Abstract
Pseudoaneurysm (PSA) of the visceral arterial tree is an uncommon but highly lethal complication of pancreatic surgery and pancreatitis. Surgical and angiographic interventions are used in treatment; however, optimal therapy remains unclear. We hypothesized that the natural history of PSA is different in these discrete clinical settings. From 1995–2005, 37 patients with PSA were treated: 13 after pancreatic surgery and 24 in the setting of pancreatitis. Postoperative patients most frequently presented with bleeding (92%), either from the gastrointestinal (GI) tract or a surgical drain. In this group, the diagnosis was most commonly made by angiography (77%), and 62% had a pancreatic fistula. In patients with pancreatitis, abdominal pain was the only presenting symptom in 62%, and GI bleeding was present in 29%. Eighty-seven percent had an associated pseudocyst or fluid collection. Interventional radiologic therapy successfully arrested hemorrhage in all 35 patients in whom it was employed. There were four false negative angiograms, and two patients required repeated interventions for rebleeding. The overall mortality was 14%. Pseudoaneurysms present differently in these two clinical settings, but transcatheter intervention is the first treatment of choice in clinically stable patients. Early recognition and prompt angiographic occlusion leads to improved outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pseudoaneurysm (PSA) of the visceral arterial tree may arise both in the postoperative period after pancreatic surgery and in the setting of acute and chronic pancreatitis.1,2 Although uncommon, massive hemorrhage from PSA has long been recognized as the most rapidly lethal complication in both of these clinical scenarios, with reported mortality rates ranging from 25 to 50%.1,2 Most reports of PSA consist either of small case series or small subgroups of patients with bleeding complications reported in the context of larger series of pancreatic surgery. The rarity of PSA and heterogeneity of the associated inflammatory states, combined with the frequently urgent nature of its presentation, have made it difficult to define the optimal clinical management.
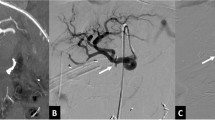
Recent advances in interventional radiology techniques have led to more widespread application of angiography and embolization for treatment of PSA. (Fig. 1) Although recent small series using transcatheter embolization have demonstrated good outcomes, some authorities continue to advocate surgical intervention.3,4 Over the past decade at a tertiary pancreatic referral center, we have treated a large number of patients with PSA both after pancreatic surgery and in the setting of pancreatitis, increasingly by utilizing interventional radiology techniques. The purpose of this review was to compare the clinical presentation, diagnosis, and treatment of patients with visceral PSA in these two discrete settings. We hypothesized that the presentation and clinical course may be different in these two groups of patients. In addition, we sought to clarify the role of angiographic intervention in treatment of this potentially devastating condition.
Methods
This retrospective analysis was approved by the Indiana University institutional review board (study number 0405-55). The study time period was 1995–2005. Patients presenting to Indiana University with the diagnosis of PSA of the visceral arterial tree (ICD-9 codes 442.83 and 442.84) were identified from a discharge database and cross-validated against a separate, prospectively collected interventional radiology database (HI-IQ). Clinical factors were abstracted from review of patient charts. SAS version 9.0 (SAS Institute, Cary, NC) was used to perform all the statistical analysis. Pearson chi-square or Fisher’s exact test was used as appropriate to compare categorical variables; Wilcoxon rank-sum test was used to compare median values of continuous data. A two-sided P value of less than 0.05 was considered significant.
Results
Thirty-seven patients with PSA were identified, 13 after pancreatic surgery (POSTOP) and 24 with PSA arising in the setting of pancreatitis (PANC). Indications and operations performed on patients in the POSTOP group are shown in Table 1; patient demographics and clinical variables are shown in Table 2. The mean age was younger in the PANC group (46 vs 62, p < 0.01), and there were similar numbers of male and female patients in each group. Associated inflammatory states identified in both groups of patients were significantly different. In 8 of 13 (62%) patients in the POSTOP group, the clinical course was complicated by a pancreatic fistula, whereas no patient in the PANC group had a fistula. The median time until PSA presentation in the POSTOP group was 22 days (range 2–90 days). In contrast, 22 patients (92%) in the PANC group had recurrent episodes of acute pancreatitis (acute-on-chronic pancreatitis), whereas only two developed PSA during their first bout of pancreatitis. The principal etiology of pancreatitis in the PANC group was alcohol (n = 19, 79%). Other causes of pancreatitis were pancreas divisum (n = 1), gallstones (n = 1), hypertriglyceridemia (n = 1), postendoscopic retrograde cholangiopancreatography (post-ERCP) (n = 1), and idiopathic (n = 1). Twenty-one patients (87%) had an associated pancreatic pseudocyst or fluid collection.
Clinical Presentation
Twelve of 13 (93%) patients in the POSTOP group presented with bleeding—either from the gastrointestinal (GI) tract (n = 7) or with visible blood in a surgically placed drain (n = 7, Table 2). One patient had both GI bleeding and blood in the surgical drain, and one patient in this group presented with hypotension alone. In the PANC group, only seven (29%) patients presented with GI bleeding; the vast majority of these patients (n = 15, 62%) presented with increasing abdominal pain. Two patients (8%) in the PANC group presented with hypotension alone.
Diagnosis
In the POSTOP group, the first diagnostic test employed was endoscopy in four patients, angiography in four, abdominal computed tomography (CT) in two, operative exploration in two, and percutaneous transhepatic cholangiography in one. In the PANC group, the initial diagnostic test was abdominal CT in 18 and endoscopy in 5. The details of initial work-up before transfer to our hospital were unclear in one patient. In the POSTOP group, PSA was definitively diagnosed by abdominal CT in 2 patients (15%) and by angiography in 10 (77%). One patient in this group underwent emergent operation for refractory hypotension; the diagnosis of PSA was made intraoperatively. In the PANC group, the definitive diagnosis of PSA was made in 16 patients (67%) with abdominal CT scan and by angiography in 5 (21%). Two patients (8%) were diagnosed by ultrasound—one endoscopic and one transabdominal. One patient in the PANC group was diagnosed at the time of ERCP when the PSA was inadvertently lacerated during attempted endoscopic drainage of a pancreatic pseudocyst. The diagnosis of PSA was confirmed by angiography in 36 of 37 patients and by direct visual inspection at the time of operation in one.
Treatment
Transcatheter interventional therapy was ultimately successful in controlling hemorrhage in all patients (35/35) in whom it was employed. (Table 3) Thirty-four patients had treatment of the PSA by coil embolization, and one patient’s treatment was by exclusion of the PSA with a covered stent. One patient in each group required repeat embolization for recurrent hemorrhage (2 of 35, 6%). Three additional patients in the PANC group underwent repeated angiography without embolization for suspicion of continued hemorrhage. Two patients did not receive transcatheter treatment of PSA: one patient in the POSTOP group underwent immediate operation (without angiography) because of hemodynamic instability, and one patient in the PANC group underwent angiography identifying a PSA without embolization. Initial angiography was falsely negative in 4 of 35 patients (11%), 3 in the POSTOP group and 1 in the PANC group. The arteries involved by PSA are shown in Table 3. Complications of angiographically directed treatment occurred in 6 of 35 patients (17%), and included splenic abscess (n = 2), rebleeding (n = 2), hepatic abscess, and hemobilia. One patient with splenic abscess was initially treated with percutaneous drainage, however, subsequently underwent splenectomy. No other patient required operative treatment of interventional complications.
Outcomes
The mean length of stay after definitive interventional treatment of PSA was 14 days (range 2–57) in the POSTOP group and 17 days (range 2–76) in the PANC group (p = 0.79). Four patients in the POSTOP group died (31%), one of uncontrolled hemorrhage and three of sepsis and multiorgan system failure after successful angiographic control of PSA hemorrhage (Table 2). All four patients who died underwent operation: one for hemorrhage, one to evacuate intraabdominal hematoma and relieve abdominal compartment syndrome, and two for debridement and control of enteric and pancreatic fistula.
Eight patients in the POSTOP group had pancreatic fistulas. Two of these patients died of multiorgan system failure. Two patients with necrotizing pancreatitis and disconnected pancreatic duct underwent distal pancreatectomy/splenectomy to achieve fistula control. One patient had a fistula after pancreaticoduodenectomy that had closed by the time of presentation with PSA. Three patients had active fistulas at the time of presentation with PSA. All three of these patients manifest their PSA approximately 3 weeks status/postpancreaticoduodenectomy, and all had controlled, low-output fistulas that eventually resolved with conservative management.
In the PANC group, 12 patients underwent surgery directed toward resolving the pancreatic inflammatory process during the same hospital admission as treatment of their PSA. These operations included distal pancreatectomy/splenectomy (n = 7), pseudocystenterostomy (n = 2), necrosectomy (n = 2), and duodenal-preserving pancreatic head resection (n = 1). Two additional patients in this group underwent operation during the same hospital admission as treatment of their PSA for complications unrelated to the pancreatic process (drainage of intraabdominal abscess and total abdominal colectomy for fulminant Clostridium difficile colitis). Ten patients were discharged from the hospital after transcatheter therapy for their PSA without definitive therapy for their pancreatitis-related inflammatory process. One patient died of cerebrovascular accident unrelated to angiography or PSA.
Discussion
This large contemporary review of patients with visceral arterial PSA arising in two discrete clinical situations highlights differences in clinical presentation and diagnosis between the two groups and emphasizes the utility of transcatheter therapy in successful treatment. Patients in the postoperative period more frequently presented with GI bleeding or blood visible in a surgical drain, an uncontrolled situation often associated with hypotension and active blood loss requiring prompt diagnosis and treatment. In contrast, patients with PSA arising in the setting of pancreatitis most commonly presented with increasing abdominal pain caused by bleeding within the confines of a thick-walled pseudocyst cavity, without associated hypotension of active blood loss. Given these differences in clinical presentation, it is not surprising to note that the most common method of diagnosis was by angiography in the POSTOP group and by abdominal CT in the PANC group.
Pseudoaneurysm was frequently associated with pancreatic fistula (62%) in POSTOP patients and with a pseudocyst or acute fluid collection (87%) in the PANC group. Identification of these associated inflammatory conditions in these two discrete clinical settings should alert treating physicians to the potential presence of underlying PSA and may allow early intervention. The most important finding of this study is the 100% success rate of angiographic intervention in controlling PSA hemorrhage. This success should not be considered absolute by any means, given the 11% false negative rate, 6% need for repeat angiographic intervention because of recurrent bleeding, and overall 14% mortality rate in this series. Nonetheless, when compared to the dismal outcomes of patients undergoing emergent attempts at operative control, both in this series and historically, angiographic intervention provides a far more effective treatment modality for PSA in patients who are clinically stable.
The incidence of major arterial hemorrhage in the postoperative setting after pancreatic surgery ranges from 2 to 5%.5–7 Early postoperative bleeding (less than 24 h) is generally related to intraoperative technical factors, whereas major hemorrhage from PSA usually occurs several weeks postoperatively. The median time to PSA presentation of 22 days in the POSTOP group is in accordance with the median times of 18–27 days reported in the literature.1,7 The precise incidence of PSA formation in the setting of pancreatitis is more difficult to estimate. Several relatively large series have suggested that this complication may occur in 10–17% of patients, which is perhaps more common than previously appreciated.7–9 The outcome of patients suffering major hemorrhage in either of these clinical scenarios has historically been dismal. Mortality from postoperative hemorrhage ranges from 18–60%,1,7,10,11 and mortality from PSA hemorrhage in the setting of pancreatitis is approximately 20%.2,4,8,12 Notably, these data are from an era when angiographic embolization was infrequently applied, and operation was often the primary therapeutic intervention. The overall mortality of 14% in the current study represents a marked improvement compared to historical data and may be related to our aggressive use of angiography and embolization as primary therapy.
Early recognition of the clinical signs and symptoms associated with PSA allows prompt diagnosis and intervention. Bleeding, either from the GI tract or from surgically placed drains, has long been recognized as a harbinger of PSA in postoperative patients. The fact that 12 of 13 postoperative patients in this series presented with some form of bleeding reinforces this sign. Shankar and Russell13 initially described the so-called “sentinel bleed,” the finding of a small amounts of blood in an operative drain or a small hematemesis closely preceding a major hemorrhagic event. Sentinel bleeding has subsequently been reported with relative frequency in the presence of PSA.1,7 Although the retrospective nature of this review did not allow us to clearly delineate the incidence of sentinel bleed in our POSTOP group, the presence of any form of bleeding in a postoperative patient clearly demands immediate attention and prompt investigation to rule out PSA.
In contrast to the bleeding almost always observed in POSTOP patients, only 29% of patients in the PANC group presented with bleeding, and 62% presented solely with increasing abdominal pain. Our observed frequency of pain as the only presenting symptom is quite similar to that reported in the literature.4,8 The pain is often described as “crescendo” and different than the “usual” pain of pancreatitis.
The presence of an active inflammatory process in the retroperitoneum logically contributes to the formation of PSA. Sixty-two percent of patients in the POSTOP group had a pancreatic fistula, and 82% of patients in the PANC group had associated pseudocyst or acute fluid collection. Both fistula and pseudocyst/fluid collection were recognized as being frequently associated with PSA formation.1,2,4,7,8,10 Thus, the presence of pancreatic fistula or pseudocyst should heighten the clinician’s awareness to the potential for PSA formation.
Angiography proved the diagnosis of PSA in 77% of POSTOP patients, whereas more patients in the PANC group were diagnosed by abdominal CT. This difference is likely a reflection of the more urgent nature of presentation in POSTOP patients. In a clinically stable patient, it is certainly reasonable to obtain CT or endoscopy as an initial method of diagnosis; however, in the situation with a high degree of suspicion for PSA, angiography offers both diagnostic and therapeutic capability.
Transcatheter embolization has increasingly been used to treat PSA arising both postoperatively and in the setting of pancreatitis.1,3,12,14,15 The accuracy of angiography in identifying the source of arterial hemorrhage is reported to be 94–100%, and the efficacy of embolization in arresting hemorrhage has ranged from 64–78%.1,2,12,14 The 100% success in control of hemorrhage in this series highlights improvements in technique and experience and is likely to have significantly contributed to the decreased mortality we observed relative to historical data. The clinician must be aware of the real potential for false negative angiograms, which occurred in 11% of patients in this series and were far more frequent in POSTOP patients. This problem may, in part, be related to vasoconstriction in the setting of acute hemorrhage and highlights the need for meticulous angiographic evaluation particularly in the postoperative setting.
Angiography is an invasive and not completely benign procedure. Complications of angiography generally include bleeding, hematoma, femoral artery PSA, dissection, atheroembolism, thrombosis, contrast reaction, renal failure, and access site infection. Complications of embolization generally include abscess, organ failure, nontarget embolization with ischemia/infarction, procedural failure, and death.2,14 Complications of angiography and embolization in this series included splenic and hepatic abscess and the need for repeated intervention for rebleeding. Nonetheless, when compared to the high morbidity and mortality associated with primary operative intervention for treatment of PSA, angiography and embolization clearly are the first choice for intervention in patients who are clinically stable.
Once control of bleeding is secured, attention must be directed toward the associated inflammatory condition, i.e., pancreatic fistula in the POSTOP patients and pancreatic pseudocyst in the PANC patients. The formation of PSA is related to the persistent inflammatory process, which weakens the arterial wall. In the setting of POSTOP patients, adequate external control of an associated pancreatic fistula is paramount to prevent episodes of rebleeding, continued intraabdominal sepsis, and death. Adequate control implies effective fistula drainage, which can generally be achieved by percutaneous methods but occasionally requires reoperation.
A more difficult question relates to the best clinical management of patients with pancreatitis and a pancreatic pseudocyst in whom bleeding from the PSA was angiographically controlled. Long-term data regarding the incidence of rebleeding after successful angiographic control are severely lacking. However, even in the short-term, rebleeding was documented to occur in 18 to 37% of patients.2,12 Twenty-one (87%) patients in the current series developed PSA in the setting of a pseudocyst or acute fluid collection, and an additional two had acute pancreatic necrosis. Thus, a full 96% had an active inflammatory process in the retroperitoneum. Leaving this process in situ may perpetuate irritation of visceral arteries leading to enlargement of existing PSA or new PSA formation. On the other hand, in the setting of chronic pancreatitis, operation directed at the inflammatory focus (resection or drainage) can be problematic. Dense adhesions from repeated bouts of inflammation obliterate normal anatomy. In addition, splenic or portal vein thrombosis with either sinistral or portal hypertension, combined with frequently identified comorbidities such as hepatic cirrhosis and malnutrition, complicate the indications for and timing of definitive surgical treatment.
In this series, 10 patients with PSA arising in the context of pancreatitis were dismissed from the hospital without definitive treatment of their pancreatic disease; 2 underwent operation for a separate abdominal process and 12 underwent operation to address their primary pancreatic inflammatory process. Unfortunately, retrospective comparison of these subgroups of patients offers little guidance in determining the optimal management of this difficult clinical situation, principally because of unsatisfactory end points. Length-of-stay data are intrinsically biased because of operative intervention. The need for readmission or future operation may occur even after satisfactory treatment of the pseudocyst and simply be related to progression of this complex pathologic process. In addition, the strong possibility of selection bias exists, as common comorbidities such as hepatic cirrhosis may have precluded operation in some. The optimal approach for dealing with this challenging clinical scenario remains unclear at present, and the infrequency of this problem makes it unlikely that prospective evaluation will be undertaken. In the absence of better data, treatment of concomitant pancreatic inflammatory disease (i.e., pseudocyst) in a patient with an adequately treated PSA should be approached on a case-by-case basis. In a physiologically fit patient, we favor an aggressive operative approach to eliminate continuing retroperitoneal inflammation. In patients with multiple confounding medical conditions or ongoing physiologic or nutritional derangement, limited treatment by controlling the PSA would appear to be adequate.
Conclusions
PSA of the major visceral arteries is a potentially lethal condition afflicting patients in the postoperative period after pancreatic surgery and in the setting of pancreatitis. The presence of a postoperative pancreatic fistula or pseudocyst in the setting of pancreatitis is commonly associated with PSA formation and should elevate the clinician’s level of suspicion. The occurrence of GI bleeding or blood in surgical drains in the postoperative patient or the acute onset of increasing pain or unexplained hypotension in the patient with pancreatitis should stimulate prompt investigation. Angiography with embolization is the preferred initial therapeutic modality as early angiographic intervention optimizes outcomes. Aggressive control of pancreatic fistulae in the postoperative patients with a PSA is mandatory although the best treatment of pancreatic pseudocysts after angiographic control of PSA is not completely clear; therapy in this setting should be individualized.
References
Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, et al. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg 1998;133(10):1099–10102.
Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg 2005;190(3):489–495.
Beattie GC, Hardman JG, Redhead D, Siriwardena AK. Evidence for a central role for selective mesenteric angiography in the management of the major vascular complications of pancreatitis. Am J Surg 2003;185(2):96–102.
de Perrot M, Berney T, Buhler L, Delgadillo X, Mentha G, Morel P. Management of bleeding pseudoaneurysms in patients with pancreatitis. Br J Surg 1999;86(1):29–32.
Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg 2003;7(2):209–219.
Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg 2004;139(7):718–725; discussion 725–727.
de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Lameris JS, van Gulik TM, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 2005;241(1):85–91.
Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005;137(3):323–328.
Waltman AC, Luers PR, Athanasoulis CA, Warshaw AL. Massive arterial hemorrhage in patients with pancreatitis. Complementary roles of surgery and transcatheter occlusive techniques. Arch Surg 1986;121(4):439–443.
Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004;199(2):186–191.
Tien YW, Lee PH, Yang CY, Ho MC, Chiu YF. Risk factors of massive bleeding related to pancreatic leak after pancreaticoduodenectomy. J Am Coll Surg 2005;201(4):554–559.
Boudghene F, L’Hermine C, Bigot JM. Arterial complications of pancreatitis: diagnostic and therapeutic aspects in 104 cases. J Vasc Interv Radiol 1993;4(4):551–558.
Shankar S, Russell RC. Haemorrhage in pancreatic disease. Br J Surg 1989;76(8):863–866.
Gambiez LP, Ernst OJ, Merlier OA, Porte HL, Chambon JP, Quandalle PA. Arterial embolization for bleeding pseudocysts complicating chronic pancreatitis. Arch Surg 1997;132(9):1016–1021.
Shibata T, Sagoh T, Ametani F, Maetani Y, Itoh K, Konishi J. Transcatheter microcoil embolotherapy for ruptured pseudoaneurysm following pancreatic and biliary surgery. Cardiovasc Interv Radiol 2002;25(3):180–185.
Acknowledgement
The authors wish to thank Kristen Richards for secretarial support and Jian Yu for statistical expertise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zyromski, N.J., Vieira, C., Stecker, M. et al. Improved Outcomes in Postoperative and Pancreatitis-related Visceral Pseudoaneurysms. J Gastrointest Surg 11, 50–55 (2007). https://doi.org/10.1007/s11605-006-0038-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-006-0038-2