Abstract
Background
Bleeding pseudoaneurysm (PSA) is a rare but potentially lethal complication of chronic pancreatitis (CP). It requires expeditious management by a multidisciplinary team. The study aims to report our experience with PSA in the background of CP.
Methods
All the patients, who underwent intervention for CP-related PSA between August 2007 and December 2020 in the Department of Surgical gastroenterology, Institute of Postgraduate Medical Education and Research, Kolkata, India were retrospectively reviewed.
Results
Of the total 26 patients, 25 (96%) were men with a median age of 38 (11–63) years. The most commonly involved vessel was the splenic artery (n = 18, 69%). The interval between onset of GI bleed and intervention was 7 (0–120) days. Embolization was attempted in 11(42%) patients and was successful in six patients. Surgery was performed in 20 (77%) patients including five patients after failed embolization. The most commonly performed operation was distal pancreatectomy with splenectomy. The median operating time was 216 (115–313) minutes. The median intraoperative blood loss was 325 (100–1000) ml. Seventeen (85%) patients' required intraoperative blood transfusion. Four patients in the embolization group and five patients in the surgical group developed procedure-related complications. The most common postoperative complication was wound infection followed by pancreatic fistula. There was no procedure-related death. Over a median follow-up of 24 (6–122) months, no patient developed recurrent hemorrhage.
Conclusions
Both embolization and surgery play an important role in the management of PSA. The choice of procedure depends upon the local availability and feasibility of a particular technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic pancreatitis (CP) is a long-standing, progressive disease characterized by persistent, chronic inflammation of the pancreas leading to permanent duct deformity and deterioration of endocrine and exocrine function [1]. Patients with CP may develop several local complications like pseudocyst, biliary stricture, splenic vein thrombosis with left-sided portal hypertension, duodenal obstruction, and pseudoaneurysm (PSA) formation of the peripancreatic arteries. The incidence of PSA in the background of CP varies from 4 to 10%. It is a potentially lethal complication of CP particularly when it ruptures [2, 3]. The most frequently involved vessel is the splenic artery, followed by the gastroduodenal, pancreaticoduodenal, and hepatic arteries [4]. For patients who fail to receive appropriate treatment, the mortality rate may be as high as 90%. Even with a rapid diagnosis and immediate therapy, the reported mortality rate still ranges from 15 to 50%, depending on the patient's clinical condition, characteristics, and site of bleeding lesion and intervention employed [5, 6]. The ideal treatment of bleeding pancreatic PSA associated with CP stays questionable. Previously, almost all patients were managed surgically. But, due to high postoperative morbidity and mortality, researchers tried to use alternative methods like endovascular or percutaneous angioembolization with an acceptable success rate. Angioembolization is associated with significantly lower morbidity and mortality. In the current era, surgery for PSA is performed only when angioembolization fails or the patient remains hemodynamically unstable even after adequate resuscitation. Surgery is also required as first-line therapy in centers where round-the-clock facilities for angioembolization are not available. Being a middle-income country, this facility is not available in most of the state-run hospitals of India. Our center is one of these hospitals where round-the-clock facilities for angioembolization are not available and we have to rely on surgical expertise most of the time. The aim of the study is to report our experience with PSAs in the background of CP where a large subset of patients was managed surgically.
Methods
This is a retrospective observational study. Data of all patients who had undergone intervention for abdominal visceral artery pseudoaneurysms between August 2007 and December 2020 were retrieved from our prospectively maintained Gastrointestinal Surgery database. All patients who underwent any intervention for peripancreatic arterial PSA in the background of chronic pancreatitis in our institution were included in the present study. Patients who developed PSA other than peripancreatic ones, or developed PSA due to other causes (post cholecystectomy, n = 2; liver abscess, n = 2; acute necrotizing pancreatitis, n = 1) were excluded.
This study was approved by the institutional ethics committee (Memo number: IPGME&R/RAC/250, dated-26th August 2021). Informed patient consent was waived by the ethics committee as the data were anonymized and the retrospective nature of the study.
Diagnosis of chronic pancreatitis (CP) was made on the basis of clinical features and the identification of pancreatic ductal and/or parenchymal changes (calcification, atrophy, ductal dilatation) on triphasic computed tomography (CT) scan of the abdomen. PSA was diagnosed by clinical parameters and radiological investigations. Clinical suspicions were raised when a patient, who is a known case of CP presented with G I bleeding, an increase in the size of a pseudocyst, increased intensity of abdominal pain with hemodynamic instability (hypotension, tachycardia), or rapidly worsening anemia. Ultrasonography with Doppler study and contrast-enhanced CT were used as screening investigations. Suspected cases were confirmed by CT angiography. The location of the PSAs was noted and their size was measured by the maximum diameter in any plane.
The line of management was decided by a multidisciplinary team including gastroenterologists, radiologists, and gastrointestinal surgeons. Angio-embolisation was considered to be the first-line management in a hemodynamically stable patient. In patients with hemodynamic instability, or cases where angioembolization had failed, or presenting with rebleed after angioembolization, or presenting in odd hours (Interventional radiology facility in our institute is not available from 5 p.m. to 8 a.m.), surgical intervention was performed.
Embolization technique
Embolization was performed by endovascular interventions or by image-guided percutaneous interventions after assessing the preoperative CT angiography.
-
a.
Endovascular embolization
Endovascular embolizations using coils or glue or in combination were performed under a Digital subtraction angiography (DSA) machine, Philips Allura Xper FD20 biplane, or IITV unit (Philips). Using USG guidance, the Right/Left common femoral artery was accessed using Seldinger's technique and usually a 5F sheath was deployed. The suspected visceral artery on CT angiography (Celiac/SMA/IMA artery) was cannulated with 5F Cobra/SIM1 Catheter (Cook Medical) and an angiogram was performed which reveals the pseudoaneurysm, its vessel of origin, size, any other feeder, and distal branch. In some patients, where anatomy was not clear or in doubt, we performed a 3D rotational angiogram with reconstruction to plan our endovascular management. After localizing the artery of origin, it was super selectively cannulated by 2.7F Pro great microcatheter placed coaxially through the diagnostic catheter, and a pseudoaneurysm is confirmed. If distal vessel cannulation was achieved then Super selective coil embolization was performed by a sandwich technique by deploying coils both distal as well as proximal to the pseudoaneurysm. Metallic vascular push coils (Hilal coils by Cook medical and vortex coils by Boston Scientific) are being used (0.018 compatible) of varying diameters depending upon the vessel caliber. If distal vessel cannulation could not be achieved then superselective glue embolization of the aneurysmal sac done by slowly injecting glue (N-butyl cyanoacrylate) and lipiodol mixture in variable concentrations depending on angiographic flow dynamics. Sometimes the combination of coil and glue embolization was done depending upon the requirement. Check angiography of suspected artery done routinely to look for the obliteration of the pseudoaneurysm, any evidence of non-target embolization, or any recruitment of the sac from other branches of parent vessels. In addition, a check angiogram of other visceral arteries was performed to rule out any residual filling of the pseudoaneurysm or other source of bleeding or non-target embolization. At the end of the procedure, vascular access sheath was removed and hemostasis was achieved by manual compression for at least 30 min.
-
b.
Percutaneous embolization
The decision of the percutaneous versus endovascular approach for embolization of visceral artery pseudoaneurysm was made based on a preprocedural CT abdominal angiogram. Only those pseudoaneurysms were not amenable for endovascular approach due to unfavorable vascular anatomy or with prospective delay in endovascular intervention due to unavoidable circumstances and the patient needed urgent embolization as a life-saving procedure. The sac was localized under USG guidance and color Doppler imaging and Volume were measured. NBCA glue and Lipiodol mixed (50%) solution prepared approximately of the same volume that of the sac. An 18 G needle is placed into the sac under USG guidance and a contrast angiogram (aneurysmogram) was taken using nonionic low osmolar contrast medium (Iopamide) under fluoroscopy to look for any active contrast extravasation. After priming the needle with 5% Dextrose solution, the aneurysm was embolized with continuous injection of already prepared 50% glue under fluoroscopic guidance. The embolization end-point is achieved by complete opacification of the aneurysmal sac with the earliest sign of contrast spillage. Immediately post glue embolization, Doppler imaging was done to see any residual patent lumen which if present was embolized by repeating the same method with another needle. CT abdominal angiogram was performed on the next day to look for embolization status (partial/complete) of the sac, feeding artery, and any non-target embolization.
Surgical intervention
The type of surgery performed was dependent on the site of PSA, associated pathology, and the hemodynamic parameters of the patients at the time of surgery. Distal pancreatectomy with splenectomy and proximal ligation of splenic artery was performed when the lesion was situated in the splenic artery. A longitudinal opening of the pancreatic duct with pancreaticojejunostomy was added if the patent had intractable pain for CP and hemodynamic stability was achieved intraoperatively. If the PSA was situated close to the splenic hilum and the patient had no significant pain, splenectomy was performed. Bleeding within the pseudocyst was controlled by intracystic ligation followed by internal drainage of the pseudocyst. Ductal abnormalities were tackled by including the longitudinally opened pancreatic duct within the anastomosis. Gastroduodenal artery PSA was controlled by proximal ligation just below the origin from the common hepatic artery and distal control was achieved by ligation of pancreaticoduodenal arcade or intracystic ligation.
Definitions
Chronic pancreatitis was defined on the basis of Marseille criteria of 1984 [7]. Death during the hospital stay or within 90 days after the intervention was the definition of perioperative mortality utilized. Postoperative complications were graded using the Clavien-Dindo classification [8]. Pancreatic fistulae, post pancreatectomy hemorrhage, and delayed gastric emptying were defined and classified according to the criteria of International Study Group on Pancreatic Surgery (ISGPS) guidelines [9,10,11]. Diabetes mellitus (DM) was defined in fasting blood sugar was more than 126 mg/dL and serum glycosylated hemoglobin A1c (HbA1c) was more than 6.5%.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation or median with range. Dichotomous variables were expressed as a percentage.
Results
Abdominal visceral artery PSAs were identified in 36 patients. Out of these 36 patients, 26 (72%) developed peripancreatic pseudoaneurysm (PSA) and met our inclusion criteria. Twenty-five (96%) of them were men with a median age of 38 (11–63) years. Alcohol abuse was identified in 19 (73%) patients and the remainder had idiopathic CP. Fourteen (54%) patients were smokers. Diabetes was confirmed in six (23%) patients. One (4%) patient was hypertensive. The median interval between onset of symptoms of CP and manifestation of gastrointestinal bleed due to PSA was 36 (0–120) months. All patients suffered from chronic abdominal pain and five (19%) had a prior history of hospitalization for pain. Twenty-five patients (96%) presented with features of upper GI hemorrhage. One patient (4%) was diagnosed during routine evaluation of painful CP. Gastrointestinal (GI) hemorrhage was manifested either in the form of melena (n = 10) or hematemesis (n = 8), or both (n = 7). The median interval between onset of GI bleed and intervention was 7 (0–120) days. Three (12%) patients presented with massive hemorrhage evidenced by rapidly worsening anemia and hemodynamic instability (tachycardia, hypotension). The mean hemoglobin level was 7.53 ± 2.47 g/dL. One (4%) patient had previously undergone the Frey procedure 6-month before the diagnosis of PSA.
Ultrasonography (Fig. 1) of the abdomen along with a doppler study detected peripancreatic PSA in six (23%) patients. Contrast-enhanced computed tomography (Fig. 2) detected and localized lesions in 19 (73%) cases. All the suspected cases who were hemodynamically stable were confirmed by CT angiography (Fig. 3) (n = 23, 100%). The splenic artery (n = 18, 69%) was the most common vessel involved, followed by the gastroduodenal artery (n = 7, 27%), and left gastric artery (n = 1, 4%). In all the cases only a single site of lesion was identified. The mean size of PSA was 22.87 ± 14.93 mm. Only one patient had giant PSA (> 50 mm). Ten (38%) patients had associated pseudocysts and 11 (42%) cases had radiological evidence of left-sided portal hypertension.
Aggressive management was done for all the patients. Twenty-two (85%) patients required blood transfusions. The median blood transfusion requirement was 3 (1–12) units.
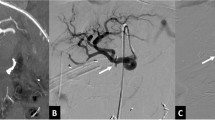
Embolization was performed as the first line of management in 11 (42%) patients. Among the endovascular procedures, coil embolization (Fig. 4) was the most commonly performed procedure (n = 10, 91%), followed by injection of glue in one patient (9%). The median procedure time was 107 (32–135) minutes. None of the patients had non-target embolization. Embolization failed to stop bleeding in three (27%) patients and rebleeding from a successfully embolised PSA was seen in two (18%) patients. Rebleeding happened 3 weeks and 6 months after the endovascular intervention. Both these scenarios were managed surgically and an attempt for re-embolization was not taken. Embolization-induced complications developed in 4/11 (36%) patients. One patient developed colonic ischemia and was managed by resection of the transverse colon. One patient developed radiological evidence of splenic infarct 8-week post-procedure. Another patient developed a splenic abscess 6-days post-procedure and was treated with antibiotics. One patient developed acute renal failure requiring hemodialysis. All four patients recovered well.
Twenty (77%) patients underwent surgical intervention. Fifteen (58%) patients received surgery without any prior angioembolization. Emergency surgery was performed in 17 (85%) patients three (15%) patients and semi-elective surgery in three (12%) patients. Depending on the location of the pseudoaneurysm and associated features, the type of surgery was decided. The type of surgery performed is presented in Table 1. The most commonly performed operation was distal pancreatectomy and splenectomy. The most commonly performed additional procedure was the Frey procedure (n = 7, 35%). The median operating time was 216 (115–313) minutes. The median intraoperative blood loss was 325 (100–1000) ml. 17 (85%) patients required intraoperative blood transfusion. Wedge resection of the stomach was done for one patient for intraoperative gastric injury. Eight postoperative complications developed in five (25%) patients. Three (15%) patients developed type B pancreatic fistula. Wound infection developed in four (20%) patients. One patient developed rebleeding on the 11th postoperative day and was managed by re-exploration and ligation of the bleeder. The median postoperative hospital stay was 8 (5–25) days. There was no surgery or endovascular procedure-related mortality. After a median follow-up of 24 (6–122) months, no patient developed recurrent hemorrhage.
Discussion
This retrospective study has demonstrated that CP-related pseudoaneurysms can be managed with acceptable perioperative complications. Both embolization and surgery are effective treatments. The choice of procedure depends on the clinical scenario as well as the local availability of particular expertise.
PSA is a rare but life-threatening complication of CP. The overall incidence varies from 4 to 10% [2, 3]. In the present series, the incidence of PSA was 3.95% which is similar to that of contemporary studies [12,13,14]. PSA results from erosion of pancreatic or peripancreatic vessels by leaked pancreatic enzymes, particularly associated with severe inflammation. Sometimes, a pseudocyst may erode the peripancreatic vessel resulting in a large pseudoaneurysm [12]. The splenic artery is most commonly involved, followed by the gastroduodenal, pancreaticoduodenal, and hepatic arteries [12, 14]. Similarly in the present study, the most commonly involved vessel was the splenic artery (n = 18, 69%).
Most patients are diagnosed after a rupture of PSA. Once ruptured, it may bleed into the upper gastrointestinal tract or into the pancreatic duct. In both scenarios, the patient usually presents with upper G I bleeding. Rarely, it ruptures into the peritoneal or retroperitoneal cavity leading to hemodynamic instability with attendant high mortality. Only a small subset of patients is diagnosed during routine evaluation of painful chronic pancreatitis. In the present series, one patient was diagnosed during routine evaluation of painful CP. Since, GI bleeding in patients with CP has several causes like stress-related peptic ulcer disease, analgesic-induced erosive gastritis, left-sided portal hypertension with gastric varices, and PSA, it is difficult to differentiate clinically PSA from other causes of G I bleeding. So, all patients with G I bleeding in the background of CP should be evaluated and managed expeditiously. A triphasic CT scan should be the first-line imaging modality after an upper GI endoscopy. CT scan can diagnose PSA as well as other local complications with high sensitivity and specificity. But, small PSAs can be missed. Hence CT-angiography is considered as the gold-standard investigation with higher diagnostic accuracy for PSAs [4, 5, 14]. In the present study, contrast-enhanced CT and CT-angiography were found to be accurate in diagnosing PSA in 73% and 100% of patients respectively. We observed that 11 (42%) patients had associated pseudocyst which is similar to that of other studies [14,15,16].
Early detection and expeditious management of PSA are of utmost importance for improved patients' survival. Without appropriate treatment, the mortality rate may be as high as 90% [17]. Even with expeditious management, the reported mortality rate ranges from 5 to 15% [17]. After stabilization of hemodynamic parameters, the patient should undergo definitive management (either embolization or surgery). Nowadays embolization is regarded as the first-line management for bleeding PSA with success rates of 67–97% and mortality rates of 4–19% [18,19,20,21]. The success rate of embolization varied according to the bleeding site, with an 80% success for PSA around the pancreatic head and 50% for those of the splenic artery [17]. The traditional surgical treatment with vessel ligation or pancreatic resection leads to higher morbidity and mortality in patients who are often hemodynamically compromised. Thus, surgery is indicated only if embolization fails or is not feasible [16, 22]. Location of the PSA is a major issue in the selection of the type of surgery. Distal pancreatectomy and splenectomy should be performed for bleeding from the splenic artery or its branches. On the other hand ligation of bleeding, vessels are preferred when the lesion is situated in the head of the pancreas. Pancreaticoduodenectomy is used in selected patients in whom other less invasive procedures are not feasible. Bleeding pseudocysts are managed by intracystic ligation followed by internal drainage of the pseudocyst. In the present series, 20 (77%) patients were managed surgically without 90-day mortality, and only 1 patient developed re-bleeding in the perioperative period.
The study has some strengths and limitations. The strength is that it is one of the largest case series where a large number of patients were successfully treated surgically with no perioperative mortality. The drawbacks are: first, it is a retrospective study. Second, contrary to contemporary series, very few patients were managed by embolization. In the present study, only 11 (42%) patients underwent embolization and it was successful in only 6 patients. This can be explained by limited local resources and expertise for endovascular management of PSA.
Conclusion
Bleeding pseudo-aneurysm (PSA) is a life-threatening complication of chronic pancreatitis with high rates of mortality if left untreated. Reasonable outcomes can be obtained with expeditious management. Although, embolization is the first-line treatment, surgery still plays an important role particularly if embolization fails or is not feasible. On the experienced hand, surgery can be performed with acceptable perioperative morbidity and mortality.
References
Ahmed SA, Wray C, Rilo HL et al (2006) Chronic pancreatitis: recent advances andongoing challenges. Curr Probl Surg 43(3):135–238. https://doi.org/10.1067/j.cpsurg.2005.12.005
Kiviluoto T, Kivisaari L, Kivilaakso E, Lempinen M (1989) Pseudocystsin chronic pancreatitis: surgical results in 102 consecutivepatients. Arch Surg 124:240–243. https://doi.org/10.1001/archsurg.1989.01410020114019]
Balachandra S, Siriwardena AK (2005) Systematic appraisal of themanagement of the major vascular complications of pancreatitis. Am J Surg 190:489–495. https://doi.org/10.1016/j.amjsurg.2005.03.009 (PMID: 16105542)
Gambiez LP, Ernst OJ, Merlier OA, Porte HL, Chambon JP, Quandalle PA (1997) Arterial embolization for bleeding pseudocystscomplicating chronic pancreatitis. Arch Surg 132:1016–1021. https://doi.org/10.1001/archsurg.1997.01430330082014 (PMID: 9301616)
El Hamel A, Parc R, Adda G, Bouteloup PY, Huguet C, Malafosse M (1991) Bleeding pseudocysts and pseudoaneurysmsin chronic pancreatitis. Br J Surg 78:1059–1063. https://doi.org/10.1002/bjs.1800780910 (PMID: 1933185)
Singer MV, Gyr K, Sarles H (1985) Revised classification of pancreatitis. Report of the Second International Symposium on the Classification of Pancreatitis in Marseille, France, March 28-30, 1984. Gastroenterology 89(3):683–685
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: anew proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161(3):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Wente MN, Veit JA, Bassi C et al (2007) Postpancreatectomy hemorrhage (PPH): aninternational study group of pancreatic surgery (ISGPS) definition. Surgery 142(1):20–25. https://doi.org/10.1016/j.surg.2007.02.001
Wente MN, Bassi C, Dervenis C et al (2007) Delayed gastric emptying (DGE) afterpancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768. https://doi.org/10.1016/j.surg.2007.05.005
Hsu JT, Yeh CN, Hung CF, Chen HM, Hwang TL, Jan YY, Chen MF (2006) Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol 11(6):3. https://doi.org/10.1186/1471-230X-6-3 (PMID: 16405731; PMCID: PMC1361773)
Mallick B, Malik S, Gupta P, Gorsi U, Kochhar S, Gupta V, Yadav TD, Dhaka N, Sinha SK, Kochhar R (2018) Arterial pseudoaneurysms in acute and chronic pancreatitis: clinical profile and outcome. JGH Open 3(2):126–132. https://doi.org/10.1002/jgh3.12116 (PMID: 31061887; PMCID: PMC6487818)
Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD (2005) Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 137(3):323–328. https://doi.org/10.1016/j.surg.2004.10.009 (PMID: 15746787)
el Hamel A, Parc R, Adda G, Bouteloup PY, Huguet C, Malafosse M (1991) Bleeding pseudocysts and pseudoaneurysms in chronic pancreatitis. Br J Surg 78:1059–1063
Bender JS, Bouwman DL, Levison MA, Weaver DW (1995) Pseudocystsand pseudoaneurysms: surgical strategy. Pancreas 10:143–147
Chiang KC, Chen TH, Hsu JT (2014) Management of chronic pancreatitis complicated with a bleeding pseudoaneurysm. World J Gastroenterol 20(43):16132–16137. https://doi.org/10.3748/wjg.v20.i43.16132
Udd M, Leppäniemi AK, Bidel S, Keto P, Roth WD, Haapiainen RK (2007) Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg 31(3):504–510. https://doi.org/10.1007/s00268-006-0209-z
Kim J, Shin JH, Yoon HK et al (2015) Endovascular intervention for management of pancreatitis-related bleeding: a retrospective analysis of thirty-seven patients at a single institution. Diagn Interv Radiol 21(2):140–147. https://doi.org/10.5152/dir.2014.14085
Nicholson AA, Patel J, McPherson S, Shaw DR, Kessel D (2006) Endovascular treatment of visceral aneurysms associated with pancreatitis and a suggested classification with therapeutic implications. J Vasc Interv Radiol 17(8):1279–1285. https://doi.org/10.1097/01.RVI.0000231948.08617.04
Nykänen T, Udd M, Peltola EK, Leppäniemi A, Kylänpää L (2017) Bleeding pancreatic pseudoaneurysms: management by angioembolization combined with therapeutic endoscopy. Surg Endosc 31(2):692–703. https://doi.org/10.1007/s00464-016-5023-6
Kirby JM, Vora P, Midia M, Rawlinson J (2008) Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol 31(5):957–970. https://doi.org/10.1007/s00270-007-9138-y
Tsiotos GG, Munoz Juarez MM, Sarr MG (1996) Intraabdominal hemorrhage complicating surgical management of necrotizing pancreatitis. Pancreas 12(2):126–130. https://doi.org/10.1097/00006676-199603000-00003 (PMID: 8720657)
Funding
Nil.
Author information
Authors and Affiliations
Contributions
AD, SR: conception, design of the study, acquisition of the data, writing, drafting the manuscript, final approval of the version to be submitted. AS, SK, SD: acquisition of the data, final approval of the version to be submitted. TSM, DNB, GKD: acquisition of the data, final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
This study was approved by institutional ethics committee (Memo number: IPGME&R/RAC/250, dated- 26th August 2021).
Informed consent
Informed patient consent was waived of by the ethics committee as the data were anonymized and retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhali, A., Ray, S., Sarkar, A. et al. Peripancreatic arterial pseudoaneurysm in the background of chronic pancreatitis: clinical profile, management, and outcome. Updates Surg 74, 1367–1373 (2022). https://doi.org/10.1007/s13304-021-01208-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01208-y