Abstract
A novel silicon nanowire film anode was successfully prepared by a combination of magnetron sputtering deposition and metal-catalyzed electroless etching technology. Scanning electron microscopy revealed the formation of a Si film composed of nanowires with a diameter of ~70 nm and lengths of ~3.5 μm. As-prepared Si nanowire film is directly grown on current collectors without binders and carbon additives, which provides a good contact and adhesion of them to current collector. Furthermore, the defined spacing of nanoscale Si nanowire allows Si to undergo large volume change during the alloying/dealloying process without loss of its integrity. These structural features of the resulting Si nanowire make it a promising anode for lithium-ion batteries with remarkably improved electrochemical performance compared with the Si film-based electrode prepared without metal-catalyzed electroless etching process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicon is an attractive anode material for lithium-ion batteries due to its high theoretical capacity of 4200 mAh g−1. Furthermore, silicon has other advantages such as low cost, abundance in nature, and excellent environmental friendliness [1, 2]. Despite these advantages, silicon anode still faces serious problems, preventing its application, related to its tremendous volume changes (400%) during the alloying/dealloying of Li with Si, resulting in mechanical disintegration of the electrode, and thus rapid capacity fading upon cycling [3].
In order to address the problem of the large volume change of Li-Si alloy and its mechanical rapture, tremendous efforts have been devoted to design of various Si nanostructures, such as Si nanowires, hollow Si nanostructure, and Si nanoparticles [4,5,6,7,8,9,10,11,12,13]. In most of these studies, the electrodes were prepared by preparing a slurry of active material mixed with a polymer binder and a conducting additive, which was further coated onto a metallic current collector [14, 15]. However, non-conductive and electrochemically inactive binder materials decrease the electrode conductivity, and reduce its volumetric and gravimetric energy density [16].
The binder-free film Si anodes were considered to avoid such disadvantages, featuring a good adhesion upon deposition, providing short ion diffusion passes and high conductivity, and prepared without use of binders and conductive agents [6, 17, 18]. Among all preparation techniques, the magnetron sputtering has been proven to be a simple and an efficient one to produce high-quality thin Si films [19,20,21]. Takamura et al. [22] demonstrated that an amorphous silicon thin film with a thickness of 50 nm, prepared by this method, exhibited a stable capacity as high as 3600 mAh g−1 upon cycling for 200 cycles. However, the stability rapidly deteriorated when the thicker film is used, for example, Si film with thickness of 0.5-1 μm could be cycles 200 cycles, but only 50 cycles could be done for the 1.8 μm films. This performance deterioration for the thicker Si films (>1 μm) is attributed to the increased length of the Li diffusion paths, higher electrical resistance, and larger internal stress of Li insertion/extraction [23,24,25,26].
Recently, metal-catalyzed electroless etching (MCEE) has been introduced to prepare silicon nanowires as a simple and a low-cost fabrication technique with mild conditions and wafer-scale production. In general, MCEE is carried out using polished silicon wafers with various crystal orientations and resistivity [27,28,29]. In contrast, preparation of porous silicon by metal-catalyzed electroless etching of amorphous silicon film, obtained by magnetron sputtering deposition, is seldom reported.
In this work, we combine magnetron sputtering deposition with metal-catalyzed electroless etching technology to produce silicon nanowire film, which can be used as an anode material for LIBs. As-prepared Si film has a large thickness of 3.5 μm and exhibited a high initial capacity above 3000 mAh g−1 with appealing cycling stability (90% capacity was retained after 100 cycles).
Experimental
The fabrication process of Si nanowire film is schematically presented in Fig. 1. A uniform silicon film was deposited on a Cu current collector (substrate) using magnetron sputtering (Beijing Logon Sci. & Tech. Co., Ltd) with a 5N-pure silicon target material. The base pressure in the sputtering chamber was approximately 5 × 10−3 Pa and was maintained at approximately 0.45 Pa during deposition. A dc voltage was applied to induce the silicon deposition. This process was conducted for 5 h and 3.5 μm thick silicon film was deposited on the collector. The film thickness and weight were measured by a surface profiler and an electric microbalance (XP6U, Mettler-Toledo Inc.), respectively.
MCEE technique was used to fabricate silicon film. Before etching, the collector was sealed by anti-acid tape and then cleaned with deionized (DI) water, acetone, and ethanol, respectively to remove the impurities, then rinsed in DI water for several times. The cleaned silicon film was immediately immersed into HF/AgNO3 solution (0.01 M AgNO3, 1 M HF) in a sealed reactor and silicon film was obtained after 5 min. As-prepared silicon film was immersed in diluted nitric acid (10%) for 30 min to remove residual silver particles. Further, the silicon film was treated with buffered 0.036 M HF solution and rinsed with DI water for several times. After drying, the silicon film on Cu current collector was cut into 1 cm−2 disk electrodes and transferred into vacuum oven and dried at 105 °C. The weight of Si nanowire in the electrode is 0.9 mg cm−2.
The samples morphology was investigated using scanning electron microscopy (FE-SEM, Leo-1530, Zeiss), and their interior structure was examined by means of transmission electron microscopy (TEM, JEM-2100F, JEOL).
The electrochemical performance of Si nanowire film electrode was investigated using coin-type cells (CR2032). The cell was composed of lithium metal counter electrode and Si film working electrode separated by a microporous polypropylene separator soaked in 1 mol L−1 solution of LiPF6 in 1:1 (v/v) ethylene carbonate/diethyl carbonate (EC/DEC) electrolyte. The coin cells were assembled in an MBraun glove box filled with high purity argon (99.9995% purity). The cells were tested galvanostatically on a multichannel battery tester (BT-2000, Arbin Instruments) between 0.01 and 2 V vs. Li+/Li electrode. Applied currents and specific capacities were calculated on the basis of the weight of silicon in the electrode.
Results and discussion
Figure 2a shows the surface SEM image of the pristine Si film on the copper foil. The surface of the film was quite rough with grain sizes ranging between 1 and 5 μm. Metal-catalyzed electroless etching technique remarkably changes the morphology and structure of the pristine Si film. One can see from Fig. 2b and c that after sliver was deposited onto the Si surface via metal-catalyzed electroless etching reaction in a HF/AgNO3 solution, the Si nanowire was successfully synthesized on the surface of Cu substrate. It can be also seen that the etching direction was almost perpendicular to the general surface of Cu foil. From a cross-section SEM (Fig. 2c) and HRTEM (Fig. 2d) of the as-prepared film, Si nanowires with an average length of 3.5 μm and an average diameter of ~70 nm could be observed. Amorphous Si nanowire was studied by SAED, and its pattern is presented in the inset of Fig. 2d. This nanowire structure could provide buffering for the Si expansion and favor good stability of the electrode materials, which was confirmed in the following battery tests.
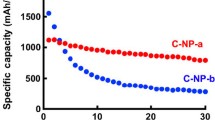
The electrochemical performance of pristine Si film and Si nanowire film electrode in galvanostatic charge/discharge cycle tests is shown in Fig. 3a. At a current density of 200 mA g−1, the capacity degradation is almost negligible in the Si nanowire electrode, which maintains a reversible capacity of 2840 mAh g−1 after 100 cycles, demonstrating a good capacity retention. In contrast, discharge capacity of pristine Si film electrode drops to 1695 mAh g−1 by the 50th cycle, which is only about a half of that nanowire counterpart. Furthermore, Fig. 3a data demonstrate a higher coulombic efficiency for the Si nanowire film, above 99% (except the 1st cycle). We believe that this significant cyclability improvement mainly stems from the nanowire structure of the electrode, which could provide buffering voids to accommodate the Si expansion upon cycling.
Figure 3b shows the 1st, 10th, and 100th galvanostatic discharge/charge curves of the Si nanowire film electrode cycled at a current density of 200 mA g−1. A flat lithiation plateau is observed at the initial cycle, yielding a high discharge capacity of 3158 mAh g−1. A reversible capacity of 3008 and 2840 mAh g−1 were obtained at the 10th and 100th cycles, respectively. During these cycles, cathodic plateau around 0.2 V could be observed, which is consistent with the lithiation process of amorphous Si. Meanwhile, an anodic plateau around 0.4 V can be observed during the delithiation process, which is related to the phase transition from LixSi phase to the amorphous Si. Furthermore, the potential profiles almost overlap with each other, suggesting the completion of the activation stage/process and reaching the electrochemical stability in operation of the Si nanowire electrode [30].
The rate capability tests, as depicted in Fig. 3c, reveal an excellent performance stability of the Si nanowire film at various current densities. After the initial activation cycle, the Si nanowire delivers a high reversible discharge capacity of 2830 mAh g−1 at a current density of 250 mA g−1. Although, there is a gradual capacity reduction with the current density increase, a reversible capacity of 1523 mAh g−1 could be sustained even at a current density of 3200 mA g−1. More importantly, after the current density was returned to 250 mA g−1, a capacity around 2790 mAh g−1 could still be restored. This superb rate performance can be attributed to the nanowire structure of the Si electrode prepared in this work, facilitating the infiltration and circulation of the electrolyte in its bulk, thus favoring a rapid ion transport.
The structural evolution of silicon films upon charge-discharge cycling are illustrated in Fig. 4, where the left panel image is for the Si-Cu electrode, fabricated by magnetron sputtering deposition on the Cu foil current collector, while the right panel represents the Si nanowire film on the Cu foil prepared through metal-catalyzed electroless etching. The pristine Si film typically has a rough surface, while, after the metal-catalyzed electroless etching process, the highly developed nanowire structure is formed. During charge-discharge cycling, the pristine Si film undergoes a significant volume expansion and pulverizes into discontinuous particles and only small amounts of silicon stay on the current collector after cycling. As for the Si nanowire film, the hierarchical nanowire structure provides a sufficient accommodation for the volume change of silicon during the lithiation/delithiation processes, and Si stays tightly connected to the Cu foil current collector, which results in the high capacity and excellent cyclability.
Conclusions
In summary, the Si nanowire film anode was prepared by a combination of magnetron sputtering deposition with metal-catalyzed electroless etching (MCEE). As obtained nanowire structure due to its unique nanowire structure provides a sufficient accommodation for the volumetric expansion and shrinkage of silicon film upon cycling, which allows for achieving the high capacity and excellent cyclability. The prepared anode delivers the initial reversible discharge capacity of 3158 mAh g−1 and maintains a capacity of 2840 mAh g−1 after 100 cycles when cycled at a current density of 200 mA g−1, and 1523 mAh g−1 at a current density of 3200 mA g−1.
References
Zhang YG, Li Y, Li HP et al (2016) Electrochemical performance of carbon-encapsulated Fe3O4 nanoparticles in lithium-ion batteries: morphology and particle size effects. Electrochim Acta 216:475–483
Li M, Li J, Li K et al (2013) SiO2/cu/polyacrylonitrile-C composite as anode material in lithium ion batteries. J Power Sources 240:659–666
Ma H, Cheng F, Chen J et al (2007) Nest-like silicon nanospheres for high-capacity lithium storage. Adv Mater 19:4067–4070
Park MH, Kim MG, Joo J et al (2009) Silicon nanotube battery anodes. Nano Lett 9:3844–3847
Cui L, Yang Y, Hsu CM et al (2009) Carbon-silicon core-shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett 9:3370–3374
Rong J, Masarapu C, Ni J et al (2010) Tandem structure of porous silicon film on single-walled carbon nanotube macrofilms for lithium-ion battery applications. ACS Nano 4:4683–4690
Tang YY, Xia XH, Yu YX et al (2013) Cobalt nanomountain array supported silicon film anode for high-performance lithium ion batteries. Electrochim Acta 88:664–670
Wang JP, Wang CY, Zhu YM et al (2015) Electrochemical stability of optimized Si/C composites anode for lithium-ion batteries. Ionics 21:579–585
Zhang YQ, Xia XH, Wang XL et al (2012) Silicon/graphene-sheet hybrid film as anode for lithium ion batteries. Electrochem Commun 23:17–20
Tao HC, Yang XL, Zhang LL et al (2015) Reduced graphene oxide/porous Si composite as anode for high-performance lithium ion batteries. Ionics 21:617–622
Tang H, Zhang J, Zhang YJ et al (2015) Porous reduced graphene oxide sheet wrapped silicon composite fabricated by steam etching for lithium-ion battery application. J Power Sources 286:431–437
Yong YQ, Fan LZ (2013) Silicon/carbon nanocomposites used as anode materials for lithium-ion batteries. Ionics 19:1545–1549
Kim H, Han B, Choo J et al (2008) Three-dimensional porous silicon particles for use in high-performance lithium secondary batteries. Angew Chem 120:10305–10308
Bo L, Liu Y, Xu Y (2014) Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J Power Sources 267:469–490
Zhang Y, Bakenov Z, Zhao Y et al (2013) Three-dimensional carbon fiber as current collector for lithium/sulfur batteries. Ionics 20:803–808
Yue H, Wang S, Yang Z et al (2015) Ultra-thick porous films of graphene-encapsulated silicon nanoparticles as flexible anodes for lithium ion batteries. Electrochim Acta 174:688–695
Zhao Y, Yin F, Zhang Y et al (2015) A free-standing sulfur/nitrogen doped carbon nanotube electrode for high performance lithium/sulfur batteries. Nanoscale Res Lett 10:450
Zhang Y, Wei Y, Li H et al (2016) Simple fabrication of free-standing ZnO/graphene/carbon nanotube composite anode for lithium-ion batteries. Mater Lett 184:235–238
Vlček J, Kormunda M, ČiŽek J et al (2003) Reactive magnetron sputtering of Si–C–N films with controlled mechanical and optical properties. Diam Relat Mater 12:1287–1294
Yuan Z, Yang J, Wang X et al (2011) Characterization and properties of quaternary Mo-Si-C-N coatings synthesized by magnetron sputtering technique. Surf Coat Technol 205:3307–3312
Hwang CM, Park JW (2011) Electrochemical characterizations of multi-layer and composite silicon-germanium anodes for Li-ion batteries using magnetron sputtering. J Power Sources 196:6772–6780
Takamura T, Ohara S, Uehara M et al (2004) A vacuum deposited Si film having a Li extraction capacity over 2000 mAh/g with a long cycle life. J Power Sources 129:96–100
Takamura T, Uehara M, Suzuki J et al (2006) High capacity and long cycle life silicon anode for Li-ion battery. J Power Sources 158:1401–1404
Ohara S, Suzuki J, Sekine K et al (2004) A thin film silicon anode for Li-ion batteries having a very large specific capacity and long cycle life. J Power Sources 136:303–306
Yoshio M, Tsumura T, Dimov N (2005) Electrochemical behaviors of silicon based anode material. J Power Sources 146:10–14
Song S, Striebel KA, Reade RP et al (2003) Electrochemical studies of nanoncrystalline Mg2Si thin film electrodes prepared by pulsed laser deposition. J Electrochem Soc 150:A121–A127
Bang B, Kim H, Song H et al (2011) Scalable approach to multi-dimensional bulk Si anodes via metal-assisted chemical etching. Energy Environ Sci 4:5013–5019
Peng KQ, Jie JS, Zhang WJ, Lee ST (2008) Silicon nanowires for rechargeable lithium-ion battery anodes. Appl Phys Lett 93:033105
Peng KQ, Wang X, Li L, Hu Y, Lee ST (2013) Silicon nanowires for advanced energy conversion and storage. Nano Today 8:75–97
Li HP, Wei Y, Zhang YG et al (2016) In situ sol-gel synthesis of ultrafine ZnO nanocrystals anchored on graphene as anode material for lithium-ion batteries. Ceram Int 42:12371–12377
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21406052, 51602111), the Program for the Outstanding Young Talents of Hebei Province (Grant No. BJ2014010), Ministry of Human Resources and Social Security of China (Grant No. CG2015003002), Guangdong Province Grant (No. 2014A030308013, 2014B090915005, 2015A030310196, 2015B050501010, 14KJ13), the Pearl River S&T Nova Program of Guangzhou (Grant No. 201506040045), Guangdong Innovative Research Team Program (No. 2011D039), PCSIRT Project No. IRT13064. ZB acknowledges a support from the targeted program №0115РК03029 from the Ministry of Education and Science of the Republic of Kazakhstan and assistance from Ms. Nurzhanat Sherimova from Pavlodar State University, Kazakhstan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 324 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Huang, L., Zhang, Y. et al. Novel silicon nanowire film on copper foil as high performance anode for lithium-ion batteries. Ionics 24, 373–378 (2018). https://doi.org/10.1007/s11581-017-2219-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2219-2