Abstract
Fe-doping Li3V2(PO4)3/C material was successfully synthesized from combustion synthesis precursors. The Li3V2(PO4)3 is layered by amorphous carbon with a porous structure and doped with Fe, which can improve the Li+ transfer rate and conductivity. The 1% Fe-doped products used as cathode electrode for lithium-ion batteries exhibit enhanced electrochemical performance. In 3.0~4.8 V, it has a specific discharge capacity of 180 mAh g−1 after 20 cycles at 0.1 C, 142.5 mAh g−1 after 500 cycles at 1 C, and 132.5 mAh g−1 after 500 cycles at 10 C. Moreover, it shows stabilized specific discharge capacity of 65.9 mAh g−1 after 500 cycles at a rate of 20 C, and the capacity retention is 98%. Thus, it could infer the Fe-doping Li3V2(PO4)3/C material is a permission candidated material for application in lithium-ion batteries with high performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) employed in hybrid electric vehicle energy storage device have been attracting great attention for their light weight, high safety, high energy, and long cycle durability without a memory effect [1, 2]. To develop desired electrode materials with high performance including excellent rate capability, high reversible discharge capacity and cycle stability are crucial for LIBs, especially for cathode materials [2, 3]. In recent decades, in order to achieve the increasing demand performance, various kinds of cathode materials have been researched and developed, such as lithium transition-metal phosphates. Due to the good structural stability, high theoretical specific capacity, and operating voltage [4, 5], lithium transition-metal phosphates have attracted significant attention in LIBs with high performance. Among the transition-metal phosphate compounds, Li3V2(PO4)3 (monoclinic) is the most prospective material ascribed to its acceptable ionic mobility, high theoretical specific capacity, operating voltage, and thermodynamically stable structure [6, 7]. However, the separated [VO6] octahedral arrangements result in the poor electronic conductivity of Li3V2(PO4)3 (2.4–10−7 S cm−1), which indeed limits its extensive application [8, 9]. Great efforts have been devoted to resolve those problems, such as coating Li3V2(PO4)3 with a carbon layer [10, 11], reducing its particle size [12, 13] and cation doping [14, 15]. Among them, cation doping is a facility and effective method to enhance the electrochemical properties of Li3V2(PO4)3 [16]. Up to now, a great number of cations, such as Zr3+, Mn2+, Al3+, Cu2+, Mg2+, Fe3+, Ge4+, and Cr3+ have been employed as dopants for Li3V2(PO4)3 and achieved some good results [14,15,16,17,18,19,20].
Previously, several methods have been adopted to synthesize cation doping Li3V2(PO4)3, which include hydrothermal technique, spray pyrolysis method, solid-state strategy, and sol-gel approach. For example, Park et al. [19] have prepared Mn-doping Li3V2(PO4)3 by the spray pyrolysis method. The electric conductivity of Li3V2(PO4)3 has been increased ascribed to the Mn doping, and the cathode electrode performances have been improved. Liu et al. [20] used two methods (the first step is the sol-gel method) to fabricate Fe-doping Li3V2(PO4)3 with improvement of electrochemical performance. However, these methods usually consist of redundant steps, such as a long time for reaction, washing several times, and calcination at various stages to prepare the final product. Thus, it is valuable to develop a facile synthesis route with low cost and efficiency for practical application. Up to now, we find no literature report on the preparation of Fe-doped LVP by combustion synthesis. In this work, we offer a facile route for the synthesis of Fe-doping Li3V2(PO4)3 using solution combustion synthesis. Compared with conventional synthesis methods, this method (solution combustion synthesis) is effective and economic due to time-saving processing and economic and simple experimental set-up [21,22,23]. Electrochemical measurement results imply the as-prepared Fe-doping Li3V2(PO4)3 is a permission candidated cathode material for application in lithium-ion batteries with high performance.
Experimental
Synthesis
In detail, 0.03 mol lithium nitrate (LiNO3), 0.02 mol ammonium metavanadate (NH4VO3), 0.02 mol ammonium dihydrogen phosphate (NH4H2PO4,), ferric nitrate (Fe (NO3)3·9H2O), 2 g glucose (C6H12O6·H2O,), and 0.07 mol glycine (NH2CH2COOH) were dissolved in 200 ml deionized water. All the chemicals were analytical reagent grade (Shanghai Zhanyun Chemical Co., Ltd.). The ratio between Fe and Li3V2(PO4)3 (0.02 mol ammonium metavanadate could prepare 0.01 mol Li3V2(PO4)3) was 0~2 (wt%). The whole process for preparation precursors takes 15–20 min. Then, the precursors prepared by combustion synthesis were heated at 800 °C for 5 h with N2 atmosphere, and then Fe-doping Li3V2(PO4)3 could be gotten.
Characterizations
The prepared samples were characterized with X-ray diffraction (XRD, MXP21VAHF) at room temperature, X-ray photoelectron spectra (XPS, PerkinElmer), scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
Electrochemical measurements
The electrodes were fabricated by a mixture of Fe-doping Li3V2(PO4)3 materials (80 wt%), acetylene black (10 wt%), and poly (10 wt%). The compounds were scattered in N-methyl-2-pyrrolidone (NMP) to form sizing agent, depositing on an Al foil. All the fabricated electrodes were stoved at 80 °C for 10 h in a vacuum oven. The half cells (CR2032-type) were fabricated in an Ar-filled glove box. The electrolyte was a non-aqueous solution with 1 M LiPF6 dissolved in a mixture of 1:1:1 of ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethylene methyl carbonate (EMC). The charge and discharge properties of the cells with different current densities were tested in 3.0–4.8 V. For the capacity rate testing, the current gradually changed from 0.5 to 1, 2, 5, and 10 C and decreased to 0.5 C. Cyclic voltammetry (CV) curves were studied with CHI710D (Chenhua, Shanghai) electrochemical workstation at the rate of 0.1 mV s−1.
Results and discussion
Figure 1a demonstrates the XRD results of the as-prepared products. All diffraction peaks can be labeled as the crystalline monoclinic Li3V2(PO4)3 (JCPDS 97-009-6962), and no other peaks could be found. It indicated the Li3V2(PO4)3 has been successfully prepared by reduction combustion synthesis precursors at a temperature of 800 °C for 5 h. Figure 1b shows the chemical information of samples characterized by XPS. According to Fig. 1b, the Li, P, C, V, O, N, and Fe elements could be found. The ~ 55.7 eV band is due the contribution of Li+ in Li3V2(PO4)3 [24]. The ~ 140 eV band is attributed to 2P3/2 in (PO4)3 [25]. The peak at 531.5 eV was the O1s spectrum generated by (PO4)3 [26]. The peak located at 285 eV is corresponding C1s in carbon [27]. The ~ 400 eV peak confirms the N1s in samples [28]. In the high-resolution XPS of V2p for 0 wt% and 1 wt% samples, as shown in Fig.1c, the V2p with a binding energy of around 517 eV ascribed to V2p, which correspond to V3+ in Li3V2(PO4)3 [29]. Additionally, the high-resolution XPS of Fe2p for 0 wt% and 1 wt% samples are shown in Fig.1d. It can be found that new peaks of Fe2p with a binding energy around 711 eV [30] appeared in the Fe-doped sample and no characteristic peak of Fe in 0% sample. The XPS results indicate the Fe-doped Li3V2(PO4)3 has been successfully prepared.
Figure 2 illustrates the SEM characterizations of all samples. As illustrated in Fig.2, the 0% sample and 2% sample has a bulk structure and composed of irregular particles. The 1% sample has a porous structure and composed of a sheet structure, which is favorable for lithium ion transmission [31]. By comparison, the Fe-doped Li3V2(PO4)3 have less agglomeration than pure Li3V2(PO4)3, particularly for the 1% sample. This is due to the Fe source (ferric nitrate) which was added in the sample. In this work, it used solution combustion method (SCS) to synthesize precursors. For the SCS, ferric nitrate is the oxidant in the system. When ferric nitrate was induced in the system, the precursors were prepared by the reaction, and the ratio of fuel and oxidant was increased. It could affect the chemical energy and gases liberated in the reaction process [32, 33]. Because of the dispersant effect of more gases on the products [34], the 1% sample has a porous structure. When the Fe content was added to the 2% sample, the surpassing oxidation in the reaction system and the surpassing energy generated in the system, and the particles began to grow and agglomerated. So, the morphology of the product has been changed. Figure 3 exhibits the original images of 1% Fe-doped Li3V2(PO4)3/C powders and the map distribution of V and Fe elements. It is visible in Fig. 3b that the powders have homogeneous distribution of V and Fe elements. Because all raw materials were dissolved in water, all elements in the reaction system uniformly mixed at a molecular or atomic level. And the elements in the samples could distribute homogeneously.
To further observe the structure and morphology of the products, TEM images of pure and Fe-doping Li3V2(PO4)3 products are displayed in Fig. 4. It is clear that pure Li3V2(PO4)3 (Fig. 4a) and 2% Fe-doping Li3V2(PO4)3 (Fig. 4c) have a bulk structure, and the 1% Fe-doped Li3V2(PO4)3 (Fig. 4b) shows a highly porous structure with interconnected macropores. High-resolution transmission electron microscope (HRTEM) investigations of 1% Fe-doped Li3V2(PO4)3 (Fig. 4d–f) demonstrate the modified Li3V2(PO4)3 are covered with amorphous carbon, which can improve the electron conductivity of the products. To study the crystalline nature of Li3V2(PO4)3, electron diffraction of Li3V2(PO4)3 was performed. Figure 4f exhibits the selected electron diffraction pattern, and the distinctive diffraction patterns confirm the highly crystalline nature of the 1% Fe-doped Li3V2(PO4)3. The clear diffraction spots further represent the monoclinic crystal system of Li3V2(PO4)3 phase which is consistent with the XRD results.
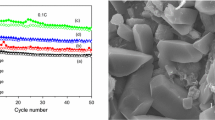
Figure 5 a exhibits the circulation performance of different Fe content samples at 0.1 C in 3.0–4.8 V. It is clear that the discharge capacity of neat Li3V2(PO4)3 after 20 cycles was only 85 mAh g−1. The Fe-doped samples have a higher capacity than pure Li3V2(PO4)3 due to the incorporation of Fe3+ which improved electrical conductivity and structural stability. The 1% sample presents the highest discharge capacity of 180 mAh g−1 and the retention is 91.4%. In comparison with the 1% sample, the 2% sample shows a lower capacity with poor capacity retention. Due to too much Fe3+ substitution in Li3M2(PO4)3 lattice, the closet bond lengths of Li1–O, Li2–O, and Li3–O contacts are significantly reduced. The binding interaction between O and Li gradually increases, reducing the insertion/extraction mobility of lithium ions during discharge/charging [35, 36]. Therefore, with the increase in Fe amount, an excess of Fe3+ in the Li3V2(PO4)3 lattice decreases the electrochemical activities and contributes to the lower electrochemical properties of the electrodes. Figure 5b shows the rate performance of the samples at different rates increasing from 0.5 to 10 C and then reducing to 0.5 C each 5 cycles. It obviously shows that the discharge capacity is decreased as the current rate increases which indicates the electrochemical activities of Li3V2(PO4)3 is determined by ion diffusion. And the 1% sample electrode possesses the capacity of 145, 138, 125, 88, and 143 mAh g−1 at 0.5 C, 1 C, 2 C, 5 C, 10 C, and 0.5 C, respectively.
EIS measurements can reveal diffusion process information during charge/discharge process [37]. The kinetic properties of undoped and Fe-doped samples were studied by EIS. Figure 6 presents the Nyquist plots of all the three Li3V2(PO4)3 electrodes, and each plot composed of a small intercept at high frequency (corresponding to the ohm resistance of electrolyte (Re)), a depressed semicircle at medium frequency (including the charge transfer resistance between the electrode and electrolyte interface (Rct) and the double-layer capacitance between electrolyte and electrode (Cdl)), and a linear part at low frequency (associated with the Warburg resistance Zw) [38, 39]. It is obvious that the 1% Fe-doped sample shows the lowest Rct. The result is in good agreement with the electrochemical performance obtained in Fig. 5. The improvement is due to the appropriate Fe doped into the Li3V2(PO4)3 crystal lattice which can increase the degree of disorder in the lattice and enhance the electronic transfer conductivity.
To further study the electrochemical reaction during cycles, the CV curves of the 1% sample electrode in 3.0–4.8 V at a scanning rate of 0.1 mV s−1 are presented in Fig.7. Because of formation and structure rearrangement of the solid electrolyte interface (SEI) film, the CV curves were tested after five cycles[40-41]. The curves present four oxidation peaks and three reduction peaks. The oxidation peaks located at ~ 3.63 (A1) and 3.72 V (A2) are corresponding to the first Li+ extraction from Li3V2(PO4)3 in two steps ascribed to the ordered phase at mixed V3+/V4+, and the third anodic peak (A3) about 4.13 V is attribute to the second Li+ extraction from Li2V2(PO4)3 [42-44]. The first two Li+ extractions related to the V3+/V4+ couple. The fourth oxidation peak (A4) at about 4.63 V generates by the removal of the third Li+ (LiV2(PO4)3 → V2(PO4)3), which attributed to the V4+/V5+ couple [1]. The border shape with lower densities of peak A4 is ascribed to the lower electronic/ionic conductivity of V2(PO4)3, and, therefore, it has slower reaction kinetics and lower electrochemical activity. Moreover, the peak around B3 is due to the insertion of two Li+ into V2(PO4)3 by a solid solution process, which is ascribed to the V5+/V4+ couple. The reduction peaks located at B2 and B1 are ascribed to a two-step insertion of the first Li+ transform Li2V2(PO4)3 to Li2.5V2(PO4)3 and then to Li3V2(PO4)3, and it corresponds to the V4+/V3+ pairs. To study the high current rate and circulation performance of the 1% sample, the electrodes in 3.0–4.8 V at different current densities for 500 cycles are shown in Fig. 7b. At the current of 1 C, 10 C, and 20 C, the initial special capacity is 175.7, 142.0, and 67.0 mAh g−1, respectively, and the capacity was still 142.5, 132.5, and 65.9 mAh g−1 after 500 cycles, respectively. The results indicate that the material has good circulation performance. The capacity of the cathode decreases with current rate increasing. Due to the low current density, the polarization of the electrode is also small, and the charging and discharging time is long enough for Li+ to be inserted/extracted, so the specific capacity is higher. However, when the current density is too high, the charging and discharging time is too short, which leads to the incomplete insertion/extraction for Li+, so the specific capacity will decrease accordingly. In general, the circulation performance of Fe-doped Li2V2(PO4)3 has been greatly improved.
Conclusions
Fe-doping Li3V2(PO4)3/C materials were successfully synthesized from combustion synthesis precursors. Because appropriate Fe doped into the Li3V2(PO4)3 crystal lattice which could increase the degree of disorder in lattice and enhance the electronic transfer conductivity, the 1% Fe-doped composites as cathode electrode material for LIBs exhibit enhanced electrochemical performance. In 3.0~4.8 V, it has a specific discharge capacity of 180 mAh g−1 at the rate of 0.1 C after 20 cycles, 142.5 mAh g−1 at 1 C, and 132.5 mAh g−1 10 C after 500 cycles. Moreover, it shows stabilized specific discharge capacity of 65.9 mAh g−1 after 500 cycles at the rate of 20 C, and the capacity retention is 98%. Thus, it could infer the Fe-doping Li3V2(PO4)3/C material is a potential cathode material for application in LIBs with high performance. Furthermore, this work provides a new insight into the construction of cation doped Li3M2(PO4)3 for improving electrochemical performance.
References
Sun P, Zhao X, Chen R, Chen T, Ma L, Fan Q, Lu H, Hu Y, Tie Z, Jin Z, Xu Q, Liu J (2016) Li3V2(PO4)3 encapsulated flexible free-standing nanofabric cathodes for fast charging and long life-cycle lithium-ion batteries. Nanoscale 8:7408–7415
Chen L, Yan B, Xu J, Wang C, Chao Y, Jiang X, Yang G (2015) Bicontinuous structure of Li3V2(PO4)3 clustered via carbon nanofiber as high-performance cathode material of Li-ion batteries. ACS Appl Mater Interfaces 7:13934–13943
Mao WF, Fu YB, Zhao H, Ai G, Dai YL, Meng DC, Zhang XL, Qu DY, Liu G, Battaglia VS, Tang ZY (2015) Rational design and facile synthesis of Li3V2(PO4)3@C nanocomposites using carbon with different dimensions for ultrahigh-rate lithium-ion batteries. ACS Appl Mater Interfaces 7:12057–12066
Whittingham MS (2014) Ultimate limits to intercalation reactions for lithium batteries. Chem Rev 114:1414–11443
Wei Q, Xu Y, Li Q, Tan S, Ren W, An Q, Mai L (2016) Novel layered Li3V2(PO4)3/rGO&C sheets as high-rate and long-life lithium ion battery cathodes. Chem Commun 52:8730–8732
Wei Q, An Q, Chen D, Mai L, Chen S, Zhao Y, Hercule KM, Xu L, Minhas-Khan A, Zhang Q (2014) One-pot synthesized bicontinuous hierarchical Li3V2(PO4)3/C mesoporous nanowires for high-rate and ultralong-life lithium-ion batteries. Nano Lett 14:1042–1048
Kang J, Mathew V, Gim J, Kim S, Song J, Bin Im W, Han J, Lee JY, Kim J (2014) Pyro-synthesis of a high rate nano-Li3V2(PO4)3/C cathode with mixed morphology for advanced Li-ion batteries. Sci Rep 4:4047
Wang S, Zhang Z, Deb A, Yang C, Yang L, Hirano S (2014) Nanostructured Li3V2(PO4)3/C composite as high-rate and long-life cathode material for lithium ion batteries. Electrochim Acta 143:297–304
Rajagopalan R, Zhang L, Dou SX, Liu H (2016) Lyophilized 3D lithium vanadium phosphate/reduced graphene oxide electrodes for super stable lithium ion batteries. Adv Energy Mater 6:1501760
Liao Y, Li C, Lou X, Hu X, Ning Y, Yuan F, Chen B, Shen M, Hu B (2018) Carbon-coated Li3V2(PO4)3 derived from metal-organic framework as cathode for lithium-ion batteries with high stability. Electrochim Acta 271:608–616
Lin X, Shen Z, Han T, Liu J, Huang J, Zhou P, Zhang H, Liu J, Li J, Li J (2018) Hydrogel assisted synthesis of Li3V2(PO4)3 composite as high energy density and low-temperature stable secondary battery cathode. J Alloy Compd 739:837–847
Tang Y, Rui X, Zhang Y, Lim TM, Dong Z, Hng HH, Chen X, Yan Q, Chen Z (2013) Vanadium pentoxide cathode materials for high-performance lithium-ion batteries enabled by a hierarchical nanoflower structure via an electrochemical process. J Mater Chem A 1:82–88
Naoi K, Kisu K, Iwama E, Sato Y, Shinoda M, Okita N, Naoi W (2015) Ultrafast cathode characteristics of nanocrystalline-Li/carbon nanofiber composites. J Electrochem Soc 162:A827–A833
Kim S, Zhang Z, Wang S, Yang L, Penner-Hahn JE, De A (2018) Electrochemical and structural investigation of Mg-doped Li3V(2-2x/3) Mgx (PO4)3. J Power Sources 396:491–497
Kalaga K, Sayed FN, Rodrigues MF, Babu G, Gullapalli H, Ajayan PM (2018) Doping stabilized Li3V2(PO4)3 cathode for high voltage, temperature enduring Li-ion batteries. J Power Sources 390:100–107
Cheng Y, Feng K, Zhou W, Zhang H, Li X, Zhang H (2015) A Bi-doped Li3V2(PO4)3/C cathode material with an enhanced high-rate capacity and long cycle stability for lithium ion batteries. Dalton Trans 44:17579–17586
Zhang Y, Su Z, Ding J (2017) Synthesis and electrochemical properties of Ge-doped Li3V2(PO4)3/C cathode materials for lithium-ion batteries. J Alloy Compd 702:427–431
Yan J, Fang H, Jia X, Wang L (2018) Copper incorporated in Li3V2(PO4)3/C cathode materials and its effects on high-rate Li-ion batteries. J Alloy Compd 730:103–109
Park J, Kim J, Park WB, Sun Y, Myung S (2017) Effect of Mn Li3V2−xMnx (PO4)3 as high capacity cathodes for lithium batteries. ACS Appl Mater Interfaces 9:40307−40316
Liu X, Zhao Y, Kuang Q, Li X, Dong Y, Jing Z, Hou S (2016) Mixing transition-metal phosphates Li3V2-xFex (PO4)3 (0≤x≤2): the synthesis, structure and electrochemical properties. Electrochim Acta 196:517–526
Varma A, Mukasyan AS, Rogachev AS, Manukyan KV (2016) Solution combustion synthesis of nanoscale materials. Chem Rev 116:14493–14586
Manukyan KV, Chen YS, Rouvimov S, Li P, Li X, Dong S, Liu X, Furdyna JK, Orlov A, Bernstein GH, Porod W, Roslyakov S, Mukasyan AS (2014) Ultrasmall α-Fe2O3 superparamagnetic nanoparticles with high magnetization prepared by template-assisted combustion process. J Phys Chem C 118:16264
Cao Z, Qin M, Zuo C, Gu Y, Jia B (2017) Facile route for synthesis of mesoporous graphite encapsulated iron carbide/iron nanosheet composites and their electrocatalytic activity. J Colloid Interface Sci 491:55–63
Cui K, Hu S, Li Y (2016) Nitrogen-doped graphene nanosheets decorated Li3V2(PO4)3/C nanocrystals as high-rate and ultralong cycle-life cathode for lithium-ion batteries. Electrochim Acta 210:45–52
Zhang LL, Liang G, Peng G, Jiang Y, Fang H, Huang YH, Croft MC, Ignatov A (2013) Evolution of electrochemical performance in Li3V2(PO4)3/C composites caused by cation incorporation. Electrochim Acta 108:182–190
Li Q, Wen Z, Fan C, Zeng T, Han S (2018) Chemical reaction characteristics, structural transformation and electrochemical performances of new cathode LiVPO4F/C synthesized by a novel one-step method for lithium ion batteries. RSC Adv 8:7044–7054
Wang C, Guo Z, Shen W, Zhang A, Xu Q, Liu H, Wang Y (2015) Application of sulfur-doped carbon coating on the surface of Li3V2(PO4)3 composites to facilitate Li-ion storage as cathode materials. J Mater Chem A 3:6064–6072
Wang C, Shen W, Liu H (2014) Nitrogen-doped carbon coated Li3V2(PO4)3 derived from a facile in situ fabrication strategy with ultrahigh-rate stable performance for lithium-ion storage. New J Chem 38:430–436
Si Y, Su Z, Wang Y, Ma T, Ding J (2015) Improved electrochemical properties of (1- x)LiFePO4$x Li3V2(PO4)3/C composites prepared by a novel sol-gel method. New J Chem 39:8971–8977
Morgan D, Ceder G, Saidi MY, Swoyer J, Huang H, Adamson G (2002) Experimental and computational study of the structure and electrochemical properties of LixM2(PO4)3 compounds with the monoclinic and rhombohedral structure. Chem Mater 14:4684–4693
Yue Y, Liang H (2017) Micro- and nano-structured vanadium pentoxide (V2O5) for electrodes of lithium-ion batteries. Adv Energy Mater 7:1602545
Cao Z, Qin M, Jia B, Gu Y, Chen P, Volinsky AA, Qu X (2015) One pot solution combustion synthesis of highly mesoporous hematite for photocatalysis. Ceram Int 41:2806–2812
Deshpande K, Mukasyan A, Varma A (2004) Direct synthesis of iron oxide nanopowders by combustion approach: reaction mechanism and properties. Chem Mater 16:4896–4904
Cao Z, Qin M, Jia B, Zhang L, Wan Q, Wang M, Volinsky AA, Qu X (2014) Facile route for synthesis of mesoporous Cr2O3 sheet as anode materials for Li-ion batteries. Electrochim Acta 139:76–81
Patoux S, Wurm C, Morcretta M, Rousse G, Masquelier C (2003) A comparative structural and electrochemical study of monoclinic Li3Fe2(PO4)3 and Li3V2(PO4)3. J Power Sources 119–121:278–284
Zhang LL, Sun HB, Yang XL, Li M, Li Z, Ni SB, Tao HC (2016) Natural graphite enhanced the electrochemical performance of Li3V2(PO4)3 cathode material for lithium ion batteries. J Solid State Electrochem 20:311–318
Zhang L, Li Z, Yang X, Ding X, Zhou Y, Sun H, Tao H, Xiong L, Huang Y (2017) Binder-free Li3V2(PO4)3/C membrane electrode supported on 3D nitrogen doped carbon fibers for high-performance lithium-ion batteries. Nano Energy 34:111–119
Wu J, Xu M, Tang C, Li G, He H, Li CM (2018) F-doping effects on carbon-coated Li3V2(PO4)3 as a cathode for high performance lithium rechargeable batteries: combined experimental and DFT studies. Phys Chem Chem Phys 20:15192–15202
Kim S, Zhang ZX, Wang SL, Yang L, Cairns EJ, Penner-Hahn JE, Deb A (2016) Electrochemical and structural investigation of the mechanism of irreversibility in Li3V2(PO4)3 cathodes. J Phys Chem C 120:7005–7012
Sun HB, Zhang LL, Yang XL, Huang YH, Li Z, Zhou YX, Ding XK, Liang G (2016) Effect of Fe-doping followed by C+SiO2 hybrid layer coating on Li3V2(PO4)3 cathode material for lithium-ion batteries. Ceram Int 42:16557–16562
Saidi MY, Barker J, Huang H, Swoyer JL, Adamson G (2003) Performance characteristics of lithium vanadium phosphate as a cathode material for lithium-ion batteries. J Power Sources 119:266–272
Yin SC, Grondey H, Strobel P, Anne M, Nazar LF (2003) Electrochemical property: structure relationships in monoclinic Li3-yV2(PO4)3. J Am Chem Soc 125:10402–10411
Ren MM, Zhou Z, Li YZ, Gao XP, Yan J (2006) Preparation and electrochemical studies of Fe-doped Li3V2(PO4)3 cathode materials for lithium-ion batteries. J Power Sources 162:1357–1362
Li Z, Zhang LL, Yang XL, Sun HB, Huang YH, Liang G (2016) Superior rate performance of Li3V2(PO4)3 co-modified by Fe-doping and RGO-incorporation. RSC Adv 6:10334–10340
Funding
This work was supported by the Applied Basic Research Programs of Sichuan Province (no. 2018JY0130, no. 2019JY0684) and the Applied Basic Research Programs of Panzhihua (no. 2018CY-G-11).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cao, Z., Zuo, C., Cui, X. et al. Synthesized Fe-doping Li3V2(PO4)3/C cathode material from combustion synthesis precursors with enhanced electrochemical performance. Ionics 25, 5709–5716 (2019). https://doi.org/10.1007/s11581-019-03107-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03107-0