Abstract
Purpose
To investigate the effects of renal denervation (RDN) on left ventricular (LV) mass, myocardial strain and diastolic function in patients with treatment-resistant arterial hypertension by cardiac magnet resonance imaging on a 12-month follow-up.
Materials and methods
Sixteen patients (38% female) were examined before and 12 months after RDN. LV morphology and strain were analyzed. Diastolic function was determined by early (EPFR) and atrial peak filling rates (APFR) derived from differential volume–time-curve analysis. Clinical visits included 24-h ambulant blood pressure monitoring (ABPM).
Results
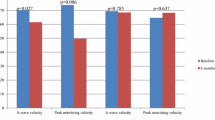
Twelve months after RDN LV mass decreased from 80 ± 21 g/m2 to 74 ± 20 g/m2 (P < 0.05). Global radial (35 ± 12% vs. 41 ± 10%, P < 0.05) and longitudinal strain improved (− 15 ± 4% vs. − 17 ± 3%, P < 0.05). Global circumferential strain (− 16 ± 5% vs. − 18 ± 4%, P = 0.12) remained unchanged. The parameter of diastolic LV function PFRR (EPFR/APFR) improved following RDN (0.9 ± 0.4 vs. 1.1 ± 0.5, P < 0.05). Individual changes of LV mass were associated with an increase of EPFR (r = − 0.54, P < 0.05) and a reduction of APFR by trend (r = 0.45, P = 0.08). Systolic ABPM showed a decrease by trend (152 mmHg vs. 148 mmHg, P = 0.08).
Conclusions
After RDN we observed a reduction of LV mass, improvement of global strain and diastolic function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arterial hypertension (AHT) is worldwide one of the leading risk factors for death and morbidity [1]. Furthermore, chronic AHT can lead to cerebral, cardiac, renal, and vascular end-organ damage including left ventricular (LV) hypertrophy and diastolic dysfunction [2]. LV hypertrophy is not only the expression of myocardial effort, but rather predictive of increased risk of heart failure [3, 4] and an independent risk factor for cardiovascular morbidity and mortality [5, 6]. Accordingly, reduction of LV hypertrophy by antihypertensive therapy is associated with improved cardiovascular outcome [7,8,9]. Beside lifestyle changes and pharmacological therapy the bilateral catheter-based radiofrequency ablation of renal nerves (renal denervation, RDN) can be an additional approach for antihypertensive strategy under certain conditions [10]. Indeed, the SYMPLICITY HTN-3 trial created doubts about the effect of RDN on blood pressure reduction [10]. However, these results should be evaluated carefully considering its potential limitations such as low experience of most interventionalists and a short run-in period after drug adaptation [11]. Latest results of the sham-controlled SPYRAL HTN-OFF MED study clearly demonstrated the blood pressure-lowering efficacy of RDN [12]. The majority of data demonstrate a decrease of blood pressure in patients with resistant AHT treated by RDN without relevant side effects [12, 13]. There is broad echocardiographic evidence for additional reduction of LV hypertrophy after RDN [14,15,16]. To date, only few studies have confirmed short-term beneficial effects of RDN on LV hypertrophy by cardiac magnet resonance imaging (CMR) in a 6-month follow-up [17,18,19,20]. The only study investigating the effect of RDN by CMR up to 12 months after the procedure has demonstrated a statistically insignificant trend for LV mass reduction [21].
The aim of this study was to investigate the effects of RDN on LV mass, myocardial strain and diastolic function in patients with treatment-resistant arterial hypertension by CMR on a 12-month follow-up.
Methods
Study population
Patients with resistant AHT were screened for additional treatment with catheter-based RDN at a single institution. The study was approved by the local ethics committee and complied with the Declaration of Helsinki. All patients participated voluntarily and gave their written informed consent. According to current guidelines, resistant AHT was defined as blood pressure > 140/90 mmHg despite the intake of at least three antihypertensive drugs including a diuretic [2]. Eligible for RDN were patients with systolic office blood pressure > 160 mmHg despite optimal medical therapy at its maximum tolerable dosage and after exclusion of secondary forms of AHT. Main exclusion criteria were estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2, significant stenosis and prior stenting or dilatation of renal arteries, myocardial infarction < 6 months before RDN, diabetes mellitus type 1 and persisting atrial fibrillation. Following an interdisciplinary discussion by a cardiologist, electrophysiologist, nephrologist, and radiologist bilateral RDN was performed in 56 patients (Supplemental Figure E1). The SymplicityTM Renal Denervation System (Medtronic, Dublin, Ireland) was used for RDN as described previously [22]. In short, a standard protocol via femoral access was followed [23]. The denervation was performed bilaterally aiming for a maximum number of ablation points and ablation in main branch arteries. 16 patients had a baseline and a 12-month follow-up CMR and were included in the current study. Additionally, the patients underwent office and 24-h ambulant blood pressure monitoring (ABPM) as well as blood sampling at the same time points.
CMR protocol
CMR was performed on a 1.5 T scanner equipped with a 5-channel cardiac-phased array receiver coil (Achieva, Philips Medical Systems, Best, the Netherlands) 4 ± 11 days before the RDN procedure. The CMR protocol included standard ECG-triggered steady-state free-precession cine CMR (25 phases of the cardiac cycle) in short axis for volumetry of the LV and right ventricle (RV) and myocardial mass measurement with the following typical imaging parameters: acquired voxel size 1.98 × 1.80 × 6 mm3, reconstructed voxel size 1.36 × 1.36 × 6 mm3, gap 4 mm, 9–10 slices for full LV coverage, echo time = 1.67 ms, time to repetition = 3.34 ms, flip angle = 60°, parallel acquisition technique = SENSE (factor 2).
CMR data analysis
Two observers, who were blinded to the timing of CMR, independently and blindly analyzed each CMR using the cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Alberta, Canada). CMR-derived cardiac volumes and myocardial mass were indexed to the subject’s calculated body surface area (BSA) and are given as the mean of the two observers’ measurements. The equation 0.20247 × height (m)0.725 × weight (kg)0.425 was used to determine the BSA [24]. Endo- and epicardial borders of LV and RV were manually delineated on short-axis cine images to measure cardiac volumes and mass [25]. LV mass was determined in end-diastole. Trabeculae and papillary muscles were included in the ventricular volumes and excluded from the myocardial mass for reproducibility.
LV strain was analyzed with cine CMR images using Segment feature-tracking software version 2.1.R.6108 (Medviso, Lund, Sweden). This software analyzes myocardial strain by computing interframe deformation fields using an endocardial tracking strategy based on non-rigid image registration [26]. Global peak systolic radial (RS), longitudinal (LS) and circumferential (CS) LV strains were measured on three long-axis and three short-axis cine slices. Endo- and epicardial contours on end-diastolic images were automatically propagated by the software throughout the cardiac cycle generating myocardial strain [26].
Diastolic function was assessed with a dedicated software (CMRtools®, Cambs, UK) as previously reported [27]. Briefly, LV 3D volumetry was performed by delineation of the LV endocardial borders in end-systolic, end-diastolic, and mid-diastolic short axis views (Fig. 1). Trabeculae and papillary muscles were excluded from the LV cavity and the 3D volumetry allowed the construction of time–volume curves from all time frames of the cardiac cycle. The differential time–volume curve of LV results in three peaks characterized by the systolic peak contraction rate, the diastolic early peak filling rate (EPFR) and the atrial peak filling rate (APFR) (Fig. 2) [27]. EPFR and APFR assessed by CMR reflect the early (E) and atrial (A) transmitral peak filling velocities determined by echocardiography (Fig. 2) [27]. In correspondence to the echocardiographic E/A ratio, the peak filling rate ratio (PFRR = EPFR/APFR) is the equivalent to characterizing diastolic filling patterns using CMR and the two parameters have been previously shown to correlate in normal subjects and in patients with cardiomyopathy [27, 28].
3D volumetry of the left ventricle illustrating delineation of endocardial borders (yellow) during diastole (a) and systole (b). Endocardial borders were assessed for all slices and all cardiac phases in short axis orientation. The analysis software generates a 3D wire model of the left ventricle (c). The mitral valve descent was taken into account by tracking the mitral valve plane in two-chamber and four-chamber views
Temporal (25 phases, dashed line) and differentiated (squares, solid line) LV volume of a patient with resistant hypertension analyzed by standard LV volumetry excluding trabeculae and papillary muscles from the LV cavity before (a) and after (b) renal denervation procedure. Temporal differentiation of the LV volume curve (squares, left axis) is characterized by the early peak filling rate (EPFR) and the atrial peak filling rate (APFR) during diastole. EPFR increased and APFR decreased after RDN
Statistical analysis
Continuous variables are described by mean ± standard deviation. Categorical variables are presented as absolute numbers and percentages. Bland–Altman analysis was used to determine agreement between the two investigators [29]. The intraclass correlation coefficient was calculated to analyze the interrater reliability. Differences were compared using a paired two-tailed t test for continuous variables and Fisher’s exact test comparisons of categorical variables. Correlations were computed using the Pearson correlation coefficient. A P value < 0.05 was considered statistically significant. All analyses were performed using GraphPad Prism version 6.00 (GraphPad Software, San Diego, CA, USA) and SPSS for Windows, version 21.0 (IBM SPSS Statistics, Armonk, NY, USA).
Results
Patient demographics
Baseline demographics of the study population are shown in Table 1. Mean systolic office blood pressure was elevated with 168 ± 17 mmHg despite the intake of 5.1 ± 1.4 antihypertensive drugs at baseline (Table 1). All patients were under therapy with a diuretic. The majority of patients additionally received ACE (angiotensin-converting enzyme) inhibitors or Angiotensin II receptor blockers (ARBs) (75%), moxonidine or clonidine (81%), beta-blockers (81%), and calcium antagonists (63%). The intake of aldosterone antagonists was less (31%) and only a minority of patients were under therapy with direct renin inhibitors (13%) (Table 1). There were no differences in demographics, prevalence of classical cardiovascular risk factors, and the total amount of antihypertensive drugs between patients with baseline CMR only and those with CMR at baseline and on a 12-month follow-up. Only the intake of moxonidine or clonidine was more frequent in the group with CMR follow-up (Supplemental Table E1).
Blood pressure and laboratory parameters
There was no difference in systolic office blood pressure at baseline (168 ± 17 mmHg) vs. 12 months after RDN (170 ± 41 mmHg, P = 0.98). Diastolic blood pressure also remained unchanged (Table 2). 24-hour ABPM showed a trend for decrease in daytime systolic blood pressure (150 ± 21 mmHg vs. 145 ± 23 mmHg; P = 0.08). The number of antihypertensive medications did not differ over time (5.1 ± 1.2 vs. 4.6 ± 1.2, P = 0.16). There was no change in renal function (indicated by serum creatinine and cystatin C) or N-terminal pro-brain natriuretic peptide 12 months after RDN (Table 2). There were no differences in blood pressure and laboratory parameters between patients with only a baseline CMR and patients with complete CMR follow-up as shown in Supplemental Table E2.
Reproducibility of LV mass and end-diastolic volume measurements
Bland–Altman analysis revealed very good reproducibility of LV mass and end-diastolic volume measurements with high interobserver agreement, without systematic over- or underestimation and narrow absolute and relative limits of agreement (Table 3 and Fig. 3).
Cardiac mass, volumes, and function after RDN
12 months following RDN BSA-indexed LV mass decreased compared to baseline (80 ± 21 g/m2 vs. 74 ± 20 g/m2; P < 0.05) as shown in Table 4. Indexed LV end-diastolic volume (P = 0.82), end-systolic volume (P = 0.09), stroke volume (P = 0.48) and ejection fraction (P = 0.19) did not differ between baseline and follow-up CMR (Table 4). Indexed RV end-diastolic volume (P = 0.97), end-systolic volume (P = 0.45), stroke volume (P = 0.61) and ejection fraction (P = 0.65) also remained unchanged (Table 4). LV ejection fraction was slightly higher in patients with only a baseline CMR than in patients with complete CMR follow-up (62 ± 8% vs. 59 ± 13%, P < 0.05), but all other CMR parameters of morphology and function were similar as shown in Supplemental Table E3.
FT-CMR LV strain analysis and correlation with LV mass
Global radial (35 ± 12% vs. 41 ± 10%, P < 0.05) and longitudinal strain (− 15 ± 4% vs. − 17 ± 3%, P < 0.05) improved 12 months after RDN (Table 4). Global circumferential strain (− 16 ± 5% vs. − 18 ± 4%, P = 0.12) did not change significantly. Indexed LV mass 12 months after RDN correlated significantly with global longitudinal strain (r = 0.60, P < 0.05), but not with global radial (r = − 0.32, P = 0.23) and circumferential strain (r = 0.12, P = 0.66). Radial strain was higher in patients with only a baseline CMR than in patients with complete CMR follow-up (45 ± 12% vs. 35 ± 12%, P < 0.05), but the values of longitudinal and circumferential strain were similar as shown in Supplemental Table E3.
CMR-derived LV diastolic function analysis
12 months after RDN passive LV diastolic filling characterized by EPFR showed a trend for increase (144 ± 44 ml/s/m2 vs. 156 ± 53 ml/s/m2; P = 0.18), but did not reach significance (Table 4). The active left ventricular filling after atrial contraction characterized by APFR remained unchanged (170 ± 37 ml/s/m2 vs. 156 ± 42 ml/s/m2; P = 0.31). The global parameter of LV diastolic function PFRR (EPFR/APFR) showed an increase from 0.9 ± 0.4 to 1.1 ± 0.5 (P < 0.05; Table 4). Decrease of LV mass was associated with an increase in EPFR (r = − 0.54; P < 0.05; Fig. 4a). Also LV mass reduction and APFR were associated by trend (r = 0.45, P = 0.08; Fig. 4b). No association was found for changes in LV mass and PFRR (r = − 0.11, P = 0.68; Fig. 4c). Parameters of diastolic function were similar between the patients with only a baseline CMR and the patients with complete CMR follow-up as shown in Supplemental Table E3.
Correlation of LV morphology and diastolic function
12 months after RDN, indexed LV mass was not associated with EPFR (r = − 0.11, P = 0.69), APFR (r = 0.39, P = 0.14) and PFRR (r = − 0.08, P = 0.76). Indexed LVEDV significantly correlated with EPFRi (r = 0.63, P < 0.01) and APFRi (r = 0.49, P < 0.05), but not with PFRR (r = 0.22, P = 0.40).
Discussion
The current study analyzed the effects of RDN procedure on LV morphology and function in patients with treatment-resistant arterial hypertension on a 12-month follow-up by CMR. First, we found a decline of LV myocardial mass 12 months after RDN, while the antihypertensive medication remained constant. Second, global radial and longitudinal strain increased after RDN and global circumferential strain did not change. Third, there was an increase of the peak filling rate ratio (PFRR) revealing an improvement of diastolic function.
The observed reduction in LV mass is in concordance with echocardiographic studies at 6–12 months [15, 17, 30, 31] and even up to 24-months follow-up after renal denervation [16]. LV mass decrease after RDN measured by CMR has been observed in studies with a maximum follow-up of 6 months [17,18,19,20]. The only study providing CMR data at 12 months showed only a trend of LV mass reduction [21]. The observed 8% decrease of indexed LV mass in our study is in the same range as described by other CMR studies at 6 months (reduction of 6–14%) [17,18,19,20] and superior to the assumed reduction after 12 months (3 ± 11%, P = 0.09) [21]. Most echocardiographic studies indicate improvement of diastolic function after RDN with divergent results of diastolic parameters [15, 16, 30, 31]. Others have not been able find differences in diastolic function over time [18]. Assessment of diastolic function by CMR has been shown to be at least complementary to echocardiography [32]. We observed an improvement of diastolic function by CMR after RDN procedure as indicated by the global parameter of LV diastolic function peak filling rate ratio (PFRR). Additionally, improved passive diastolic filling pattern was associated with a decrease in LV mass. Mahfoud et al. reported indirect CMR evidence for diastolic function improvement after RDN based on an increase of circumferential strain in a subgroup of patients with contractile dysfunction (i.e., circumferential strain ≥ − 20 before RDN) [20]. In our cohort we also detected an improvement in myocardial contractility 12 months after RDN as measured by global radial and longitudinal strain, but the improvement of global circumferential strain did not reach statistical significance.
Despite the observed effects on improved LV mass and diastolic function only a statistically not significant reduction in blood pressure could be found in the examined cohort. According to the literature, 1 year after RDN at least 30% of the patients can still be classified as non-responders for reduction in blood pressure [20, 23]. In the current study about 50% showed a decrease in systolic blood pressure by 24-h ABPM of < 5 mmHg and could therefore be classified as non-responders. The reason for this higher rate of non-responders is unclear. It might be influenced by the recruitment of patients prior to RDN, but could also be due to the small sample size. Causal procedural factors seem to be unlikely since the mean number of ablation points was 5.4 ± 1.4 for the right and 5.3 ± 1.4 for the left renal artery, which is slightly higher [33] or in the same range [15] as in previous RDN studies with significant blood pressure reduction.
Consistently, several studies confirmed a LV mass reduction after RDN independently from changes in blood pressure [15, 16, 19,20,21, 30, 31]. It is well known that cardiac sympathetic activity is increased in hypertensive LVH [34] and diminished in patients after RDN independently from blood pressure [35]. Similar beneficial effects due to the reduction of the sympathetic burden after RDN are also suspected for improved glucose metabolism [36], obstructive sleep apnea [37], heart failure [38], and cardiac arrhythmias [39]. This also seems to be due to a reduction of sympathetic activity [36,37,38,39]. Thus, a direct effect of RDN through a decrease in sympathetic activity on myocardial structure and function irrespective of the blood pressure reduction could be assumed in the current study. On the other hand, the majority of antihypertensive drug therapies also lead to a decrease in LV hypertrophy, improvement of diastolic function, and better prognosis due to the achievement of blood pressure goals [8, 40]. Thus, further and preferably sham-controlled studies are needed to discriminate the effect of blood pressure and sympathetic activity on LV hypertrophy as well as diastolic function to confirm a potential benefit of RDN beyond medical drug therapy.
Furthermore, since LV hypertrophy and diastolic dysfunction are two of the main consequences of hypertensive heart disease [41] and may lead to heart failure and worse prognosis [5, 6], the CMR-based detection of patients suffering from structural and functional impairment by hypertensive heart disease could be used to identify patients, who may benefit from a RDN procedure.
There are limitations inherent to the present study. First, the sample size is small and may therefore explain the lack of statistical significance for blood pressure reduction, diastolic LV filling patterns and global circumferential strain. However, due to the high accuracy, reproducibility and interobserver agreement of CMR analyses, the main functional and structural results of our study seem to be conclusive. Second, this is an observational single-arm study. Thus, further studies should integrate a sham-procedure arm and may also investigate potential improvement of prognosis in larger multi-center cohorts. Third, several patients were lost in follow-up. This could have lead to a potential bias within the study population. However, no relevant discrepancies were found in patients with baseline CMR only and those with baseline and 12-month follow-up CMR. Therefore, a relevant selection bias seems to be unlikely.
Conclusions
Renal denervation procedure may improve LV hypertrophy, global myocardial strain and diastolic function on a 12-month follow-up as assessed by CMR. These effects seem to be independent from blood pressure values. However, follow-up sham-controlled studies are warranted to confirm these observations and to analyze the long-term effects of reduced LV mass and improved myocardial strain and diastolic function in patients with treatment-resistant arterial hypertension.
Abbreviations
- ABPM:
-
Ambulant blood pressure monitoring
- APFR:
-
Atrial peak filling rate
- BSA:
-
Body surface area
- BMI:
-
Body mass index
- CMR:
-
Cardiac magnetic resonance
- EF:
-
Ejection fraction
- EPFR:
-
Early peak filling rate
- FT-CMR:
-
Feature-tracking cardiac magnetic resonance
- LV:
-
Left ventricle/left ventricular
- LVH:
-
Left ventricular hypertrophy
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- PFRR:
-
Peak filling rate ratio
- RV:
-
Right ventricle
References
GBD 2013 Risk Factors Collaborators, Forouzanfar MH, Alexander L, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–323. https://doi.org/10.1016/S0140-6736(15)00128-2.
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357. https://doi.org/10.1097/01.hjh.0000431740.32696.cc.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–622.
Okin PM, Oikarinen L, Viitasalo M, et al. Serial assessment of the electrocardiographic strain pattern for prediction of new-onset heart failure during antihypertensive treatment: the LIFE study. Eur J Heart Fail. 2011;13(4):384–91. https://doi.org/10.1093/eurjhf/hfq224.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–6. https://doi.org/10.1056/NEJM199005313222203.
Bombelli M, Facchetti R, Carugo S, et al. Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens. 2009;27(12):2458–64. https://doi.org/10.1097/HJH.0b013e328330b845.
Muiesan ML, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E. Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens. 1995;13(10):1091–5.
Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB. Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens. 2002;15(12):1021–8.
Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292(19):2343–9. https://doi.org/10.1001/jama.292.19.2343.
Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. https://doi.org/10.1056/NEJMoa1402670.
Lüscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN-3: confused at the higher level? Eur Heart J. 2014;35(26):1706–11. https://doi.org/10.1093/eurheartj/ehu195.
Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–70. https://doi.org/10.1016/S0140-6736(17)32281-X.
Davis MI, Filion KB, Zhang D, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62(3):231–41. https://doi.org/10.1016/j.jacc.2013.04.010.
Lu D, Wang K, Liu Q, Wang S, Zhang Q, Shan Q. Reductions of left ventricular mass and atrial size following renal denervation: a meta-analysis. Clin Res Cardiol. 2016;105(8):648–56. https://doi.org/10.1007/s00392-016-0964-2.
de Sousa Almeida M, de Araújo Gonçalves P, Branco P, et al. Impact of renal sympathetic denervation on left ventricular structure and function at 1-year follow-up. PLoS ONE. 2016;11(3):e0149855. https://doi.org/10.1371/journal.pone.0149855 (Joles JA, ed).
Tsioufis C, Papademetriou V, Dimitriadis K, et al. Long-term effects of multielectrode renal denervation on cardiac adaptations in resistant hypertensive patients with left ventricular hypertrophy. J Hum Hypertens. 2016;30(11):714–9. https://doi.org/10.1038/jhh.2015.127.
Palionis D, Berukstis A, Misonis N, et al. Could careful patient selection for renal denervation warrant a positive effect on arterial stiffness and left ventricular mass reduction? Acta Cardiol. 2016;71(2):173–83. https://doi.org/10.2143/AC.71.2.3141847.
McLellan AJA, Schlaich MP, Taylor AJ, et al. Reverse cardiac remodeling after renal denervation: atrial electrophysiologic and structural changes associated with blood pressure lowering. Heart Rhythm. 2015;12(5):982–90. https://doi.org/10.1016/j.hrthm.2015.01.039.
Doltra A, Messroghli D, Stawowy P, et al. Potential reduction of interstitial myocardial fibrosis with renal denervation. J Am Heart Assoc. 2014;3(6):e001353. https://doi.org/10.1161/JAHA.114.001353.
Mahfoud F, Urban D, Teller D, et al. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi-centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014;35(33):2224–31. https://doi.org/10.1093/eurheartj/ehu093.
Verloop WL, Vink EE, Spiering W, et al. Effects of renal denervation on end organ damage in hypertensive patients. Eur J Prev Cardiol. 2015;22(5):558–67. https://doi.org/10.1177/2047487314556003.
Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–7. https://doi.org/10.1161/HYPERTENSIONAHA.110.163014.
Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128(2):132–40. https://doi.org/10.1161/CIRCULATIONAHA.112.000949.
Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–11. (discussion 312–3).
Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15(1):35. https://doi.org/10.1186/1532-429X-15-35.
Morais P, Marchi A, Bogaert JA, et al. Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm: assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson. 2017;19(1):24. https://doi.org/10.1186/s12968-017-0333-y.
Schoennagel BP, Fischer R, Grosse R, et al. Peak filling rates assessed by CMR imaging indicate diastolic dysfunction from myocardial iron toxicity. JACC Cardiovasc Imaging. 2016;9(11):1353–4. https://doi.org/10.1016/j.jcmg.2015.10.017.
Nacif MS, Almeida ALC, Young AA, et al. Three-dimensional volumetric assessment of diastolic function by cardiac magnetic resonance imaging: the multi-ethnic study of atherosclerosis (MESA). Arq Bras Cardiol. 2017;108(6):552–63. https://doi.org/10.5935/abc.20170063.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10.
Schirmer SH, Sayed MMYA, Reil J-C, et al. Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol. 2014;63(18):1916–23. https://doi.org/10.1016/j.jacc.2013.10.073.
Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59(10):901–9. https://doi.org/10.1016/j.jacc.2011.11.034.
Westenberg JJM. CMR for assessment of diastolic function. Curr Cardiovasc Imaging Rep. 2011;4(2):149–58. https://doi.org/10.1007/s12410-011-9070-z.
Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. https://doi.org/10.1016/S0140-6736(09)60566-3.
Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108(5):560–5. https://doi.org/10.1161/01.CIR.0000081775.72651.B6.
Donazzan L, Mahfoud F, Ewen S, et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol. 2016;105(4):364–71. https://doi.org/10.1007/s00392-015-0930-4.
Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123(18):1940–6. https://doi.org/10.1161/CIRCULATIONAHA.110.991869.
Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58(4):559–65. https://doi.org/10.1161/HYPERTENSIONAHA.111.173799.
Davies JE, Manisty CH, Petraco R, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162(3):189–92. https://doi.org/10.1016/j.ijcard.2012.09.019.
Romanov A, Pokushalov E, Ponomarev D, et al. Pulmonary vein isolation with concomitant renal artery denervation is associated with reduction in both arterial blood pressure and atrial fibrillation burden: data from implantable cardiac monitor. Cardiovasc Ther. 2017;35(4):e12264. https://doi.org/10.1111/1755-5922.12264.
Devereux RB, Wachtell K, Gerdts E, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292(19):2350–6. https://doi.org/10.1001/jama.292.19.2350.
Georgiopoulou VV, Kalogeropoulos AP, Raggi P, Butler J. Prevention, diagnosis, and treatment of hypertensive heart disease. Cardiol Clin. 2010;28(4):675–91. https://doi.org/10.1016/j.ccl.2010.07.005.
Acknowledgements
We thank Christiane Brodersen for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All procedures were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments.
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Figure E1:
Patient flowchart. (JPG 461 kb)
Supplemental Table E1:
Demographics and drug therapy of patients with a baseline CMR only (Group 1, N=30) compared to patients with baseline and 12-month follow-up CMR (Group 2, N=16). (DOCX 36 kb)
Supplemental Table E2:
Baseline comparison of blood pressure and laboratory parameters of patients with a baseline CMR only (Group 1, N=30) and patients with baseline and 12-month follow-up CMR (Group 2, N=16). (DOCX 34 kb)
Supplemental Table E3:
Baseline CMR analysis of patients with a baseline CMR only (Group 1, N=30) compared to patients with baseline and 12-month follow-up CMR (Group 2, N=16). (DOCX 35 kb)
About this article
Cite this article
Tahir, E., Koops, A., Warncke, M.L. et al. Effect of renal denervation procedure on left ventricular mass, myocardial strain and diastolic function by CMR on a 12-month follow-up. Jpn J Radiol 37, 642–650 (2019). https://doi.org/10.1007/s11604-019-00854-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-019-00854-y