Abstract
The present work was carried out to investigate the abnormal trend of electrochemical properties of solid biopolymer electrolytes (SBEs) system-based carboxymethyl cellulose (CMC) blended with polyvinyl alcohol (PVA)-doped NH4Cl. The SBEs system was prepared via solution casting technique and analyzed through Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), X-ray diffraction (XRD) analysis, and electrical impedance spectroscopy (EIS). Complexation was observed with the changes of peaks at 1065 cm−1, 1598 cm−1, 2912 cm−1, and 3396 cm−1 that corresponds to C–O–C, C=O of COO− stretching, C–H stretching, and O–H stretching, respectively, of CMC/PVA blend system upon the addition of NH4Cl. The decrease of the amorphousness and the increase of weight loss demonstrated the abnormal observation of the ionic conductivity when (1–5 wt%) NH4Cl was added in the SBEs system which was lower than the un-doped SBEs system. It was also observed that the highest ionic conductivity at 8.86 × 10−5 Scm−1 was achieved by the sample containing 6 wt% of NH4Cl. The temperature dependence of the SBEs system is found to be governed by the Arrhenius rule. Through the IR deconvolution technique, the conductivity of CMC/PVA-NH4Cl SBEs system was shown to be primarily influenced by the ionic mobility and diffusion coefficient of the ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer electrolytes have been reported to possess a variety of desirable properties and hence have been extensively utilized in most electrochemical devices [1]. Solid biopolymer electrolytes (SBEs) have been introduced using natural materials which are not harmful to the environment and consequently have gained due attention among researchers owing to their unique properties, namely high ionic conductivity, good electrode-electrolytes contact apart from their inherent advantage over liquid- or gel-based electrolytes such as low self-discharge in batteries, and no leakage [2, 3]. Besides that, SBEs also play an important role in solid-state ionic due to their potential application in capacitors, fuel cells, and batteries [4, 5]. To develop the SBEs system, different types of polymers were studied widely from natural and synthetic source. Natural polymers, e.g., starch, chitosan [6], and carboxymethyl cellulose (CMC) [7], are favorable in the preparation of polymer-based electrolytes due to the abundance nature of such polymers. Apart from natural polymers, synthetic polymers such as polyvinyl alcohol (PVA) [8], polyethylene oxide (PEO) [9], and polyvinylidene fluoride (PVdF) [10] are also suitable to be applied in the SBEs system as a result of its remarkable ionic conductivity.
The most common method adopted by researchers to prepare thin film is the casting technique [4, 11, 12]. In order to enhance the ionic conductivity, several methods were used by researchers including polymer blending, co-polymer grafting, as well as the addition of ceramic and plasticizer [4, 13]. One of the viable techniques with ease preparation to increase the conductivity of biopolymer electrolytes is via the blending method [14]. Polymer blending is a well-used technique that employs a low-cost conventional technology in which the modification of properties is needed to increase the ionic conductivity of polymer electrolytes [15]. Furthermore, the preparation of polymer blends is fairly straightforward, and it is also noteworthy to mention that the physical properties such as the mechanical strength, segmental polymer chain, and crystallinity and thermal properties of the electrolyte system can be controlled or tailored [16, 17].
In the present work, CMC is selected as the primary host polymer due to its superior properties that are also environmental and human friendly. In addition, it is also a highly effective additive that has been utilized in various fields of applications to improve the quality of product and processing properties from cosmetics, pharmaceuticals, industrial textile, and paper to edible products [18, 19]. As reported by Kamarudin et al. [20], CMC is a polysaccharide derived from the reaction involving the hydroxyl (–OH) group and sodium monochloroacetate in an alkaline medium that is also known as etherification. Due to this process, CMC bears two characteristics of functional group, namely carboxylate anion (COO−) and hydroxyl group (–OH) which can offer multi-point interaction between ionic dopants in polymer electrolyte system. On the other hand, polyvinyl alcohol (PVA) is used as a secondary host polymer in a polymer-blended system due to the existence of many hydroxyl groups on the PVA backbone chain [21, 22]. PVA possesses photo-stable and good dimensional stability upon the irradiation of UV-visible light. Additionally, the large energy of inter-cohesive that is obtained from the highly polar alcohol (–OH) group contributes towards its low permeability to oxygen [23].
CMC/PVA is a polymer blending that can be used as a dressing material since it allows the healing process to be observed, is semi-transparent, and is also flexible blend [24, 25]. NH4Cl is chosen as the dopant in the present system since NH4+ is believed to be responsible for the ionic conduction in SBEs and has also been demonstrated as good proton donors in polymer-salt complexes [26]. This work mainly focusses on investigating the structural properties of a CMC/PVA-NH4Cl system with the aim of gaining an understanding of the irregularity trend of the ionic conduction that is characterized through Fourier transform infrared (FTIR) spectroscopy, thermogravimetric analysis (TGA), X-ray diffraction (XRD) analysis, and electrical impedance spectroscopy (EIS).
Experimental method
Sample preparation
CMC and PVA (~ 85% hydrolysis) were obtained from Acros Organic Co. (M.W. 90,000) and Merck Co. (M.W. 70,000), respectively. The 80/20 ratio of CMC/PVA was used as biopolymer-blended host and was dissolved in distilled water (highest conducting sample as reported in [27]. Then, a varied amount of NH4Cl from the range of 1 to 10 wt% was added to the CMC/PVA-blended solution and stirred continuously until a homogenous solution was attained. The solution was then cast into several Petri dishes and left to be dried further at room temperature until the film was formed.
Characterization
Fourier transform infrared spectroscopy
The infrared spectra were used to characterize the complexation of CMC/PVA-NH4Cl SBEs system and it was recorded using a PerkinElmer Spectrum 100 spectrometer. The spectrometer contains an attenuated total reflection (ATR) accessory with a germanium crystal. The infrared light was passed through the sample with a frequency in the range of 700 to 4000 cm−1 and a 2 cm−1 spectrum resolution was configured when the sample was placed on the germanium crystal. OriginPro 8 was used to analyze the FTIR spectra and peak deconvolution. The FTIR spectra deconvolution process was accomplished by using line-based correction and curve fitting. The Gaussian and Lorentzian functions were utilized for curve fitting purpose.
Thermogravimetric analysis
Mettler Toledo TGA-DSC was used to carry out the TGA. The measurements were recorded in a nitrogen gas atmosphere at a flow rate of 20 ml min−1. The samples from different compositions were heated from 30 to 800 °C at a heating rate of 10 °C min−1 and a mass of ~ 5 mg.
X-ray diffraction
A Rigaku MiniFlex II diffractometer was used to characterize the XRD patterns of CMC/PVA-NH4Cl SBEs system. The sample was placed onto a sample slide after being cut into suitable sizes. The sample was directly scanned at 2θ between 5° and 80° using Cu Kα radiation.
Electrical impedance spectroscopy
The ionic conductivity of the blended CMC/PVA doped with various compositions of NH4Cl-based solid biopolymer electrolytes was analyzed by using HIOKI 3532-50 LCR Hi-TESTER with applied DC voltage of 1 V. The SBEs system was tested at different frequencies and temperature ranges of 50 Hz to 1 MHz and 303 K to 353 K, respectively. The SBEs sample was subsequently cut into smaller sizes with approximately 2-cm diameter of the sample contact surface and flanked between two stainless steel electrodes. From the imaginary impedance (Zi) versus real impedance (Zr) of Cole-Cole plot, the bulk resistance (Rb) value is obtained and the ionic conductivity, σ, was determined using the following equation:
where t is the thickness of the sample and A (cm2) is the cross-sectional area of the biopolymer electrolyte film. A digital thickness gauge (DML3032) was used to measure the thickness of the SBEs system which was found to be in the range between 0.011 and 0.013 cm.
Result and discussion
FTIR spectroscopy analysis
Figure 1 shows the FTIR spectra of blended CMC/PVA doped with various compositions of NH4Cl at different wavenumber regions. Based on Fig. 1a, the vibrational band at 1065 cm−1 was attributed by the ether linkage (C–O–C) stretching the vibration of pure CMC [28], and a slight shifting of the wavenumber was observed from 1065 to 1063 cm−1 and a decrease in intensity upon the addition of (1–3 wt%) NH4Cl was noticed. Moreover, it was also found that when the addition was beyond 3 wt%, the wavenumber increased to 1066 cm−1 as well as its intensity. The shift in wavenumber was expected within the region of 1065 cm−1 due to the presence of oxygen which is known as a highly electronegative atom from the C–O–C group, and this creates an opportunity for the interaction to happen between CMC/PVA blend with H+ of NH4Cl. In addition, the changes of the intensity at C–O–C substructure COO− of CMC/PVA blend could be due to either the protonation of H+ that has or has not occurred, and this in turn, might affect the ionic conductivity and crystallinity of the SBEs system [19]. Meanwhile, Fig. 1b depicts the functional group of hydroxyl (–OH) with a bending vibration at 1323 cm−1 [29, 30]. Apparently, the peak intensity at this band has decreased for samples containing 1 to 3 wt% and increased again when more than 4 wt% NH4Cl was added. This phenomenon may be elucidated by the substitution of –OH group with proton (H+) from NH4Cl to form H–OH in the present system [15]. A similar observation was made by a research carried out by Samsudin et al. [28], in which it was reported that the changes in intensity of peak in the wavenumber occurred when the composition of dopant increases. These changes could be attributed by the coordination of NH4+ ion with polar group present in CMC in the event that NH4Cl was added.
Figure 1c illustrates another noticeable complexation, in which the wavenumber of 1598 cm−1 that corresponds to C=O of COO− from the CMC/PVA blend stretching is seen to shift to a lower wavenumber, i.e., 1577 cm−1. This could be due to the lone pair electron possessed by oxygen that attracts the NH4Cl molecule to be attached to it and hence induces the protonation process [31]. It is believed that this observation is due to the coordination interaction of COO− functional group in CMC/PVA blend and [H+]–[NH3+] which reflects the protonation between cation and carboxylate group of CMC/PVA blend.
It is important to note at this juncture that the concentrations of H+ rise with the increasing composition of NH4Cl and consequently creating more ions to be migrated towards the CMC. This migration initiates the ion transition from one site to another site via the formation of H bonding [16]. Ion exchange happens between complexed sites and is also known as the Grotthuss mechanism, where the interaction process occurs through structure diffusion [32]. The NH4+ ions contain two hydrogen atoms which bond with an equal strength, while the bonding strength of the remaining two hydrogen atoms is weak and strong, respectively. The weaker H bonding from NH4+ is more easily dissociated and H+ can jump from one site to another leaving a vacant site in which another H+ ion from the neighboring site could fill the space [33]. As the present work utilizes PVA which is partially hydrolyzed, there is an appearance of a new shoulder peak at wavenumber 1726 cm−1 that belongs to the C=O stretching in the acetate group of PVA [34]. However, the peak begins to disappear after 4 wt% of NH4Cl was added in the complexes. According to Ramlli et al. [4] and Rajendran et al. [35], the position and intensity of polar polymer groups are expected to change due to the protonation of H+ ion from the ammonium salt that jumps to the host polymeric backbone. This interaction between ammonium salt and host polymer compels more electron to move towards the blended CMC/PVA to form hydrogen through C=O.
Based on Fig. 1d, there are two peaks observed represented by C–H stretching and O–H stretching [35, 36]. It could be seen that upon the addition of 4 wt% NH4Cl in the present system, the stretching C–H at 2912 cm−1 has shifted to a lower wavenumber and peak broadened. Meanwhile, another shifting was observed at the O–H stretching region where the wavenumber shifted to a much lower, from 3296 to 3277 cm−1 (0 until 6 wt%). This is attributable to the interaction of H bonding at the O–H functional group. This is agreed by Shukur et al., [16] which has reported that the same interaction between starch and chitosan has involved at the O–H group. The observation of the present investigation is in good agreement to that of Shukur et al. [15] with regard to the coordination of NH4+ ions with polar groups found in PVA as NH4Cl is introduced into the system. The summarization of the peak changes in wavenumber for SBEs system is tabulated in Table 1.
Thermogravimetric analysis
Figure 2 depicts the thermal spectra of the present SBEs system with three distinct decomposition stages. Based on Fig. 2, it could be observed that the initial weight loss for SBEs system from 30 to 260 °C is due to the evaporation or dehydration of moisture, impurities, and residual solvent which is mainly to the designated molecule of O–H in the CMC/PVA backbone [36]. The presence of small amount of moisture attributed by the distilled water as solvent in the SBEs system contributed to the initial weight loss [37]. The observation of the initial weight loss (~ 30 °C to 260 °C) is similar to that as reported by Ramesh et al. [38] and Ahmad et al. [15] where the initial drop is due to the evaporation of moisture and the transition of the polymer electrolyte system that tends to absorb moisture from the surrounding. Based on Table 2, it was found that the first maximum decomposition temperature for SBEs system is at 250 °C, 236 °C, 215 °C, 232 °C, and 206 °C with 16.19%, 16.38%, 14.73%, 15.23%, and 12.29% of weight loss, respectively.
The second maximum decomposition temperature was observed from 250 to 330 °C for all samples in the SBEs system. This sudden drop in weight loss may be due to the decomposition of carboxylate (–COO−) group in the present SBEs system [39]. It could be observed that when NH4Cl was added in the CMC/PVA SBEs system, the sample that contains the lowest composition provided the highest weight loss in comparison to the un-doped CMC/PVA SBEs system. The increase in weight loss at this region was expected to lead towards the decrease in ionic conductivity [40]. The greatest weight loss is also believed to be due to the disintegration of the intermolecular and partial breaking of the molecular structure [41]. As reported by Anjali et al. [42] and Biswal et al. [43], the decarboxylation of the polymer chain occurs at the temperature between 300 and 340 °C, and our finding is in line with their works. Apart from that, according to Liew et al. [44] and Yang et al. [45], the second weight loss is due to the elimination of the side groups of the polymer backbone of PVA at about 250–350 °C.
On the other hand, it shows that the sample with 6 wt% has the lowest weight loss and the highest in maximum decomposition temperature. This observation gives the impression that heat resistivity caused the present sample to sustain its original form even when it is being heated at high temperature [46]. Therefore, it is apparent that the sample with 6 wt% NH4Cl has a good thermal stability that, in turn, affects the amorphousness of the system and hence affects the ionic conductivity. The final weight loss stage that transpired to all sample-based SBEs system is at the temperature range between 520 and 610 °C. This is maybe due to the degradation of the biopolymer complexes backbone [47, 48]. It can be seen that prolonged heating of the present sample beyond the decomposition temperature would result in carbonization and ash formation at 610 °C to 800 °C in SBEs system [39, 44].
X-ray diffraction analysis
The XRD analysis is important in determining the behavior of crystallinity and amorphous phase of a SBEs system. Figure 3 shows the XRD spectra for CMC/PVA-doped NH4Cl with various compositions. The nature of the microstructure of this SBEs system is observed through the peak and intensity changes. Based on Fig. 3, the decrease in relative intensity of broad peak with the increase of NH4Cl takes place at the range between 10° and 52°. This may be attributed to the inter- or intra-interaction between the CMC/PVA and the NH4Cl. Bond breaking and bond forming processes result in an increase of amorphousness in the SBEs system [6, 49]. Broad peak is interpreted as an amorphous hump and is the typical characteristic of the most optimum conducting electrolyte system. Thus, the change in intensity and the broad nature of the peaks was triggered by the complexation between polymer and dopant via formation of hydrogen bonding within the CMC/PVA-NH4Cl solid biopolymer electrolyte system [29, 50] which will eventually induce for more amorphous region of the polymer matrix [51]. As ion concentration in the SBEs system increases, both fractions of amorphous phase and charge carriers increased simultaneously [52]. The measurement of crystallite size value (Table 3) in the present system is to identify any changes of amorphous phase in SBEs system via the Debye-Scherrer method [8, 53, 54] based on information from full width at half maximum (FWHM) which was retrieved via OriginPro 8.0 software.
where λ is the wavelength of the X-ray used which is 1.5406 Å, β is the full width at half maximum (FWHM) of the peak, and θ is the Bragg diffraction angle.
Based on the calculated crystallite size, it is evident that there is an increment in crystallinity in comparison with the un-doped SBEs system upon the introduction of NH4Cl. The increased crystallinity could be due to the instability of the ion dispersion between the polymer host and the dopant that, in turn, caused the peak intensity of the SBEs sample to be higher (high in crystallite size). As reported by Liew et al. [55], self-cross linkage is formed at lower concentration of salt in polymer electrolyte system due to some salt trapped in the entanglements and thus causes the peak intensity to gradually increase. This situation is also evident in the present work, as the peak intensity increases at lower NH4Cl composition, the impregnation of NH4Cl weakened the interactions within the biopolymer-blended chains, and hence disrupt the ordered arrangement of the polymeric network [56]. This observation is also substantiated by the TGA, where the sample containing lower composition of NH4Cl showed higher weight loss, and thus, the probable cause of the decrease in the ionic conductivity of the SBEs system.
It is apparent that the sample that contains 6 wt% NH4Cl obtained the lowest value of crystallinity size. The decrease of the crystallinity at higher dopant composition is in agreement with the FTIR observation, where the increase in the protonation is due to the enhancement in the amorphous phase that would make H+ from [NH4]+-Cl− easily migrate towards the coordinating site in the CMC/PVA-blended backbone. Moreover, the XRD analysis also supported the findings of the TGA, where the most amorphous sample exhibited the higher decomposition temperature [54]. From the observation, it is expected that sample containing 6 wt% would give the optimum ionic conductivity of the SBEs system.
Ionic conductivity analysis
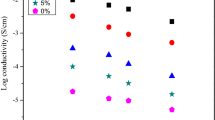
Figure 4 depicts the conductivity plot at room temperature of the CMC/PVA biopolymer blend doped with NH4Cl. It could be noted here that the ionic conductivity properties of polymer electrolytes are caused by several factors such as temperature, composition of ionic dopant, ionic, and species types [31, 46]. In the present system, it is noticeable that the ionic conductivity of the SBEs system drastically drops to a lower value in the range 1.34 × 10−7 to 1.22 × 10−6 S/cm when NH4Cl was added (1–5 wt%) into the CMC/PVA blend and it is found to be lower than the un-doped sample (9.08 × 10−6 S/cm). The significant reduction of the ionic conductivity upon the addition of 1 wt% might be triggered by the dissociation of NH4Cl [57]. Moreover, the abnormal trend could also be attributed by the electronic structure between NH4Cl and CMC/PVA blend which is similarly observed in other polymer electrolyte systems as reported by Kim et al. [58]. This indicated that the segmental mobility of ions has decreased and resulted in the reduced chain mobility thus in turn reduced the ionic conductivity of the present system [59]. The abnormal trends in the ionic conductivity are substantiated with the results attained from the FTIR spectroscopy, TGA, and XRD analysis where the changes of the structural and thermal properties at lower concentration lead to the decrement in the ionic conductivity of the present SBEs system.
Apparently, there is a sudden increase in conductivity when more than 5 wt% of NH4Cl is added. This might be attributable to the rapid jump of ions dissociation [H+]–NH3+ Cl towards the CMC/PVA backbone [60] and achieved the optimum value of ionic conductivity at 8.86 × 10−5 S/cm for the sample containing 6 wt%. As ion concentration increases, the tendency for the complexation to transpire within the CMC/PVA blend matrices is higher as more ions will migrate towards the CMC/PVA system and leads to formation of inter- or intra-interaction as discussed in the FTIR analysis in the previous section. This observation is also supported by the XRD results where the sample with 6 wt% of NH4Cl showed the lowest crystallinity of the SBEs system, hence enhances the ionic conductivity [61]. Unfortunately, the ionic conductivity began to decrease with the addition of more than 6 wt% NH4Cl and this observation might be due to the re-crystallization of sample as shown in the XRD analysis. When sample begins to re-crystallize, it would create a blocking pathway for ion migration and therefore resulted to the overcrowding of the ions in the polymer-salt complexes. Overcrowded ions are expected, and this would eventually cause the NH4Cl to re-associate, which reduces the movement of ions and limits the segmental motion of the polymer chain and on such accounts, the ionic conductivity decreases.
The temperature dependence of the ionic conductivity is shown in Fig. 5 and it is established that the ionic conductivity based on CMC/PVA SBEs system is thermally activated and stable [15, 16]. This phenomenon is further supported by the TGA result where the weight loss decreased with the increasing decomposition temperature, and hence, enhanced the ionic conductivity. As temperature increases, the thermal movement of the biopolymer chain segments, the dissociation of salts, and the amorphousness of the CMC/PVA-NH4Cl SBEs are improved, and as a result, the ionic conductivity is increased [62]. From Fig. 5, it is obvious that there was no abrupt jump at any stage, suggesting the complete amorphous nature of the structure of the biopolymer electrolytes [63]. Therefore, H+ ions might migrate through the conduction path formed by the lattice structure of the CMC/PVA blend chains [64]. The temperature dependence exhibited that the regression values for all complexes are close to unity, viz. R2 ~ 1 and obey the Arrhenius behavior [28, 65] via the following relationship:
where σo is the pre-exponential factor, Ea is the activation energy, and k is the Boltzmann constant. Table 4 shows the activation energy value of CMC/PVA-NH4Cl SBEs system.
From the calculated value of the activation energy, it is apparent that the Ea value increases and is higher than that of the un-doped sample upon the addition of 1 wt% NH4Cl composition. According to Latif et al. [66], Ea is a combination of the energy of the charge carrier creation which defects the energy of formation and ion migration. Therefore, it could be inferred that the rise in the Ea value is due to the higher amount of energy which is required to migrate the ions (H+) due to the blocking pathway (due to crystallinity) that occurred in the present sample [67, 68]. The increase of crystallinity of the present sample provides lesser free volume which in turn decreases the ionic conductivity upon the addition of NH4Cl at lower composition. It can be seen that the sample with 6 wt% of NH4Cl has a lower Ea value and it does not come to a surprise that this sample has the highest ionic conductivity, as it only requires smaller Ea in order to migrate the ions [69,70,71]. This behavior may be attributed to the increase in the amorphous phase and thermal decomposition which allows the ions to migrate easily with lesser restrictions between CMC/PVA blend and NH4Cl [72, 73].
Transport properties analysis
The IR spectra of the CMC/PVA-doped NH4Cl was deconvoluted to isolate and identify the free (H+) and contact ions [H]+–NH3Cl [21]. The wavenumbers between 1650 and 1500 cm−1 were used for deconvolution owing to the obvious changes of peak which suggests that complexation has occurred at this region [31]. The FTIR deconvolution of CMC/PVA-NH4Cl SBEs is illustrated in Fig. 6. Based on the IR deconvolution, free ions and contact ions were calculated [19, 74, 75] and tabulated in Table 5. The percentage of free ions and VTotal are determined from Eq. 4 and Eq. 5, respectively [74].
where Af is the area under the peak and Ac is the total area under the Af representing for free ions while Ac representing for contact ions. VTotal is the total volume of the SBE. Based on Table 5, it could be seen that free ions increased with the increase of NH4Cl composition. This implies that more ions are dissociated from NH4+ and become free ions, and hence essentially leads to an increment in the ionic conductivity [76]. The percentage of free ions is observed to decrease with the addition of more than 6 wt% of NH4Cl and this again might be attributed to association of ions which reduces the ionic conductivity of CMC/PVA-NH4Cl SBEs [77, 78].
Based on the free ion value, the transport properties of CMC/PVA-doped NH4Cl could be calculated. The transport properties, specifically the number of free mobile ions (ƞ), ionic mobility (μ), and diffusion coefficient of ions (D), were determined by using [74]:
where M is the number of moles of NH4Cl used in the SBEs system, NA is Avogadro’s number (6.02 × 1023), σ is the DC conductivity, e is the electric charge (1.602 × 10−19 C), k is the Boltzmann constant (1.38 × 10−23 J K−1), and T is the absolute temperature (303 K) [74]. The calculated transport property values are tabulated in Table 5.
From Table 5, it could be seen that the value of mobile ion, ƞ, increased with the increment of NH4Cl composition. Previous studies suggested that the ionic conductivity is influenced by the number of ions via the relationship based on Eq. (7) [64, 69, 74]. In the present system, the continuous ƞ values increased with the increase of NH4Cl composition and revealed that the ionic conductivity in the present system is not fully dependent with ƞ, however, appears to have a strong association with μ and D. This observation could be justified by the possible fact that the present system might be fully crowded with ions (H+) dissociated from NH4Cl and due to the higher crystallinity phase that, in turn, complicates the bond between one site and the other in the CMC/PVA blend. Similar results were observed from other polymer electrolyte systems where the effect of ionic conductivity was only demonstrated to be influenced by the ionic mobility and diffusion coefficient [58, 79].
Conclusion
A new series of solid biopolymer electrolytes (SBEs) system based on carboxymethyl cellulose (CMC)- and polyvinyl alcohol (PVA)-doped NH4Cl was successfully prepared via solution casting method. The prepared SBEs system was characterized for its structural and conduction properties by using FTIR spectroscopy, thermogravimetric analysis (TGA), X-ray diffraction (XRD) analysis, and electrical impedance spectroscopy (EIS). The IR spectra provided an insight into the possible interaction from the changes of peak at the functional group level of CMC/PVA via C–O–C and C=O from carboxylate anion group (COO−) with the addition of NH4Cl. The protonation of H+ towards the CMC/PVA blend backbone in the present system occurred through structural diffusion via Grotthuss mechanisms. The TGA revealed that the abnormal behavior of the SBEs system where early addition of NH4Cl resulted in an increase of weight loss in comparison to the un-doped system. The abnormal trend was also supported by the XRD results where the un-doped sample has a lower crystallinity size value in comparison to the initial composition of NH4Cl (1–5 wt%) which is due to the blocking pathway for protonation in the CMC/PVA SBEs system. The ionic conductivity obtained was found to be in agreement with the structural properties observation. With the addition of NH4Cl (1–5 wt%) in the SBEs system, the ionic conductivity was found to decrease from ~ 10−6 to 10−7 S/cm before achieving a maximum value of 8.86 × 10−5 S/cm for the sample that contains 6 wt% NH4Cl. The increase in the ionic conductivity to the optimum value is due to the higher ionic mobility and diffusion rate of protonation in the CMC/PVA blend which is reflected by the smaller activation energy. The results attained from the present investigation suggest that further research is needed in order to enhance the electrochemical properties of CMC/PVA-NH4Cl-based SBEs system.
References
Singh R, Polu AR, Bhattacharya B, Rhee HW, Varlikli C, Singh PK (2016) Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew Sust Energ Rev 65:1098–1117
Rani MSA, Rudhziah S, Ahmad A, Mohamed NS (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polym 6:2371–2385
Ning W, Xingxiang Z, Haihui L, Benqiao H (2009) 1-Allyl-3-methylimidazolium chloride plasticized-corn starch as solid biopolymer electrolytes. Carbohydr Polym 76:482–484
Ramlli MA, Kamarudin KH, Isa MIN (2015) Ionic conductivity and structural analysis of carboxymethyl cellulose doped with ammonium fluoride as solid biopolymer electrolytes. Am-Eurasian J Sustain Agric 9:46–52
Zhang J, Huang X, Fu J, Huang Y, Liu W, Tang X (2010) Novel PEO-based composite solid polymer electrolytes incorporated with active inorganic–organic hybrid polyphosphazene microspheres. Mater Chem Phys 121:511–518
Shukur MF, Kadir MFZ (2015) Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim Acta 158:152–165
Noor NAM, Isa MIN (2016) Ionic conductivity and dielectric properties of CMC doped NH4SCN solid biopolymer electrolytes. Adv Mater 1107:230–235
Wang J, Song S, Muchakayala R, Hu X, Liu R (2017) Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics 23:1759–1769
Sarangika HNM, Dissanayake MAKL, Senadeera GKR, Rathnayake RRDV, Pitawala HMJC (2017) Polyethylene oxide and ionic liquid-based solid polymer electrolyte for rechargeable magnesium batteries. Ionics 23:2829–2835
Liu J, Wu X, He J, Li J, Lai Y (2017) Preparation and performance of a novel gel polymer electrolyte based on poly (vinylidene fluoride)/graphene separator for lithium ion battery. Electrochim Acta 235:500–507
Ahmad NH, Isa MIN (2016) Ionic conductivity and electrical properties of carboxymethyl cellulose-NH4Cl solid polymer electrolytes. JESTEC 11:839–847
Samsudin AS, Isa MIN, Mohamad N (2011) New types of biopolymer electrolytes: ionic conductivity study on CMC doped with NH4Br. Int J Curr Eng Sci Res 1:7–11
Khiar ASA, Arof AK (2011) Electrical properties of starch/chitosan-NH4NO3 polymer electrolyte. WASET 59:23–27
Basavaraja C, Kim WJ, Kim DG (2011) Synthesis and characterization of soluble polypyrrole–poly (ɛ-caprolactone) polymer blends with improved electrical conductivities. Mater Chem Phys 129:787–793
Ahmad NH, Isa MIN (2016) Characterization of un-plasticized and propylene carbonate plasticized carboxymethyl cellulose doped ammonium chloride solid biopolymer electrolytes. Carbohydr Polym 137:426–432
Shukur MF, Ithnin R, Kadir MFZ (2014) Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 20:977–999
Tamilarasan P, Ramaprabhu S (2014) Stretchable supercapacitors based on highly stretchable ionic liquid incorporated polymer electrolyte. Mater Chem Phys 148:48–56
Ghanbarzadeh B, Almasi H, Entezami AA (2011) Improving the barrier and mechanical properties of corn starch-based edible films: effect of citric acid and carboxymethyl cellulose. Ind Crop Prod 33:229–235
Rasali NMJ, Samsudin AS (2017) Ionic transport properties of protonic conducting solid biopolymer electrolytes based on enhanced carboxymethyl cellulose-NH4Br with glycerol. Ionics 1:1–12
Kamarudin KH, Isa MIN (2013) Structural and DC ionic conductivity studies of carboxy methylcellulose doped with ammonium nitrate as solid polymer electrolytes. Int J Phys Sci 8:1581–1587
Yusuf SNF, Azzahari AD, Yahya R, Majid SR, Careem MA, Arof AK (2016) From crab shell to solar cell: a gel polymer electrolyte based on N-phthaloylchitosan and its application in dye-sensitized solar cells. RSC Adv 6:27714–27724
Yang CC, Lin SJ, Wu GM (2005) Study of ionic transport properties of alkaline poly (vinyl) alcohol-based polymer electrolytes. Mater Chen Phys 92:251–255
Sinha S, Chatterjee SK, Ghosh J, Meikap AK (2014) Dielectric relaxation and ac conductivity behaviour of polyvinyl alcohol–HgSe quantum dot hybrid films. J Phys D Appl Phys 47:275301
El-Gamal S, El Sayed AM, Abdel-Hady E (2017) Effect of cobalt oxide nanoparticles on the nano-scale free volume and optical properties of biodegradable CMC/PVA films. J Polym Environ 1:1–10
Wei QB, Fu F, Zhang YQ, Wang Q, Ren YX (2014) pH-responsive CMC/PAM/PVP semi-IPN hydrogels for theophylline drug release. J Polym Res 21:453
Ahmad NH, Isa MIN (2015) Conduction mechanism of solid biopolymer electrolytes system based on carboxymethyl cellulose--ammonium chloride. Am Eurasian J Sustain Agric 9:1–8
Saadiah MA, Samsudin AS (2018) Electrical study on carboxymethyl cellulose-polyvinyl alcohol based bio-polymer blend electrolytes. In IOP Conference Series: Mater Sci and Eng. 342:012045
Samsudin AS, Lai HM, Isa MIN (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Dai H, Huang Y, Huang H (2017) Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr Polym 185:1–11
Dai H, Huang H (2017) Enhanced swelling and responsive properties of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogel by the introduction of carclazyte. J Agric Food Chem 65:565–574
Ahmad NH, Isa MIN (2015) Structural and ionic conductivity studies of CMC based polymerelectrolyte doped with NH4Cl. Adv Mater Res 1107:247–252
Hema M, Selvasekerapandian S, Sakunthala A, Arunkumar D, Nithya H (2008) Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system. Phys B Condens Matter 403:2740–2747
Kadir MFZ, Aspanut Z, Majid SR, Arof AK (2011) FTIR studies of plasticized poly (vinyl alcohol)–chitosan blend doped with NH4NO3 polymer electrolyte membrane. Spectrochim Acta A Mol Biomol Spectrosc 78:1068–1074
Park JC, Ito T, Kim KO, Kim KW, Kim BS, Khil MS, Kim IS (2010) Electrospun poly (vinyl alcohol) nanofibers: effects of degree of hydrolysis and enhanced water stability. Polym J 42:273–276
Rajendran S, Sivakumar M, Subadevi R (2003) Effect of salt concentration in poly (vinyl alcohol)-based solid polymer electrolytes. J Power Sources 124:225–230
Liu X, Yu L, Liu H, Chen L, Li L (2008) In situ thermal decomposition of starch with constant moisture in a sealed system. Polym Degrad Stab 93:260–262
Mohamad AA, Arof AK (2007) Plasticized alkaline solid polymer electrolyte system. Mater Lett 61:3096–3099
Ramesh S, Arof AK (2001) Structural, thermal and electrochemical cell characteristics of poly (vinyl chloride)-based polymer electrolytes. J Power Sources 99:41–47
Ma XH, Xu ZL, Liu Y, Sun D (2010) Preparation and characterization of PFSA–PVA–SiO2/PVA/PAN difunctional hollow fiber composite membranes. J Membr Sci 360:315–322
Du F, Fischer JE, Winey KI (2003) Coagulation method for preparing single-walled carbon nanotube/poly (methyl methacrylate) composites and their modulus, electrical conductivity, and thermal stability. J Polym Sci B Polym Phys 41:3333–3338
Lewandowska K (2009) Miscibility and thermal stability of poly (vinyl alcohol)/chitosan mixtures. Thermochim Acta 493:42–48
Anjali T (2012) Modification of carboxymethyl cellulose through oxidation. Carbohydr Polym 87:457–460
Biswal DR, Singh RP (2004) Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym 57:379–387
Liew CW, Ramesh S, Arof AK (2014) A novel approach on ionic liquid-based poly (vinyl alcohol) proton conductive polymer electrolytes for fuel cell applications. Int J Hydrog Energy 39:2917–2928
Yang JM, Chiang CY, Wang HZ, Yang CC (2009) Two step modification of poly (vinyl alcohol) by UV radiation with 2-hydroxy ethyl methacrylate and sol–gel process for the application of polymer electrolyte membrane. J Membr Sci 341:186–194
Hirankumar G, Selvasekarapandian S, Bhuvaneswari MS, Baskaran R, Vijayakumar M (2004) AC impedance studies on proton conducting polymer electrolyte complexes (PVA+ CH3COONH4). Ionics 10:135–138
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Nithya H, Iwai Y, Kawamura J (2015) Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 21:1017–1029
Vöge A, Deimede V, Paloukis F, Neophytides SG, Kallitsis JK (2014) Synthesis and properties of aromatic polyethers containing poly (ethylene oxide) side chains as polymer electrolytes for lithium ion batteries. Mater Chem Phys 148:57–66
Shukur MF, Ithnin R, Kadir MFZ (2014) Electrical characterization of corn starch-LiOAc electrolytes and application in electrochemical double layer capacitor. Electrochim Acta 136:204–216
Yadav I, Nayak SK, Rathnam VS, Banerjee I, Ray SS, Anis A, Pal K (2018) Reinforcing effect of graphene oxide reinforcement on the properties of poly (vinyl alcohol) and carboxymethyl tamarind gum based phase-separated film. J Mech Behav Biomed Mater 81:61–71
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim Acta 55:1475–1482
Aji MP, Masturi, Bijaksana S, Khairurrijal, Abdullah M (2012) A general formula for ion concentration dependent electrical conductivities in polymer electrolytes. Am J Appl Sci 9:946–954
Mahakul PC, Sa K, Das B, Mahanandia P (2017) Structural investigation of the enhanced electrical, optical and electrochemical properties of MWCNT incorporated poly [3-hexylthiophene-2, 5-diyl] composites. Mater Chem Phys 199:477–484
Rasali NMJ, Nagao Y, Samsudin AS (2018) Enhancement on amorphous phase in solid biopolymer electrolyte based alginate doped NH4NO3. Ionics 1:1–14
Liew CW, Ramesh S (2015) Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr Polym 124:22–228
Middleton JC, Tipton AJ (2000) Synthetic biodegradable polymers as orthopedic devices. Biomaterials 21:2335–2346
Ibrahim S, Johan MR (2012) Thermolysis and conductivity studies of poly (ethylene oxide)(PEO) based polymer electrolytes doped with carbon nanotube. Int J Electrochem Sci 7:2596–2615
Kim JY, Oh MW, Lee S, Cho YC, Yoon JH, Lee GW, Jeong SY (2014) Abnormal drop in electrical resistivity with impurity doping of single-crystal Ag. Sci Rep 4:5450
Zhang H, Liu C, Zheng L, Feng W, Zhou Z, Nie J (2015) Solid polymer electrolyte comprised of lithium salt/ether functionalized ammonium-based polymeric ionic liquid with bis (fluorosulfonyl) imide. Electrochim Acta 159:93–101
Sit YK, Samsudin AS, Isa MIN (2012) Ionic conductivity study on hydroxyethyl cellulose (HEC) doped with NH4Br based biopolymer electrolytes. Res J Recent Sci 1:16–21
Samsudin AS, Isa MIN (2012) Structural and ionic transport study on CMC doped NH4Br: a new types of biopolymer electrolytes. J Appl Sci 12:174–179
Ulaganathan M, Pethaiah SS, Rajendran S (2011) Li-ion conduction in PVAc based polymer blend electrolytes for lithium battery applications. Mater Chem Phys 129:471–476
Ravi M, Pavani Y, Kumar KK, Bhavani S, Sharma AK, Rao VN (2011) Studies on electrical and dielectric properties of PVP: KBrO4 complexed polymer electrolyte films. Mater Chem Phys 130:442–448
Ahmad NH, Isa MIN (2015) Proton conducting solid polymer electrolytes based carboxymethyl cellulose doped ammonium chloride: ionic conductivity and transport studies. Int J Plast Technol 19:47–55
Kesharwani P, Sahu DK, Mahipal YK, Agrawal RC (2017) Conductivity enhancement in K+−ion conducting dry solid polymer electrolyte (SPE):[PEO: KNO3]: a consequence of KI dispersal and nano-ionic effect. Mater Chem Phys 193:524–531
Latif F, Aziz M, Katun N, Yahya MZ (2006) The role and impact of rubber in poly (methyl methacrylate)/lithium triflate electrolyte. J Power Sources 159:1401–1404
Sekhar PC, Kumar PN, Sasikala U, Rao VVRN, Sharma AK (2012) Investigations on lithium ion complexed polyvinyl chloride (PVC) solid polymer electrolyte films. IRACST-Eng Sci Technol Int J (ESTIJ) 2:908–912
Jayakrishnan P, Ramesan M (2017) Studies on the effect of magnetite nanoparticles on magnetic, mechanical, thermal, temperature dependent electrical resistivity and DC conductivity modeling of poly (vinyl alcohol-co-acrylic acid)/Fe3O4 nanocomposites. Mater Chem Phys 186:513–522
Samsudin AS, Khairul WM, Isa MIN (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non-Cryst Solids 358:1104–1112
Ahmad Z, Isa MIN (2012) Ionics conduction via correlated barrier hoping mechanism in Cmc-Sa solid biopolymer electrolytes. Int J Latest Res Sci Technol 1:70–75
Fuzlin AF, Rasali NMJ, Samsudin AS (2018) Effect on ammonium bromide in dielectric behavior based alginate solid biopolymer electrolytes. IOP Conf Ser Mater Sci and Eng 342:012080
Buraidah MH, Teo LP, Majid SR, Arof AK (2009) Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Physica B Condens Matter 404:1373–1379
Mohamed NS, Arof AK (2004) Investigation of electrical and electrochemical properties of PVDF-based polymer electrolytes. J Power Sources 132:229–234
Ramlli MA, Isa MIN (2016) Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on carboxymethyl cellulose doped with ammonium fluoride. J Phys Chem B 120:11567–11573
Chai MN, Isa MIN (2016) Novel proton conducting solid bio-polymer electrolytes based on carboxymethyl cellulose doped with oleic acid and plasticized with glycerol. Sci Rep 6:27328
Samsudin AS, Kuan ECH, Isa MIN (2011) Investigation of the potential of proton-conducting biopolymer electrolytes based methyl cellulose-glycolic acid. Int J Polym Anal Charact 16:477–485
Majid SR, Arof AK (2007) Electrical behavior of proton-conducting chitosan-phosphoric acid-based electrolytes. Phys B Condens Matter 390:209–215
Arof AK, Amirudin S, Yusof SZ, Noor IM (2014) A method based on impedance spectroscopy to determine transport properties of polymer electrolytes. Phys Chem Chem Phys 16:1856–1867
Zainuddin NK, Samsudin AS (2018) Investigation on the effect of NH4Br at transport properties in K–carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater Today Commun 14:199–209
Acknowledgments
The authors would like to thank MOHE for FRGS (RDU170115), Faculty of Industrial Sciences and Technology, Universiti Malaysia Pahang, for the help and support given for the completion of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazuki, N.F., Fuzlin, A.F., Saadiah, M.A. et al. An investigation on the abnormal trend of the conductivity properties of CMC/PVA-doped NH4Cl-based solid biopolymer electrolyte system. Ionics 25, 2657–2667 (2019). https://doi.org/10.1007/s11581-018-2734-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2734-9









