Abstract
High temperatures affect the physical properties of red sandstone seriously, especially the pores. Understanding its mechanism is of great significance in coal mining following underground gasification, geothermal energy utilization, and the deep burial of nuclear waste. Nuclear magnetic resonance (NMR) was used to detect pore structure characteristics, and scanning electron microscopy (SEM) and polarizing light microscopy (PLM) were used to mechanism of change. The transverse relaxation time (T2) and signal strengths of red sandstone treated at various temperatures were observed by NMR, and then, the pore situation can be obtained, and finally, the influence of temperature on the pore structure of red sandstone can be obtained. Microscopic photographs of the pores of red sandstone were obtained by SEM and PLM to assist in explaining the process of microstructural change, especially the influences of temperature on pore characteristics and grain morphology and distribution. The researches indicate that after the heat treatment of red sandstone at 25–1300 °C, the pore and strength characteristics change in well-defined stages. Before 500 °C, the pore diameters and distribution range increase, but the porosity and internal grain structure do not change significantly. At 500–1000 °C, red sandstone micropores contract, mesopores and macropores develop, and strength decreases. After 1000 °C, the grains that comprise sandstone melt and fill many of the pores, decreasing porosity. The proportion of micropores decreases, while mesopores and macropores increase. In addition, a large number of bubbly holes appear in and on the sandstone, presumably caused by gases such as CO2, and water vapor from dehydrating grains. The changes in pore and cementation states with temperature are the main factors affecting the tensile strength of red sandstone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The influence of high temperatures on rock properties, especially pores, is of great research value in the development and utilization of oil and gas resources, geothermal resources, and mining after the underground gasification of coal. Drying makes the cementation weaker after the rock is baked at the temperature of 200–250 °C (Rao et al. 2007). At the same time, high temperatures cause thermal expansion of grains in the rock, resulting in changes in strength and elastic modulus (Wu et al. 2013; Yin et al. 2015; Sirdesai et al. 2017b; Zhang et al. 2020). In the temperature range of 0–800 °C, Jin et al. (2019) reported that the process of change in the internal pores of sandstone is one of initiation–extension–connection–contraction. From the perspective of permeability, 500 °C is an important point of change in the internal structure of sandstone. Under 500 °C, due to the thermal expansion of grains, the internal structure is denser; subsequently, the reduction of water leads to obvious fracture development (Li et al. 2016). Higher temperature will lead to qualitative changes of clay and other grains. For example, kaolin is decomposed into metakaolin at 550 °C, and metakaolin is further decomposed at 700 °C (Tydlitát et al. 2015). In contrast, the chemical compositions of calcite and feldspar are relatively stable and only change after 800 °C and 1200 °C, respectively (Wu et al. 2013).
The pore properties of sedimentary rocks are influenced by the sedimentary environment (Morad et al. 2010; Taylor et al. 2010) and tectonic deformation (Hou et al. 2019) and directly affect rock permeability (Clarkson et al. 2012; Wei et al. 2015; Kamal et al. 2018). At present, the methods used to obtain the microscopic characteristics of rock include microbe-induced calcium carbonate deposition (MICP), low-field nuclear magnetic resonance (NMR), X-ray tomography (XCT), scanning electron microscopy (SEM) (Kogbara et al. 2015; Lai et al. 2017), and 3D digitization technology (Bao et al. 2020) and 3D print (Bao et al. 2019). The NMR technique can quantitatively describe pore changes (Kleinberg and Horsfield 1990; Kleinberg 1996). In combination with other methods, NMR results can be visualized. For example, after NMR experiment, CT can be used to obtain the water distribution image inside the saturated sample (Kogbara et al. 2015) and then combined with SEM to observe the specific microscopic morphology of the internal structure (Geng et al. 2018).
The NMR could accurately detect the pore characteristics of sandstone (Dunn et al. 2002). In addition, mercury injection is also a relatively common method detecting pores and measuring porosity (Xiao et al. 2013), but it cannot guarantee 100% mercury saturation (Fang et al. 2018), and the pressure generated during mercury injection may affect subsequent experiments (Song et al. 2017). The signals acquired by NMR are mainly transverse relaxation time (T2) and surface relaxation time (T1) (Volokitin et al. 2001). The change of T1 is not obvious (Hürlimann et al. 1994), which is not suitable for pore analysis (Liu et al. 2003).
The NMR process can accurately detect liquid water (Tziotziou et al. 2011), especially the micropores in the low saturation state (Roberts et al. 1995), not only its content but also its existing shape (Ji et al. 2020). For example, it is applied to study the influence of liquid water content in geotechnical mass on electrical properties (Watanabe and Wake 2009) and strength (Jia et al. 2019). In addition, NMR instruments for detecting 13C signals can be applied to the detection of organic matter such as coal (Abdulsamad et al. 2019). In this experiment, NMR was applied to study the pore–temperature relationship. The trend in porosity with the development of heat treatment temperature was quantitatively analyzed. In addition, with a combination of SEM and polarizing light microscope (PLM) photographs, the specific process of change in the internal microstructure and pore distribution of sandstone is qualitatively analyzed. We aim to provide a theoretical basis for using this information to benefit energy exploitation and utilization.
Material and methods
Samples
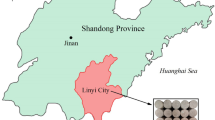
Red sandstone is widely distributed in eastern China, and samples were collected from Linyi city, Shandong Province, China, as shown in Fig. 1. Linyi is located in Mount Yimeng area, with low hilly terrain, high in the northwest and low in the southeast. The region's coal resources are small and nearly exhausted, and underground coal gasification can alleviate this problem. In order to understand the influence of high temperature on surrounding rock during gasification, the representative red sandstone in this area was selected as the research object. Some 26 sets of samples were prepared, each sample being 25 mm × 50 mm in size. The main grain composition and structural characteristics of the red sandstone are shown in Table 1.
Thermal treatment
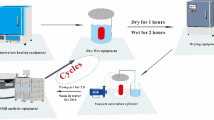
The experiment used 14 treatment groups: 1 control and 13 treated to temperatures of 100, 200, 300, 400, 500, 600, 800, 1000, 1100, 1150, 1175, 1200, and 1300 °C. Heat treatment was applied at a heating rate of 10 °C/min in a high-temperature furnace until reaching the target temperature. This was maintained for 1 h, and then, samples were allowed to cool naturally to room temperature and stored in sealed bags.
Test experiments
The NMR instrument used for T2 measurement was manufactured by Suzhou Niumag Analytical Instrument Corporation (Macromr12-150 h-1, Suzhou, China), as shown in Fig. 2. During the measurement, CPMG pulse train was used to exclude the magnetic field uniformity. The signal acquisition frequency was 250 Hz, the number of sampling points was 500,038, the waiting time for repeated sampling was 1500 ms, the echo time was 0.4 ms, and the number of echoes was 5000. The SEM images were obtained using a field emission scanning electron microscope (JSM-7610F, Japan Electron Strain Society). The PLM images were obtained with a polarizing microscope (Nikon Y-TV).
Results and analysis
NMR results
NMR is an important method for studying fluids within solids. By calculation, the T2 value can be used to characterize the pore distribution characteristics (Ausbrooks et al. 1999; Liu et al. 2018). The formula is as follows.
In the formula, T2 represents the total relaxation time, T2B represents the volume relaxation time, T2S represents the surface relaxation time, and T2D represents the diffusion relaxation time (Kleinberg et al. 1994; Hou et al. 2019). T2B and T2D can be ignored, so T2 is approximately equal to T2S (Kleinberg and Horsfield 1990; Dunn et al. 2002; Dillinger and Esteban 2014).
where ρ represents the transverse surface relaxation strength coefficient of the rock (generally 1–10 μm/s), S is the pore area, V is the pore volume, α is the pore shape coefficient (for planar, cylindrical and spherical pores, it is 1, 2 and 3, respectively), and r is the pore radius (Kleinberg 1996; Jia et al. 2020). T2 is positively correlated with pore volume and pore radius, and inversely correlated with pore area. Meanwhile, the signal strength of T2 can reflect the number of pores of different radii, so T2 can be used to explain the pore distribution (Volokitin et al. 2001).
Figure 3a shows all the T2 images obtained by NMR. All the temperatures can be divided into three stages according to these images, as shown in Fig. 3b–d. In Fig. 3b, the changes in the T2 spectral curve have three peaks. In the 100–400 °C temperature range, the first peak of T2 is at 0.74–0.94 ms with a signal intensity range of 131.51–138.08. The second peak is at 7.32–11.90 ms with a signal intensity range of 112.10–143.96. The third peak is at 191.16–270.5 ms with a signal intensity range of 9.18–17.22. Hence, the change of signal strength in the time and scope is small for three peaks. According to Fig. 3c, although the T2 spectrum still has three peaks at 600 °C, the signal strengths of its first and second peaks are 160.96 and 186.75, respectively, which are higher than those of the previous temperature curve, and T2 is obviously shifted to the right. At 800 °C and 1000 °C, only one peak appeared in the spectrum, which occurred at 5.17 ms and 4.82 ms, and the signal strengths were 321.21 and 389.64, respectively. According to Fig. 3d, the single peak at 1100 °C decreased significantly, and the peak value was 173.83 when T2 = 1.29 ms. At 1150–1200 °C, the peak intensity was reduced to 65.65 at 1200 °C, and the T2 value of the wave peak reached 44.49 ms.
Analysis
It could be got Formula (3) by deforming Formula (2). T2 was converted by Formula (3) to obtain the proportions of pores of different radii, as shown in Fig. 4a.
where ρ is 10 m/s, and α is 3 (Borgia et al. 1996).
The variation in pore radius in the figure is consistent with that of T2. When the temperature was ≤ 1100 °C, the maximum T2 value was detected at 973.79 ms. However, when the temperature increased to 1150, 1175, and 1200 °C, NMR data were collected again at T2 = 8703.59, 8703.59, and 5353.57 ms, respectively. When T2 = 973.79, 8703.59, and 5353.57 ms, the corresponding pore radii were 18.88, 174.07, and 107.07 μm, respectively. Figure 4b shows the cumulative proportional distribution of pore radii. For each stage, when the temperature was low, the distribution of pore radii was concentrated and the cumulative curve rose rapidly. With increasing temperature, the cumulative curve shifted to the right, and the curve rose slowly, which means that the range of the pore radius distribution became larger.
The change in porosity with temperature is shown in Fig. 5. The solid red line is the porosity calculated by NMR data according to Formula (4), while the dotted blue line is the porosity obtained by the traditional weighing method. According to the laboratory-measured specification of the NMR parameters of rock sample SY/T 6480–2014, the calculation formula is as follows:
where mi represents the NMR T2 spectrum amplitude of the ith T2 component of the rock sample, Mb represents the total amplitude of the T2 spectrum of the standard sample, Sb represents the number of scans of the standard sample during NMR data acquisition, S represents the number of times the rock sample was scanned when NMR data was collected, Gb represents the receiving gain of the standard sample in NMR data acquisition, g represents the receiving gain of rock samples in NMR data acquisition, Vb represents the total water content of the standard sample (cm3), and V represents the volume of the sample (cm3).
The porosity of red sandstone in natural conditions is 5.79. In the temperature range 100–500 °C, the change in porosity was in a state of equilibrium fluctuation, from 7.96 at 100 °C to 7.98 at 500 °C, and always remained below 8. With increasing temperature, the porosity rose sharply to 18.65 between 500 and 1000 °C, with an increase of 10.67, or growth rate of 1337%. When the heat treatment temperature was > 1000 °C, the porosity dropped sharply and, at 1150 °C, the porosity was 4.33, a decrease of 14.32%, and it remained < 5 in subsequent heat treatment.
According to pore radius, the pores were divided into four grades: micropores (< 10 nm), pores (10–100 nm), mesopores (100–1000 nm), and macropores (> 1000 nm) (Hodot 1966). The pore types were then converted into corresponding T2 values, being micropores (T2 < 0.52 ms), pores (0.52 ms < T2 < 5.15 ms), mesopores (5.15 ms < T2 < 51.11 ms), and macropores (T2 > 51.11 ms). This approach is not conducive to the direct analysis of NMR results, so another more appropriate classification method was adopted. Specifically, pores were divided into micropores (T2 < 10 ms), mesopores (10 ms < T2 < 100 ms), macropores (100 < T2 < 1000 ms), and fractures (T2 > 1000 ms) (Cai et al. 2013; Jin et al. 2019). According to the above criteria for dividing pores, the signal strength proportion corresponding to each T2 interval can be converted into porosity, as shown in Table 2.
Figure 6 is obtained from the cumulative changes in NMR signal strength. According to Fig. 6a, the pore radius of sandstone increases significantly and the accumulative signal strength of micropores decreases with increases in temperature, while the accumulative signal strength and temperature change of large pores show more complex variations. At temperatures < 500 °C, the position change of the curve is more complicated; however, except at 100 °C, there is no significant change in the other curves. After 500 °C, the curve experienced an initial rise and then continued to rise after a sharp decline at 1100 °C. In combination with Table 2 and Fig. 6b and c, at < 500 °C, the porosity was relatively stable, but at 500–1000 °C, the porosities of micropores and mesopores increased from 5.53 and 1.59 to 12.52 and 3.95, respectively. However, the porosity of macropores remained between 0.03 and 0.05, and the total porosity increased from 7.98 to 18.65. Above 1100 °C, the porosity of mesopores remained between 0.51 and 0.97, with a small change interval. The main change was that the micropores were reduced to 1.16, while the macropores reached 0.16. We can see a huge contrast between the micropores and macropores in Fig. 6b. However, Fig. 6c indicates that the change in sandstone porosity is the result of the joint action of pores of different sizes. As the temperature increases, the pore structure changes but the total porosity eventually decreases.
Discussion
With the rising temperature, the T2 spectrum gradually shifted to the right, and the number of crests decreased from 2 to 1 and then increased to 2 again. Thermal expansion of the grain in sandstone is the main reason for this phenomenon (Sirdesai et al. 2017b). Secondly, when the temperature was low, high-temperature evaporation of free water caused poor cementation of the clay inside the sandstone and a dehydration condensation phenomenon (Jin et al. 2019). The influence of temperature differed for different grains. The changes known to occur in different grains with temperature, according to Somerton (1992), Sirdesai et al. (2017a), and Jin et al. (2019), are shown in Table 3.
Figure 7 shows an SEM image of the sandstone’s internal structure after different temperature treatments. As sandstone is formed by the sedimentation of different types of grain detritus and aluminosilicate cementation, there are original pores in its interior, as shown in Fig. 7a. At high temperatures, thermal stress is generated in sandstone due to differences in the expansive properties of the various grains (Jin et al. 2019). Moreover, as the temperature rises, the grains in sandstone undergo physical and chemical changes that have great impacts on the pore changes (Li et al. 2016). From the SEM observations made at each temperature (Fig. 7), it could be seen that with increases in temperature, the grain morphology, structure, pore size, and distribution inside the sandstone all change greatly, which can be collectively referred to as the hot crack feature. In sandstone that has not been affected by temperature, the internal pores are in their original state and exist between grains. As the temperature approaches 300 °C, clear boundaries form between grains and the pores increase to a large size. In Fig. 7b, it can be clearly observed that the pores only exist between grains and there are no cracks through the interior of the crystal. According to Yin et al. (2015), at lower temperatures, sandstone interior cracking is caused by water evaporation, drying of aluminosilicate cement, and thermal stress. When the temperature exceeds 400 °C, the thermal expansion rate of the grains in sandstone accelerates and thermal stress becomes more severe (Yin et al. 2015). Transgranular cracks begin to appear, and fractures can be observed on the grain surface. When the temperature reaches 600 °C, the thermal expansion of quartz reaches its peak (Hartlieb et al. 2016). Differential expansion of quartz and other grains accelerates the development and size of pores. As can be seen from Fig. 7c, in addition to the pore development between the grains, some of the pores are arranged in a straight line. While these are not connected in the picture, they are probably connected to the interior. The lengths and widths of the whole pores increase obviously. In addition, the surfaces of the grains show a slight indication of cooling after high-temperature melting, but the boundaries of the particles remain obvious. As the temperature increases, the cracks between the grains continue to expand and the melted cement shrinks as it cools, creating more bubble-like pores, while the transgranular cracks further separate the grains. Pore development changes most dramatically at 1000 °C. As can be seen from Fig. 7d, the cementation that previously existed between grains is rarely observed and is replaced by pore channels with a diameter of about 15 μm. In addition, the transgranular cracks are fully developed, which divide the grains into smaller particles. There are melting marks at the edges of the fracture surfaces, and no obvious pore characteristics are found on the crystal surfaces.
After 1000 °C, the melting of grains is more obvious and there is cementation between partially melted particles. As the temperature rises further, a large number of pores of uneven size and distribution appear in the sandstone and tend to grow larger under increasing temperature. In addition, the degree of melting of primary grains increases with temperature and the proportion visible in the image decreases. As the grains cool and recrystallize, a fondant of different grain components appears on the outside of the original crystal. The primary grains are completely covered by dark grains, and there is no obvious boundary between them, although some of the unfused primary grains can still be distinguished. In the image, we can see smooth depressions left by shedding of the original grains. Compared with the pores, these depressions are relatively shallow, as shown in Fig. 7e of the 1200 °C image. (The resolution was adjusted to 100 μm after 1200 °C and 1300 °C for better comparison.) At 1300 °C, as shown in Fig. 7f, the primary grains exhibit a whole melting phenomenon, but the molten material is not completely mixed, and pores of about 20 μm diameter exist in the mineral-mixing area. Most of the area is occupied by fondant, and the inner part is developed with super-large pores about 300 μm in diameter with smooth inner walls.
SEM is effective in showing the effects of high temperatures on sandstone; however, it has certain limitations such as providing poor visual descriptions of transgranular cracks. This phenomenon can be compensated for by using PLM photographs.
Figure 8 shows a PLM image of the inside of the sandstone after high-temperature treatment. PLM images allow visual observation of the development of pores in the sandstone. Figure 8a shows the sandstone in its original state, the morphology of grain fractures, and the distribution of cementation between them, which have obvious differences and boundaries. At lower temperature of heat treatment, the grains still remain intact, the cementation is evenly distributed among the crystals, and the overall state is similar to undisturbed sandstone. At 600℃, as shown in Fig. 8b, cracks with bifurcation appear in the crystal, but there is no trace of melting at the edges. The distribution of intercrystalline cementation has also changed, and the color of the cementation is darker at the corners of the crystals, which are farther apart from each other. At 1100 °C, the edges of the grains are obviously ablated and grain clusters can be observed on some crystal surfaces. A large number of transgranular cracks appeared, but the broken crystal remained in its original position, and no melting and displacement occurred in the crystal. The center of the larger black spot in the crystal is not filled, being annular, while the smaller black spot is bubble-like, as shown in Fig. 8c. At 1200 °C, only a large number of bubbles with black edges can be seen inside the sandstone and crystal clusters exist on the inner wall of the bubbles. The rest of the grains are basically mixed after melting. The positions of the original grains can be distinguished, but the morphology has been completely changed, as shown in Fig. 8d.
The initial content of micropores is relatively small, but as higher temperatures reduce the water content (Rao et al. 2007), micropores increase in number. The pores are readily filled with molten grains, so the higher the temperature, the more the molten grains and the fewer the micropores. Mesopores were the most common pore type in undisturbed rock. Before 1000 °C, as grain expansion increased the micropores, some became connected (Li et al. 2016), resulting in increases in mesopores. There was also expansion or closure of mesopores in this process, but the general trend was to increase. After 1000 °C, the change in mesopores reached a dynamic equilibrium. At this time, the water generated by dehydroxylation (Jin et al. 2019) of the grains inside the sandstone was gasified and did not spill out in time, forming the punctate pores visible in Fig. 7e–f and the bubble-like area in Fig. 8d. Before 1100 °C, thermal cracking was the main factor affecting macropores (Wu et al. 2013), because there was no large-scale grain melting in this temperature range, as shown in Figs. 7c, d and 8c. When feldspar and quartz reached the melting temperature limit, gases such as water vapor that could not be released converged under the action of fluid pressure, and the development of macropores peaked. Therefore, a large number of bubble-like pores appeared in the SEM and PLM photographs, and the smooth inner walls of these pores are proof of the existence of gas.
Figure 9a shows the change in the stress peak of sandstone in the tensile test. As can be seen from the image, the peak stress remained between 8 and 10 kN before 400 °C. From 400 °C to 1000 °C, the stress decreased to 1.51 kN and the growth rate was − 0.0291 kN/°C at 400–600 °C and − 0.00535 kN/ °C at 600–1000 °C. At 1000–1175 °C, the stress peak increased sharply to 15.83 kN, an increase of 14.32 kN, representing growth of 948.34% at a rate of 0.0818 kN/ °C. As can be seen from Fig. 9b, the variation in porosity calculated from NMR data is the same as that of the saturated water content obtained by the weighing method. The reason why the curve obtained by the weighing method lags that of temperature is that the drying temperature was low. This would have evaporated the free water in the internal pores of the sandstone, while structural water and combined water still existed. Combined with the tensile strength curve, we can see that the influence of temperature on the tensile strength of sandstone is phased. Before 400–500 °C, the porosity and tensile strength were relatively stable. After 500 °C, the porosity decreased and the tensile strength increased, reaching an extreme value at about 1000 °C. After that, the tensile strength increased rapidly with the sudden drop in porosity. As the rock sample was seriously deformed at 1200 °C and completely melted at 1300 °C, the experimental data of this study have no reference value. In summary, when the temperature was low, pore development and closure were stable, the sandstone structure did not change, and the tensile strength was stable. With increases in temperature, the pores gradually became larger and more numerous, the structure inside the sandstone changed, and the tensile strength decreased. When the temperature was high enough to melt most of the grains inside the sandstone (Somerton et al. 1992; Sirdesai et al. 2017a), there were complex changes in the sandstone’s internal structure and grain composition. Firstly, the high temperatures changed the grain composition of the sandstone and the intermineral cementation mode, making the cementation stronger. Secondly, most of the gas gathered in the network of holes due to liquid pressure, the remaining gas formed fewer pores, and the tensile strength increased rapidly.
Conclusions
The effect of temperature on the porosity of red sandstone was studied by NMR. Combined with SEM and PLM, changes in microstructure were directly observed, and the relationship between temperature and tensile strength was preliminarily discussed. According to the experimental data and analysis, the following conclusions can be drawn.
-
(1)
NMR can be used as an effective method to detect the internal pores of rock and soil mass, and an appropriate tool to track the change of porosity with temperature. The T2 spectrum can accurately reflect the porosity of sandstone at various temperatures, determine the pore diameter distribution, and thus, predict the influence of temperature on pore structure. Compared with other techniques, NMR has obvious advantages for its accuracy and nondestructive properties.
-
(2)
High temperatures greatly influence the pores in red sandstone. Under high temperatures, the pore radius of red sandstone tends to increase as micropores gradually form larger pores or fractures. In addition, thermal expansion and desorption of clay grains provide favorable conditions for intercrystalline pore connection and transgranular crack development, respectively, thus further increasing the development rate of large pores. Throughout the process, 500 °C and 1000 °C are the key temperatures at which pores change.
-
(3)
Pore changes can directly affect the strength of rock. According to the NMR, SEM, and PLM results, 800 °C can be used as the target treatment temperature for sandstone with high strength and a certain amount of permeability.
The high temperature causes the mineral composition inside the rock to melt, and the new cementation mode will be formed after the cooling. Such cementation has a direct effect on the strength of rock and other properties. Further experiments are needed to explain this microscopic phenomenon.
References
Abdulsamad F, Revil A, Ghorbani A, Toy V, Kirilova M, Coperey A, Duvillard P, Ménard G, Ravanel L (2019) Complex conductivity of graphitic schists and sandstones. J Geophys Res Solid Earth 124:8223–8249. https://doi.org/10.1029/2019JB017628
Ausbrooks R, Hurley N, May A, Neese D (1999) Pore-size distributions in vuggy carbonates from core images, NMR, and capillary pressure. In: SPE annual technical conference and exhibition. https://doi.org/10.2118/56506-MS
Bao H, Zhang G, Lan H, Yan C, Xu J, Xu W (2019) Geometrical heterogeneity of the joint roughness coefficient revealed by 3D laser scanning. Eng Geol. https://doi.org/10.1016/j.enggeo.2019.105415
Bao H, Xu X, Lan H, Zhang G, Yin P, Yan C, Xu J (2020) A new joint morphology parameter considering the effects of micro-slope distribution of joint surface. Eng Geol. https://doi.org/10.1016/j.enggeo.2020.105734
Borgia G, Brown S, Fantazzini P (1996) Nuclear magnetic resonance relaxivity and surface-to-volume ratio in porous media with a wide distribution of pore sizes. J Appl Phys 79(7):3656–3664. https://doi.org/10.1063/1.361194
Cai Y, Liu D, Pan Z, Yao Y, Li J, Qiu Y (2013) Petrophysical characterization of chinese coal cores with heat treatment by nuclear magnetic resonance. Fuel 108:292–302. https://doi.org/10.1016/j.fuel.2013.02.031
Clarkson C, Jensen J, Pedersen P, Freeman M (2012) Innovative methods for flow-unit and pore-structure analyses in a tight siltstone and shale gas reservoir. AAPG Bull 96(2):355–374. https://doi.org/10.1306/05181110171
Dillinger A, Esteban L (2014) Experimental evaluation of reservoir quality in Mesozoic formations of the Perth Basin (Western Australia) by using a laboratory low field nuclear magnetic resonance. Mar Pet Geol 57:455–469. https://doi.org/10.1016/j.marpetgeo.2014.06.010
Dunn K, Bergman J, Latorraca A (2002) Nuclear magnetic resonance: Petrophysical and logging applications. Nuclear magnetic resonance petrophysical and logging applications. Pergamon
Fang T, Zhang L, Liu N, Zhang L, Wang W, Yu L, Lei Y (2018) Quantitative characterization of pore structure of the carboniferous-permian tight sandstone gas reservoirs in eastern Linqing depression by using NMR technique. Pet Res 3(2):110–123. https://doi.org/10.1016/j.ptlrs.2018.06.003
Geng Y, Liang W, Liu J, Kang Z, Wu P, Jiang Y (2018) Experimental study on the variation of pore and fracture structure of oil shale under different temperatures and pressures. Chin J Rock Mech Eng 37(11):91–100. https://doi.org/10.13722/j.cnki.jrme.2018.0623 ((In Chinese))
Hartlieb P, Toifl M, Kuchar F, Meisels R, Antretter T (2016) Thermo-physical properties of selected hard rocks and their relation to microwave-assisted comminution. Miner Eng 91:34–41. https://doi.org/10.1016/j.mineng.2015.11.008
Hodot B (1966) Outburst of coal and coalbed gas (Chinese translation). China Coal Industry Press, Beijing
Hou X, Zhu Y, Wang Y, Liu Y (2019) Experimental study of the interplay between pore system and permeability using pore compressibility for high rank coal reservoirs. Fuel 254:115712. https://doi.org/10.1016/j.fuel.2019.115712
Hürlimann M, Latour L, Sotak C (1994) Diffusion measurement in sandstone core: nmr determination of surface-to-volume ratio and surface relaxivity. Magn Reson Imaging. https://doi.org/10.1016/0730-725X(94)91548-2
Ji Y, Hou J, Zhao E, Lu N, Bai Y, Zhou K, Liu Y (2020) Study on the effects of heterogeneous distribution of methane hydrate on permeability of porous media using low-field NMR technique. Am Geophys Union. https://doi.org/10.1029/2019JB018572
Jia H, Zi F, Yang G, Li G, Yang P (2019) Influence of pore water (ice) content on the strength and deformability of frozen argillaceous siltstone. Rock Mech Rock Eng. https://doi.org/10.1007/s00603-019-01943-0
Jia H, Ding S, Zi F, Dong Y, Shen Y (2020) Evolution in sandstone pore structures with freeze-thaw cycling and interpretation of damage mechanisms in saturated porous rocks. CATENA 195:104915. https://doi.org/10.1016/j.catena.2020.104915
Jin P, Hu Y, Shao J, Liu Z, Feng G, Song S (2019) Influence of temperature on the structure of pore–fracture of sandstone. Rock Mech Rock Eng. https://doi.org/10.1007/s00603-019-01858-w
Kamal M, Mahmoud M, Hanfi M, Elkatatny S, Hussein I (2018) Clay minerals damage quantification in sandstone rocks using core flooding and NMR. J Pet Explor Prod Technol. https://doi.org/10.1007/s13202-018-0507-7
Kleinberg R (1996) Utility of NMR T2 distributions, connection with capillary pressure, clay effect, and determination of the surface relaxivity parameter ρ2. Magn Reson Imaging 14(7–8):761–767. https://doi.org/10.1016/s0730-725x(96)00161-0
Kleinberg R, Horsfield M (1990) Transverse relaxation processes in porous sedimentary rock. J Magn Reson (1969) 88(1):9–19. https://doi.org/10.1016/0022-2364(90)90104-h
Kleinberg R, Kenyon W, Mitra P (1994) Mechanism of NMR relaxation of fluids in rock. J Magn Reson Ser A 108(2):206–214. https://doi.org/10.1006/jmra.1994.1112
Kogbara R, Iyengar S, Grasley Z, Masad E, Zollinger D (2015) Non-destructive evaluation of concrete mixtures for direct LNG containment. Mater Des 82:260–272. https://doi.org/10.1016/j.matdes.2015.05.084
Lai J, Wang G, Wang Z, Chen J, Fan X et al (2017) A review on pore structure characterization in tight sandstones. Earth Sci Rev 177:436–457. https://doi.org/10.1016/j.earscirev.2017.12.003
Li M, Mao X, Cao L, Pu H, Mao R, Lu A (2016) Effects of thermal treatment on the dynamic mechanical properties of coal measures sandstone. Rock Mech Rock Eng 49(9):3525–3539. https://doi.org/10.1007/s00603-016-0981-5
Liu T, Wang S, Fu R, Zhou M, Li Y, Luo M (2003) Analysis of pore throat structure in rocks by NMR spectroscopy. Petroleum geophysical exploration. https://doi.org/10.13810/j.cnki.issn.1000-7210.2003.03.022(In Chinese)
Liu M, Xie R, Guo J, Jin G (2018) Characterization of pore structures of tight sandstone reservoirs by multifractal analysis of the NMR T2 distribution. Energy Fuels 32(12):12218–12230. https://doi.org/10.1021/acs.energyfuels.8b02869
Morad S, Al-Ramadan K, Ketzer J, DeRos L (2010) The impact of diagenesis on the heterogeneity of sandstone reservoirs: a review of the role of depositional facies and sequence stratigraphy. AAPG Bull 94(8):1267–1309. https://doi.org/10.1306/04211009178
Rao Q, Wang Z, Xie H, Xie Q (2007) Experimental study of mechanical properties of sandstone at high temperature. J Cent South Univ Technol 14(s1):478–483. https://doi.org/10.1007/s11771-007-0311-x
Roberts S, Mcdonald P, Pritchard T (1995) A bulk and spatially resolved nmr relaxation study of sandstone rock plugs. J Magn Reson Ser A 116(2):189–195. https://doi.org/10.1006/jmra.1995.0007
Sirdesai N, Singh T, Ranjith P (2017a) Thermal alterations in the poro-mechanical characteristic of an Indian sandstone—a comparative study. Eng Geol 226:208–220. https://doi.org/10.1016/j.enggeo.2017.06.010
Sirdesai N, Singh T, Ranjith P, Singh R (2017b) Effect of varied durations of thermal treatment on the tensile strength of red sandstone. Rock Mech Rock Eng 50(1):205–213. https://doi.org/10.1007/s00603-016-1047-4
Somerton W (1992) Thermal properties and temperature-related behavior of rock/fluid systems. Elsevier, Amsterdam
Song Z, Liu G, Yang W, Zou H, Sun M, Wang X (2017) Multi-fractal distribution analysis for pore structure characterization of tight sandstone—a case study of the upper paleozoic tight formations in the Longdong District. Marine Petroleum Geology, Ordos Basin. https://doi.org/10.1016/j.marpetgeo.2017.12.018
Taylor T, Giles M, Hathon L, Diggs T, Braunsdorf N, Birbiglia G, Espejo I (2010) Sandstone diagenesis and reservoir quality prediction: models, myths, and reality. AAPG Bull 94(8):1093–1132. https://doi.org/10.1306/04211009123
Tydlitát V, Trník A, Scheinherrová L, Podoba R, Černý R (2015) Application of isothermal calorimetry and thermal analysis for the investigation of calcined gypsum–lime–metakaolin–water system. J Therm Anal Calorim 122(1):115–122. https://doi.org/10.1007/s10973-015-4727-5
Tziotziou M, Karakosta E, Karatasios I, Diamantopoulos G, Sapalidis A, Fardis M, Maravelaki-Kalaitzaki P, Papavassiliou G, Kilikoglou V (2011) Application of 1h nmr to hydration and porosity studies of lime–pozzolan mixtures. Microporous Mesoporous Mater 139(1–3):16–24. https://doi.org/10.1016/j.micromeso.2010.10.010
Volokitin Y, Looyestijn W, Slijkerman W, Hofman J (2001) A practical approach to obtain primary drainage capillary pressure curves from NMR core and log data. Petrophysics SPWLA J Form Eval Reserv Descr 42(4):334–343
Watanabe K, Wake T (2009) Measurement of unfrozen water content and relative permittivity of frozen unsaturated soil using NMR and TDR. Cold Reg Sci Technol 59(1):34–41. https://doi.org/10.1016/j.coldregions.2009.05.011
Wei D, Liu X, Hu X, Xu R, Zhu L (2015) Estimation of permeability from NMR logs based on formation classification method in tight gas sands. Acta Geophys 63(5):1316–1338. https://doi.org/10.1515/acgeo-2015-0042
Wu G, Wang Y, Swift G, Chen J (2013) Laboratory investigation of the effects of temperature on the mechanical properties of sandstone. Geotech Geol Eng 31(2):809–816. https://doi.org/10.1007/s10706-013-9614-x
Xiao L, Liu X, Zou C, Hu X, Mao Z, Shi Y, Guo H, Li G (2013) Comparative study of models for predicting permeability from nuclear magnetic resonance (NMR) logs in two Chinese tight sandstone reservoirs. Acta Geophys 62(1):116–141. https://doi.org/10.2478/s11600-013-0165-6
Yin T, Li X, Cao W, Xia K (2015) Effects of thermal treatment on tensile strength of laurentian granite using brazilian test. Rock Mech Rock Eng 48(6):2213–2223. https://doi.org/10.1007/s00603-015-0712-3
Zhang J, Shen Y, Yang G, Zhang H, Wang Y, Hou X, Sun Q, Li G (2020) Inconsistency of changes in uniaxial compressive strength and P-wave velocity of sandstone after temperature treatments. J Rock Mech Geotech Eng. https://doi.org/10.1016/j.jrmge.2020.05.008
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 41672279, 41972288).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Michal Malinowski (CO-EDITOR-IN-CHIEF)/Jadwiga Anna Jarzyna, prof (ASSOCIATE EDITOR).
Rights and permissions
About this article
Cite this article
Zhang, H., Sun, Q., Jia, H. et al. Effects of high-temperature thermal treatment on the porosity of red sandstone: an NMR analysis. Acta Geophys. 69, 113–124 (2021). https://doi.org/10.1007/s11600-020-00526-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11600-020-00526-w