Carbohydrate antigen 19-9 has been commonly used as a screening tool for cholangiocarcinoma, but its sensitivity is reported to be only 69 % for the diagnosis of all hepatobiliary malignancies [100]. Brush cytology and an intraductal biopsy obtained from the bile duct with eosinophilic presentation and without malignancy are important to rule out the possibility of cholangiocarcinoma. However, eosinophilic cholangitis can be difficult to differentiate from IgG4-SC or PSC. Peripheral eosinophilia has been observed in 27 % of PSC patients in Japan [101]. Moreover, mild-to-moderate peripheral eosinophilia is found in 34 % of patients with IgG4-related diseases, and an elevation in the serum concentration of IgG4 in eosinophilic cholangitis has been reported [102]. The improvement of the disease after steroid treatment has also been noted in IgG4-SC. However, based on the distinctive histopathological features of IgG4-SC, PSC and combinations of clinical and laboratory information may facilitate the differential diagnosis. The majority of the unresected cases of eosinophilic cholangitis have a diffuse type of biliary stricture, severe eosinophilia (>1000/μL), and a stable clinical course with steroid treatment [82]. Therefore, although rare, eosinophilic cholangitis should be considered in the diagnosis of bile duct strictures, particularly, in the setting of peripheral eosinophilia.
Follicular cholangitis
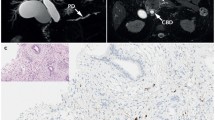
Another variant of sclerosing cholangitis is follicular cholangitis characterized by a marked periductal lymphoplasmacytic infiltrate, and the presence of numerous lymphoid follicles with reactive germinal centers (Fig. 3). This extremely rare clinicopathologic entity preferentially affects the hilar bile ducts of adults, represents radiological features suggestive of hilar cholangiocarcinoma, and may progress to cirrhosis if left untreated [103]. Since the first case reported by Aoki et al. [104], there have been only eight documented cases of follicular cholangitis [5, 12, 103–105]. It accounted for 1.1 % (two cases) in a report of 176 cases of hilar biliary stricture operations [12]. Follicular cholangitis is a disease of middle-aged to elderly patients, with a mean age of 57 years (range 42–73 years) from all reported cases, with a female-male ratio of 1.7:1. Patients usually present with a gradual progression of the disease, characterized by an elevation of serum liver enzymes at the supposed time of onset, a normal serum IgG4 concentration, and negative for antinuclear antibodies. The etiology of follicular cholangitis remains unknown, although treatment with corticosteroids may improve the disease [105].
Follicular cholangitis. a Marked inflammatory infiltration; higher magnification of the bile duct (box) is showed in b. b Periductal fibrosis with numerous variable-sized lymphoid follicles. c Lymphoplasmacytic infiltration is found under the epithelium; the biliary epithelial layer is intact. d Immunohistochemistry staining reveals some IgG4-positive plasma cells
Histopathology remains the hallmark of follicular cholangitis diagnosis. Macroscopically, affected bile ducts exhibit marked irregular wall thickening associated with bile duct dilatation. The mucosa of the bile ducts in cases of follicular cholangitis may include moderate stenosis by granular nodules that have a “cobblestone-like” appearance, appearing as granular filling defects on the cholangiogram or show diffuse, smooth narrowing [5]. Microscopically, the mucosal epithelia are usually intact, but may demonstrate inflammation or reproduction resulting in a cellular atypia appearance via bile duct brush cytology [105]. The affected bile ducts show dense periductal fibrosis with marked lymphoplasmacytic infiltration under the epithelium. In addition, there are numerous variable-sized lymphoid follicles surrounding the bile ducts, distributing focally or diffusely [12]. The collagenous fibrosis differs from the storiform fibrosis typically observed in IgG4-related disease. Lymphoid follicles are well circumscribed, surrounded by an intact mantle zone, and are usually contained a germinal center without atypical features of lymphocytes and plasma cells. These specific findings are primarily localized at the proximal extrahepatic bile ducts and hepatic hilum. The intrahepatic bile ducts are somewhat intact, but lymphoid aggregates in the small portal tracts have also been noted [103]. No abnormal findings, such as onion skin-like lesions in the intrahepatic peripheral bile ducts have been documented. Lymphoid follicles have also been observed in the gallbladder mucosa in some patients with follicular cholangitis [103]. Immunohistochemically, the infiltrating lymphocytes consist of CD3-positive T cells and CD20-positive B cells; CD4, CD8, and CD79a are also expressed. The distribution of λ-chain and κ-chain cells supports the polyclonal nature of B cells and plasma cells [103]. Bcl-2 is not expressed in the germinal center [103, 105], and IgG4-positive plasma cells are usually absent. Some cases reported by Zen et al. demonstrated focal aggregations occasionally (range 5–21/HPF), representing 2–15 % of the cells, far fewer than that expected in IgG4-related disease [46, 103]. Similar histologic findings have been observed in the pancreas and the gallbladder, which is termed follicular pancreatitis, or follicular cholecystitis, respectively [103, 106, 107].
It is extremely difficult to differentiate follicular cholangitis from hilar cholangiocarcinoma when both are based on clinical and imaging features. The majority of the reported cases were treated surgically based on a preoperative diagnosis of cholangiocarcinoma. It may be useful to perform cholangioscopy and a biopsy of the lymph gland by endoscopic ultrasound with fine-needle aspiration for the diagnosis of this disease [105]. From a cytological perspective, the presence of atypical epithelial cells may raise concerns of a neoplasm, which creates difficulty for a diagnosis. However, histologic features are easily appreciated from the core biopsies of the bile ducts and may establish a diagnosis. In addition, follicular cholangitis may mimic PSC when bile ducts are more diffusely involved. Indeed, one patient was diagnosed preoperatively with PSC and was referred to a hospital for a liver transplantation [103]. However, histopathologically, follicular cholangitis exhibits prominent lymph follicles with germinal centers, which can also be seen in PSC or IgG4-SC, but are less extensive [69]. Furthermore, follicular cholangitis lacks the histologic and immunohistochemical features of IgG4-SC and PSC. Another differential diagnosis (although extremely rare) [108], also includes low-grade B-cell lymphomas associated with prominent germinal center formation (e.g., follicular lymphoma). The germinal centers in follicular cholangitis are negative for Bcl-2 [103, 105], which is in contrast to the follicles of low-grade follicular lymphoma which are nearly always Bcl-2 positive [107]. Finally, to obtain an accurate diagnosis, follicular cholangitis should be taken into account in the differential diagnosis of hilar biliary strictures.
Conclusion
Biopsy and cytology of bile ducts and bile samples, as well as a frozen section diagnosis of the marginal bile duct from a malignancy, are the depressed pathological specimens for general pathologists. In addition, the pathological diagnosis is also possibly influenced by the radiological findings and the clinical diagnosis. The presence of malignant pathological findings indicates a definite pathological diagnosis. However, the gray zones caused by dysplastic lesions, and the degenerated changes often found in tiny specimens of the biliary tree area, as well as borderline lesions are encountered in resected surgical specimens. In contrast, although many malignant and dysplastic lesions are not found in biopsy and cytology, definite malignant lesions are often found in surgically resected specimens. Therefore, pathologists should routinely perform bold diagnoses, and it is important to communicate such findings with clinicians on a daily basis.
References
Bowlus CL, Olson KA, Gershwin ME (2016) Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol 13(1):28–37
Katabathina VS, Dasyam AK, Dasyam N, Hosseinzadeh K (2014) Adult bile duct strictures: role of MR imaging and MR cholangiopancreatography in characterization. Radiographics 34(3):565–586
Kirchner GI, Rummele P (2015) Update on sclerosing cholangitis in critically Ill patients. Viszeralmedizin 31(3):178–184
Nashed C, Sakpal SV, Shusharina V, Chamberlain RS (2010) Eosinophilic cholangitis and cholangiopathy: a sheep in wolves clothing. HPB Surg 2010:906496
Lee JY, Lim JH, Lim HK (2005) Follicular cholangitis mimicking hilar cholangiocarcinoma. Abdom Imaging 30(6):744–747
Zepeda-Gomez S, Baron TH (2011) Benign biliary strictures: current endoscopic management. Nat Rev Gastroenterol Hepatol 8(10):573–581
Kaffes AJ (2015) Management of benign biliary strictures: current status and perspective. J Hepatobiliary Pancreat Sci 22(9):657–663
Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA (2015) Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 81(1):168–176
Nanda A, Brown JM, Berger SH, Lewis MM, Barr Fritcher EG, Gores GJ, Keilin SA, Woods KE, Cai Q, Willingham FF (2015) Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol 8(2):56–65
Sadeghi A, Mohamadnejad M, Islami F, Keshtkar A, Biglari M, Malekzadeh R, Eloubeidi MA (2016) Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc 83(2):290–298 (e1)
Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH (2015) Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 82(4):608–614
Fujita T, Kojima M, Kato Y, Gotohda N, Takahashi S, Konishi M, Kinoshita T (2010) Clinical and histopathological study of “follicular cholangitis”: sclerosing cholangitis with prominent lymphocytic infiltration masquerading as hilar cholangiocarcinoma. Hepatol Res 40(12):1239–1247
Wakai T, Shirai Y, Sakata J, Maruyama T, Ohashi T, Korira PV, Ajioka Y, Hatakeyama K (2012) Clinicopathological features of benign biliary strictures masquerading as biliary malignancy. Am Surg 78(12):1388–1391
de Vries AB, Janse M, Blokzijl H, Weersma RK (2015) Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol 21(6):1956–1971
Sano H, Nakazawa T, Ando T, Hayashi K, Naitoh I, Okumura F, Miyabe K, Yoshida M, Takahashi S, Ohara H, Joh T (2011) Clinical characteristics of inflammatory bowel disease associated with primary sclerosing cholangitis. J Hepatobiliary Pancreat Sci 18(2):154–161
Aadland E, Schrumpf E, Fausa O, Elgjo K, Heilo A, Aakhus T, Gjone E (1987) Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol 22(6):655–664
Nakazawa T, Naitoh I, Hayashi K, Sano H, Miyabe K, Shimizu S, Joh T (2014) Inflammatory bowel disease of primary sclerosing cholangitis: a distinct entity? World J Gastroenterol 20(12):3245–3254
Tanaka A, Takikawa H (2013) Geoepidemiology of primary sclerosing cholangitis: a critical review. J Autoimmun 46:35–40
Weismüller TJ, Talwalkar JA, Ponsioen CY, Gotthardt DN, Marschall HU, Naess S, Holm K, Weersma RK, Lazaridis KN, Fevery J, Trivedi PJ, Schramm C, Chazouilleres O, Müller T, Farkkila M, Almer S, Pereira S, Mason AL, Floreani A, Milkiewicz P, Harley H, Pares A, de Vries L, Manser C, Gatselis N, Berg C, Lenzen H, de Valle MB, Imam M, Kirchner G, de Leuw P, Zimmer V, Fabris L, Braun F, Hirschfield GM, Marzioni M, Juran BD, Strassburg CP, Beuers U, Manns MP, Schrumpf E, Karlsen TH, Bergquist A, Boberg KM (2014) Primary sclerosing cholangitis from a global perspective—a multicenter, retrospective, observational study of the international PSC study group. J Hepatol 60(1, Supplement):S3
Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H (2014) Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci 21(1):43–50
Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H (2015) Clinical profiles of patients with primary sclerosing cholangitis in the elderly. J Hepatobiliary Pancreat Sci 22(3):230–236
Yimam KK, Bowlus CL (2014) Diagnosis and classification of primary sclerosing cholangitis. Autoimmun Rev 13(4–5):445–450
Lindor KD, Kowdley KV, Harrison ME, American College of G (2015) ACG clinical guideline: primary sclerosing cholangitis. Am J Gastroenterol 110(5):646–659 (quiz 660)
Hirschfield GM, Karlsen TH, Lindor KD, Adams DH (2013) Primary sclerosing cholangitis. The Lancet 382(9904):1587–1599
Angulo P, Maor-Kendler Y, Lindor KD (2002) Small-duct primary sclerosing cholangitis: a long-term follow-up study. Hepatology 35(6):1494–1500
Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS (2007) The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol 102(5):1042–1049
Mieli-Vergani G, Vergani D (2016) Sclerosing cholangitis in children and adolescents. Clin Liver Dis 20(1):99–111
Sarkar S, Bowlus CL (2016) Primary sclerosing cholangitis: multiple phenotypes, multiple approaches. Clin Liver Dis 20(1):67–77
Berntsen NL, Klingenberg O, Juran BD, Benito de Valle M, Lindkvist B, Lazaridis KN, Boberg KM, Karlsen TH, Hov JR (2015) Association between HLA haplotypes and increased serum levels of IgG4 in patients with primary sclerosing cholangitis. Gastroenterology 148(5):924–927 (e2)
Matsubayashi H, Igarashi K, Kishida Y, Yoshida Y, Sasaki K, Ono H (2014) Sclerosing cholangitis with thumbprint appearance and incomplete steroid response. J Dig Dis 15(10):578–582
Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, Andreassen OA, Weersma RK, Weismuller TJ, Eksteen B, Invernizzi P, Hirschfield GM, Gotthardt DN, Pares A, Ellinghaus D, Shah T, Juran BD, Milkiewicz P, Rust C, Schramm C, Muller T, Srivastava B, Dalekos G, Nothen MM, Herms S, Winkelmann J, Mitrovic M, Braun F, Ponsioen CY, Croucher PJ, Sterneck M, Teufel A, Mason AL, Saarela J, Leppa V, Dorfman R, Alvaro D, Floreani A, Onengut-Gumuscu S, Rich SS, Thompson WK, Schork AJ, Naess S, Thomsen I, Mayr G, Konig IR, Hveem K, Cleynen I, Gutierrez-Achury J, Ricano-Ponce I, van Heel D, Bjornsson E, Sandford RN, Durie PR, Melum E, Vatn MH, Silverberg MS, Duerr RH, Padyukov L, Brand S, Sans M, Annese V, Achkar JP, Boberg KM, Marschall HU, Chazouilleres O, Bowlus CL, Wijmenga C, Schrumpf E, Vermeire S, Albrecht M, Consortium U-P, Rioux JD, Alexander G, Bergquist A, Cho J, Schreiber S, Manns MP, Farkkila M, Dale AM, Chapman RW, Lazaridis KN, International PSCSG, Franke A, Anderson CA, Karlsen TH, International IBDGC (2013) Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet 45(6):670–675
Nakanuma Y, Sasaki M, Harada K (2015) Autophagy and senescence in fibrosing cholangiopathies. J Hepatol 62(4):934–945
Trivedi PJ, Hirschfield GM (2016) The immunogenetics of autoimmune cholestasis. Clin Liver Dis 20(1):15–31
Naitoh I, Zen Y, Nakazawa T, Ando T, Hayashi K, Okumura F, Miyabe K, Yoshida M, Nojiri S, Kanematsu T, Ohara H, Joh T (2011) Small bile duct involvement in IgG4-related sclerosing cholangitis: liver biopsy and cholangiography correlation. J Gastroenterol 46(2):269–276
Deshpande V, Sainani NI, Chung RT, Pratt DS, Mentha G, Rubbia-Brandt L, Lauwers GY (2009) IgG4-associated cholangitis: a comparative histological and immunophenotypic study with primary sclerosing cholangitis on liver biopsy material. Mod Pathol 22(10):1287–1295
Folseraas T, Boberg KM (2016) Cancer risk and surveillance in primary sclerosing cholangitis. Clin Liver Dis 20(1):79–98
Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA (2014) Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 79(5):783–789
Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA (2014) Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 79(6): 943–950 (e3)
Benito de Valle M, Muller T, Bjornsson E, Otten M, Volkmann M, Guckelberger O, Wiedenmann B, Sadik R, Schott E, Andersson M, Berg T, Lindkvist B (2014) The impact of elevated serum IgG4 levels in patients with primary sclerosing cholangitis. Dig Liver Dis 46(10):903–908
Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD (2006) Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol 101(9):2070–2075
Zhang L, Lewis JT, Abraham SC, Smyrk TC, Leung S, Chari ST, Poterucha JJ, Rosen CB, Lohse CM, Katzmann JA, Wu TT (2010) IgG4+ plasma cell infiltrates in liver explants with primary sclerosing cholangitis. Am J Surg Pathol 34(1):88–94
Navaneethan U, Venkatesh PG, Choudhary M, Shen B, Kiran RP (2013) Elevated immunoglobulin G4 level is associated with reduced colectomy-free survival in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis 7(2):e35–e41
Zen Y, Quaglia A, Portmann B (2011) Immunoglobulin G4-positive plasma cell infiltration in explanted livers for primary sclerosing cholangitis. Histopathology 58(3):414–422
Fischer S, Trivedi PJ, Ward S, Greig PD, Therapondos G, Hirschfield GM (2014) Frequency and significance of IgG4 immunohistochemical staining in liver explants from patients with primary sclerosing cholangitis. Int J Exp Pathol 95(3):209–215
Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, Nishino T, Ko SB, Mizuno N, Hamano H, Kanno A, Notohara K, Hasebe O, Nakazawa T, Nakanuma Y, Takikawa H, Research Committee of Ig GrD, Research Committee of Intractable Diseases of L, Biliary T, Ministry of Health L, Welfare J, Japan Biliary A (2012) Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci 19(5):536–542
Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Kloppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH (2012) Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25(9):1181–1192
Stone JH, Zen Y, Deshpande V (2012) IgG4-related disease. N Engl J Med 366(6):539–551
Brito-Zeron P, Ramos-Casals M, Bosch X, Stone JH (2014) The clinical spectrum of IgG4-related disease. Autoimmun Rev 13(12):1203–1210
Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, Okumura K, Toshima F, Toyama J, Minami T, Matsui O, Gabata T, Zen Y (2015) IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 94(15):e680
Zen Y, Kawakami H, Kim JH (2016) IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol 51(4):295–312
Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T (2013) Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol 19(43):7661–7670
Nakazawa T, Ikeda Y, Kawaguchi Y, Kitagawa H, Takada H, Takeda Y, Makino I, Makino N, Naitoh I, Tanaka A (2015) Isolated intrapancreatic IgG4-related sclerosing cholangitis. World J Gastroenterol 21(4):1334–1343
Ohara H, Nakazawa T, Kawa S, Kamisawa T, Shimosegawa T, Uchida K, Hirano K, Nishino T, Hamano H, Kanno A, Notohara K, Hasebe O, Muraki T, Ishida E, Naitoh I, Okazaki K (2013) Establishment of a serum IgG4 cut-off value for the differential diagnosis of IgG4-related sclerosing cholangitis: a Japanese cohort. J Gastroenterol Hepatol 28(7):1247–1251
Graham RPD, Smyrk TC, Chari ST, Takahashi N, Zhang L (2014) Isolated IgG4-related sclerosing cholangitis: a report of 9 cases. Hum Pathol 45(8):1722–1729
Okazaki K, Uchida K, Koyabu M, Miyoshi H, Ikeura T, Takaoka M (2014) IgG4 cholangiopathy—current concept, diagnosis, and pathogenesis. J Hepatol 61(3):690–695
Hart PA, Zen Y, Chari ST (2015) Recent advances in autoimmune pancreatitis. Gastroenterology 149(1):39–51
Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E, Frulloni L, Goto H, Hart PA, Kamisawa T, Kawa S, Kawano M, Kim MH, Kodama Y, Kubota K, Lerch MM, Löhr M, Masaki Y, Matsui S, Mimori T, Nakamura S, Nakazawa T, Ohara H, Okazaki K, Ryu JH, Saeki T, Schleinitz N, Shimatsu A, Shimosegawa T, Takahashi H, Takahira M, Tanaka A, Topazian M, Umehara H, Webster GJ, Witzig TE, Yamamoto M, Zhang W, Chiba T, Stone JH (2015) International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol 67(7):1688–1699
Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, Aderca I, Mettler TA, Therneau TM, Zhang L, Takahashi N, Chari ST, Roberts LR (2011) Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 54(3):940–948
Stone JH, Brito-Zerón P, Bosch X, Ramos-Casals M (2015) Diagnostic approach to the complexity of IgG4-related disease. Mayo Clin Proc 90(7):927–939
Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH (2015) The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 74(1):14–18
Ngwa TN, Law R, Murray D, Chari ST (2014) Serum immunoglobulin G4 level is a poor predictor of immunoglobulin G4-related disease. Pancreas 43(5):704–707
Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, Ren XS, Sato H, Nakanuma Y (2012) Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology 56(1):157–164
Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, Stone JH (2015) IgG4-related disease: clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol 67(9):2466–2475
Khosroshahi A, Cheryk LA, Carruthers MN, Edwards JA, Bloch DB, Stone JH (2014) Spuriously low serum IgG4 concentrations caused by the prozone phenomenon in patients with IgG4-related disease. Arthritis Rheumatol 66(1):213–217
Boonstra K, Culver EL, de Buy Wenniger LM, van Heerde MJ, van Erpecum KJ, Poen AC, van Nieuwkerk KM, Spanier BW, Witteman BJ, Tuynman HA, van Geloven N, van Buuren H, Chapman RW, Barnes E, Beuers U, Ponsioen CY (2014) Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology 59(5):1954–1963
Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, Kulikova M, Deshpande V, Pillai S, Stone JH (2015) Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis 74(1):190–195
Mattoo H, Mahajan VS, Della-Torre E, Sekigami Y, Carruthers M, Wallace ZS, Deshpande V, Stone JH, Pillai S (2014) De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 134(3):679–687
Smit WL, Culver EL, Chapman RW (2016) New thoughts on immunoglobulin G4-related sclerosing cholangitis. Clin Liver Dis 20(1):47–65
Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y (2004) IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 28(9):1193–1203
Zen Y, Nakanuma Y, Portmann B (2012) Immunoglobulin G4-related sclerosing cholangitis: pathologic features and histologic mimics. Semin Diagn Pathol 29(4):205–211
Harada K, Nakanuma Y (2014) Cholangiocarcinoma with respect to IgG4 reaction. Int J Hepatol 2014:803876
Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJ, Barnes E (2014) Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol 109(10):1675–1683
Straub BK, Esposito I, Gotthardt D, Radeleff B, Antolovic D, Flechtenmacher C, Schirmacher P (2011) IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch 458(6):761–765
Oh HC, Kim JG, Kim JW, Lee KS, Kim MK, Chi KC, Kim YS, Kim KH (2008) Early bile duct cancer in a background of sclerosing cholangitis and autoimmune pancreatitis. Intern Med 47(23):2025–2028
Ohtani H, Ishida H, Ito Y, Yamaguchi T, Koizumi M (2011) Autoimmune pancreatitis and biliary intraepithelial neoplasia of the common bile duct: a case with diagnostically challenging but pathogenetically significant association. Pathol Int 61(8):481–485
Hart PA, Smyrk TC, Chari ST (2015) Lymphoplasmacytic sclerosing pancreatitis without IgG4 tissue infiltration or serum IgG4 elevation: IgG4-related disease without IgG4. Mod Pathol 28(2):238–247
Lin J, Cummings OW, Greenson JK, House MG, Liu X, Nalbantoglu I, Pai R, Davidson DD, Reuss SA (2015) IgG4-related sclerosing cholangitis in the absence of autoimmune pancreatitis mimicking extrahepatic cholangiocarcinoma. Scand J Gastroenterol 50(4):447–453
Matsubayashi H, Uesaka K, Sugiura T, Ohgi K, Sasaki K, Ono H (2014) IgG4-related sclerosing cholangitis without obvious pancreatic lesion: difficulty in differential diagnosis. J Dig Dis 15(7):394–403
Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K (2001) High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344(10):732–738
Kawakami H, Zen Y, Kuwatani M, Eto K, Haba S, Yamato H, Shinada K, Kubota K, Asaka M (2010) IgG4-related sclerosing cholangitis and autoimmune pancreatitis: histological assessment of biopsies from Vater’s ampulla and the bile duct. J Gastroenterol Hepatol 25(10):1648–1655
Umemura T, Zen Y, Hamano H, Kawa S, Nakanuma Y, Kiyosawa K (2007) Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology 46(2):463–471
Hokuto D, Yamato I, Nomi T, Yasuda S, Obara S, Yamada T, Chihiro K, Nakajima Y (2015) Eosinophilic cholangitis coexisted with idiopathic thrombocytopenic purpura: report of a case. Hepatol Res 45(5):595–600
Fragulidis GP, Vezakis AI, Kontis EA, Pantiora EV, Stefanidis GG, Politi AN, Koutoulidis VK, Mela MK, Polydorou AA (2016) Eosinophilic cholangitis-a challenging diagnosis of benign biliary stricture: a case report. Medicine (Baltimore) 95(1):e2394
Leegaard M (1980) Eosinophilic cholecystitis. Acta Chir Scand 146(4):295–296
Butler TW, Feintuch TA, Caine WP Jr (1985) Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: a case report. Am J Gastroenterol 80(7):572–574
Goode EC, Simpson Bw, Rushbrook SM, Rushbrook SM (2013) A rare cause of cholangiopathy. Gastroenterology 144(7):e14–e15
Yagawa Y, Yasuda H, Sugimoto M, Koda K, Suzuki M, Yamazaki M, Tezuka T, Kosugi C, Higuchi R, Watayo Y (2008) Two cases of eosinophilic cholangitis caused biliary obstruction. Jpn J Gastroenterol Surg 41(5):533–539
Usuki N, Toyoshima M, Mikami S, Katsuyama E (2011) A case of eosinophilic cholangitis. Jpn J Clin Radiol 56(2):261–264
Oh SR, Kim D, Kim TH (2012) Eosinophilic cholangiopathy. Gastrointest Endosc 75(3):669–670
Platt ML, Kiesling VJ Jr, Vaccaro JA (1990) Eosinophilic ureteritis associated with eosinophilic cholangitis: a case report. J Urol 144(1):127–129
Grauer L, Padilla Iii VM, Bouza L, Barkin JS (1993) Eosinophilic sclerosing cholangitis associated with hypereosinophilic syndrome. Am J Gastroenterol 88(10):1764–1769
Kroemer A, Sabet-Baktach M, Doenecke A, Ruemmele P, Scherer MN, Schlitt HJ, Breidert M (2012) Eosinophilic cholangitis and wirsungitis as cause of simultaneous bile duct obstruction and pancreatitis. Z Gastroenterol 50(8):766–770
Rodgers MS, Allen JP, Koea JB, McCall JL (2001) Eosinophilic cholangitis: a case of ‘malignant masquerade’. HPB (Oxford) 3(3):235–239
Raptou G, Pliakos I, Hytiroglou P, Papavramidis S, Karkavelas G (2009) Severe eosinophilic cholangitis with parenchymal destruction of the left hepatic lobe due to hydatid disease. Pathol Int 59(6):395–398
Rosengart TK, Rotterdam H, Ranson JHC (1990) Eosinophilic cholangitis: a self-limited cause of extrahepatic biliary obstruction. Am J Gastroenterol 85(5):582–585
Matsumoto N, Yokohama K, Nakai K, Yamamoto T, Otani T, Ogawa M, Tanaka N, Iwasaki A, Arakawa Y, Sugitani M (2007) A case of eosinophilic cholangitis: imaging findings of contrast-enhanced ultrasonography, cholangioscopy, and intraductal ultrasonography. World J Gastroenterol 13(13):1995–1997
Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, Kato K, Takada T, Fukushima J, Kondo F, Takikawa H (2009) Resected case of eosinophilic cholangiopathy presenting with secondary Sclerosing cholangitis. World J Gastroenterol 15(11):1394–1397
Shanti CM, Lucas CE, Tyburski JG, Tyburski JG, Soulen RL, Soulen Rl, Lucas DR (2001) Eosinophilic abscess and eosinophilic pseudotumor presenting as bile duct masses: a report of 2 cases. Surgery 130(1):104–108
Chen WH, Yu CC, Wu CC, Jan YJ (2009) Eosinophilic cholangitis with obstructive jaundice mimicking bile duct carcinoma. J Hepatobiliary Pancreat Surg 16(2):242–245
Burnett AS, Bailey J, Oliver JB, Ahlawat S, Chokshi RJ (2014) Sensitivity of alternative testing for pancreaticobiliary cancer: a 10-y review of the literature. J Surg Res 190(2):535–547
Takikawa H (1999) Recent status of primary sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Surg 6(4):352–355
Iwamuro M, Yamamoto K, Kawamoto H, Terada R, Ogawa T, Nose S (2009) Eosinophilic cholangitis with initial clinical features indistinguishable from IgG4-related cholangitis. Intern Med 48(13):1143–1147
Zen Y, Ishikawa A, Ogiso S, Heaton N, Portmann B (2012) Follicular cholangitis and pancreatitis - clinicopathological features and differential diagnosis of an under-recognized entity. Histopathology 60(2):261–269
Aoki T, Kubota K, Oka T, Hasegawa K, Hirai I, Makuuchi M (2003) Follicular cholangitis: another cause of benign biliary stricture. Hepatogastroenterology 50(51):639–642
Fujii M, Shiode J, Niguma T, Ito M, Ishiyama S, Fujiwara A, Nose S, Yoshioka M, Mimura T (2014) A case of follicular cholangitis mimicking hilar cholangiocarcinoma. Clin J Gastroenterol 7(1):62–67
Mizuuchi Y, Aishima S, Hattori M, Ushijima Y, Aso A, Takahata S, Ohtsuka T, Ueda J, Tanaka M, Oda Y (2014) Follicular pancreatitis, report of a case clinically mimicking pancreatic cancer and literature review. Pathol Res Pract 210(2):118–122
Gupta RK, Xie BH, Patton KT, Lisovsky M, Burks E, Behrman SW, Klimstra D, Deshpande V (2016) Follicular pancreatitis: a distinct form of chronic pancreatitis—an additional mimic of pancreatic neoplasms. Hum Pathol 48:154–162
Zakaria A, Al-Obeidi S, Daradkeh S (2013) Primary non-Hodgkin’s lymphoma of the common bile duct: a case report and literature review. Asian J Surg doi:10.1016/j.asjsur.2013.09.009
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Nguyen Canh, H., Harada, K. Adult bile duct strictures: differentiating benign biliary stenosis from cholangiocarcinoma. Med Mol Morphol 49, 189–202 (2016). https://doi.org/10.1007/s00795-016-0143-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-016-0143-6