Abstract
All-solid-state lithium batteries using flexible solid electrolytes instead of combustible organic liquid electrolytes are the ultimate solution to address the safety problem of commercialized lithium ion batteries. In this study, a free-standing and thermostable polymer/plastic crystal composite electrolyte (PPCE) based on polymerized trimethylolpropane trimethacrylate (TMPTMA)-1, 6-hexanediol diacrylate (HDDA) matrix, and plastic crystal electrolyte was prepared for all-solid-state lithium batteries. The polymerized TMPTMA-HDDA-based matrix of a porous network structure coupled with plastic crystal electrolyte (PCE) in the pores reveals good compatibility. The as-synthesized PPCE possesses excellent flexible performance, thermostability, and high conductivity, showing that PPCE can reach 8.53 × 10−4 S cm−1 with 7.5 wt% monomers (PPCE-7.5%) at 25 °C under a stability electrochemical window above 5.2 V. The assembled lithium batteries Li|PPCE|LiFePO4 exhibit high capacity and highly cycling stability at room temperature, indicating great potential of all-solid-state lithium batteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, lithium ion batteries (LIBs) have attracted great attention as efficient power sources in smart phones, tablets, personal computers, digital cameras, and other portable devices due to the high energy density and long cycle life [1,2,3]. However, safety problems that initiate from the leakage and flammability of organic solvents are still the challenges and hinder the better development of LIBs [4, 5]. Thus, solid electrolytes have received increasing attention for its advantages in shape versatility and high safety [6]. Among various solid-state electrolytes, polymer electrolytes that consist of salt mixtures and solvating polymers have been extensively studied because of their excellent characteristics such as low toxicity, stability, and flexibility [7]. However, the ionic conductivity of polymer electrolytes is generally less than 10−6 S cm−1 due to the limited chain mobility [8]. The plastic crystal electrolytes composed of lithium salts and plastic crystals with solvation capability are particularly attractive because of their unusual thermal stability and ionic transport behavior [9,10,11,12]. Succinonitrile (N ≡ C-CH2-CH2-C ≡ N, abbreviated as SN) is a non-ionic and highly polar plastic crystalline organic molecule, which has a plastic crystalline phase that approximately extends from −40 to 60 °C (melting point) [13, 14]. Owing to the presence of trans-gauche isomerism about C-C bonds in the SN, SN/lithium salt electrolytes provide a high ionic conductivity more than 10−3 S cm−1 at room temperature since the trans-isomer decreases the activation energy for ionic conduction [14]. Although SN-based plastic crystal electrolytes are advantageous in delivering high ionic conductivity, the mechanical properties still to be enhanced. Combination of the SN-based plastic crystal electrolytes with a polymer matrix is considered to be an effective way to accomplish high mechanical properties. The mechanical properties of the plastic crystal electrolytes could be remarkably enhanced via adding other polymers with high mechanical strength into SN-based electrolytes, such as polyethylene oxide (PEO) [15,16,17,18], polyacrylonitrile (PAN) [19, 20], poly(vinylidenefluoride-co-hexafluopropylene) (P(VDF-HFP) [15, 21], and other polymers [22,23,24,25,26]. Unfortunately, enhanced mechanical properties would largely suppress the ionic conductivity of the plastic crystal electrolytes. Therefore, it is an urgent problem to improve both the mechanical properties and ionic conductivity of the plastic crystal electrolytes.
In this work, a free-standing and thermostable polymer/plastic crystal composite electrolyte (PPCE) membrane that consists of polymer matrix and plastic crystal electrolyte (PCE, lithium bis-trifluoromethanesulphonimide (LiTFSI) in SN) was prepared for all-solid-state lithium batteries. The polymer matrix is formed by the addition of trimethylolpropane trimethacrylate (TMPTMA) and 1,6-hexanediol diacrylate (HDDA). The results suggest an effective way to enhance the flexible performance of the PCE and meanwhile maintain the high ionic conductivity. According to the understanding of structural features, the membrane performance of PPCE (as a novel solid-state electrolyte) was investigated based on mechanical bendability, dimensional thermostability, ionic conductivity, and electrochemical stability. Finally, the electrochemical properties of the cell assembled with PPCE were examined, indicating that great potential for developing novel stable all-solid-state lithium batteries.
Experimental
The synthesis of PPCE membranes
PPCE membranes were synthesized by thermal polymerization. LiTFSI (Li (CF3SO2)2N, Sigma-Aldrich) and succinonitrile (Sigma-Aldrich) were mixed with a molar ratio of 5:95 and melted at 70 °C to get a plastic crystal electrolyte (PCE). For the preparation of the PPCE, two monomers, TMPTMA (C18H26O6) and HDDA (C12H18O4), were mixed in a weight ratio of 1:2, and then, the mixtures were blended with PCE and thermal initiator 2,2′-Azobisisoheptonitrile (ABVN, C14H24N4). The weight composition of (TMPTMA-HDDA)/PCE were 5/95, 7.5/92.5, and 10/90 (abbreviated as PPCE-5%, PPCE-7.5%, and PPCE-10%), respectively. The concentration of the ABVN was fixed at 1 wt% of the monomers. The mixture solution was cast onto a Teflon plate and then placed in an oven under 70 °C for 30 min. Finally, a free-standing, flexible polymer/plastic crystal electrolyte membrane (180 ± 10 μm) incorporated with crosslinked polymer networks was obtained. All of the above procedures were carried out in an Ar-filled glove box.
Physical characterization
The surface morphology of the PPCE membranes was observed by a field-emission scanning electronmicroscopy (FE-SEM, JSM-6330). In order to observe the unique plastic crystal behavior, the PPCE was evaluated by differential scanning calorimetry (DSC, TA 2920 modulated instrument) measurements to obtain the thermal characteristics including T pc (plastic crystal phase transition temperature) and T m (melting temperature) at a heating rate of 10 °C min−1.
Electrochemical measurement
The ionic conductivities of the PPCE membranes were measured by the electrochemical impedance spectroscopy (EIS) method with a CHI660C Electrochemical Workstation (Shanghai Chenhua). The EIS measured in the frequency range from 100 kHz to 0.1 Hz at temperatures between −20 and 80 °C with the potential amplitude of 5 mV. A piece of PPCE membrane was stuck in the middle of two stainless steel blocking electrodes to form Swagelok-type cells and then kept the cells at each test temperature for at least half an hour to reach the thermal equilibrium before EIS measurements. The electrochemical stability window of the PPCE was evaluated by linear sweep voltammetry in the potential range from 2.0 to 6.0 V (vs. Li + /Li) at a scan rate of 1.0 mV s−1 with a stainless steel blocking electrode as the working electrode and lithium-metal as the counter and reference electrode [27]. The transference numbers of lithium ion (T Li+ ) were measured by chronoamperometry on the CHI660C Electrochemical Workstation at room temperature in Swagelok-type cells using lithium-metal as both electrodes with a step potential of 10 mV.
The electrochemical characterization was evaluated by galvanostatic charge/discharge method with a unit cell (CR2032 coin-type cell) which was assembled by sandwiching the electrolyte membrane between a PCE-soaked LiFePO4 cathode and a lithium foil [7]. The sample of PPCE-7.5% was selected as the testing electrolyte membrane. The LiFePO4 cathode was prepared by casting a mixture of active materials (80 wt% LiFePO4), Super-P (15 wt%), and polyvinylidene fluoride (PVDF, 5 wt%) on an aluminum foil current collector followed by drying at 70 °C for 12 h. For comparison, conventional 2032 coin-type cells were assembled with polypropylene microporous film (Celgard 2400) as separators and 1 M LiPF6/ethylene carbonate (EC): dimethylcarbonate (DMC): ethylmethylcarbonate (EMC) (1:1:1, in volume ratio) as the electrolytes. Galvanostatic charged/discharged was tested under 0.2 C between 2.0 and 4.2 V using a cell test instrument (LAND CT2001A, Wuhan Jinnuo Electronics, Ltd.).
Results and discussion
The chemical structure and physical morphology of the major components are shown in Fig. 1. As depicted in Fig. 1a, TMPTMA is a polymer monomer with three functional groups while HDDA has a long-chain molecular structure. Through free radical polymerization, the long linear hydrocarbon chains attached to the crosslinkable acrylate groups and, thereby, the polymerized TMPTMA-HDDA would act as a compliant and flexible framework. As a result, the unique TMPTMA-HDDA polymeric network is anticipated to present a beneficial feature on the bendability of the PPCE membrane. In Fig. 1b, d, e, f, electrolyte membranes are generated by thermal polymerization reaction with the precursor of 7.5 wt% monomer mixtures and 92.5 wt% plastic crystal electrolytes (PPCE-7.5%). It is observed that the three-dimension interconnected polymeric electrolyte matrix is formed in the PPCE (Fig. 1b, c). This unique structure would not only provide good mechanical strength but also ensure the high conductivity for the PPCE [28]. Figure 1d, e shows that the PPCE membranes are free-standing even at a low concentration of polymers (PCE/TMPTMA-HDDA polymers = 92.5/7.5 w/w) by the incorporation of the TMPTMA-HDDA polymers. It is noted that the electrolyte membranes can be bent more than 180° without breaking (Fig. 1f), indicating an effective way to introduce the TMPTMA-HDDA polymers as a skeleton to improve the deformability of PPCE. The free-standing PPCE membrane with excellent flexibility can be directly assembled into cells without using commercial separators.
The thermal stability of membranes was one of the most important factors for battery safety. To attain in-depth understanding of the influence of separators on internal short-circuit failure of batteries, the Celgard 2400 separator and PPCE-7.5% membrane were stored in an oven for 0.5 h in air at 130 °C. Figure 2a, b showed the photographs of membranes before and after thermal shrinkage test at different temperatures. The area-based dimensional shrinkage (ΔA) of the PPCE-7.5% was found to be negligibly small, as compared to the Celgard 2400 separator (ΔA∼40%). This poor thermal stability of Celgard separators is considered as a major cause of the internal short-circuit failures. Therefore, from the viewpoint of the dimensional thermostability, it could be expected that the PPCE membrane would provide the excellent safety characteristic for high-power battery even at elevated temperature.
The plastic crystal behavior of SN, PCE, and PPCE-x% (x = 5, 7.5, 10) were investigated by measuring the characteristic transition temperatures T pc (the transition temperature from crystal to plastic crystal phase) and T m (the melting peak temperature) of SN in the heating. Figure 2c shows that the pristine SN has two endothermic peaks at −36 and 58 °C, which correspond to the T pc and T m , respectively. It shows an obvious reduction in T m (58 °C → 35 °C) with addition of LiTFSI in the SN, implying an isomer transition from gauche to trans [14]. All other composites (PPCE-5%, PPCE-7.5%, and PPCE-10%) exhibit the characteristic transition temperature of the PCE, indicating that the behavior of the plastic crystal is not impacted with the presence of a polymerized TMPTMA-HDDA network. It is also noticed that the addition of polymer slightly improved the T m of the PCE. This result demonstrates that PPCE can improve the safety performance of the battery and could maintain a solid state in high temperature operation.
The temperature-dependent ionic conductivity of the PCE and PPCE-x% (x = 5, 7.5, 10) is examined as shown in Fig. 3a. PCE displays remarkably high conductivity of 2.63 × 10−3 S cm−1 at room temperature and 5.69 × 10−5 S cm−1 at −20 °C. A slight decrease in ionic conductivity was observed when adding TMPTMA-HDDA polymers, mainly because the polymerized TMPTMA-HDDA is not ionic conductive but a framework for hosting PCE. In addition, the conductivity decreases with the increase of the polymer content. In the experiment, the sample of PPCE-7.5% possesses excellent free-standing and bending performance, and the ionic conductivity of the electrolytes can reach a high level of 8.53 × 10−4 S cm−1 at 25 °C. Therefore, PPCE-7.5% was used to test the lithium ion transference number and charge/discharge properties in the following experiments.
a Temperature-dependent ionic conductivities of PCE, PPCE-5%, PPCE-7.5%, and PPCE-10%. b Linear sweep voltammograms of PCE, PPCE-5%, PPCE-7.5%, and PPCE-10% on a stainless-steel working electrode and lithium metal as a counter and reference electrode. Li ion transference number of PCE (c) and PPCE-7.5% (d) at room temperature in Swagelok-type cells using Li metal as both electrodes with step potential of 10 mV
The electrochemical stability window of the PCE and PPCE-x% (x = 5, 7.5, 10) was evaluated from linear sweep voltammograms. As shown in Fig. 3b, there is no meaningful component decomposition below 5.2 V vs. Li+/Li. A considerable current appeared when the voltage exceeded 5.2 V, indicating the beginning of the decomposition in the PCE. In addition, the PPCE-x% (x = 5, 7.5, 10) shows a slight improvement in the anodic stability compared to PCE. The electrochemical working window of the PPCE-5% is higher than 5.5 V as no irreversible oxidation was observed below 5.5 V. The enhanced electrochemical stability is attributed to the improved interfacial characteristics of the PPCE, which indicates that the polymers of TMPTMA-HDDA play a positive role in the electrochemical stability and are well compatible with the electrode materials. In a word, PPCE could be applied to high-voltage LIBs due to their high anodic stability.
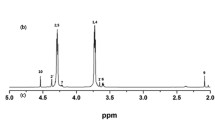
The transference numbers of lithium ions (T Li+ ) were measured for PCE and PPCE-7.5% membranes in the Swagelok-type cells (Fig. 3c, d). T Li+ were estimated by chronoamperometry by comparing the initial and final resistance values and current values with the Eq. (1) [29],
where I 0 and I SS are the currents at the initial state and steady state, respectively; R 0 and R SS represent the initial and steady-state values of Li/electrolyte interfacial resistances. The dc polarization was subsequently carried out in the measurement with a step potential (ΔV) of 10 mV. According to Eq. (1), the transference numbers of lithium ions are 0.55 and 0.57 for PCE and PPCE-7.5%, respectively. Compared with the T Li+ of PCE, there is no significant change for PPCE-7.5%. It is suggested that the addition of the polymers has no negative effect on the transference numbers of lithium ions. It is also proved that the polymerized TMPTMA-HDDA forms a three-dimensional network channel that improves the lithium ion mobility.
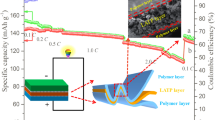
To explore the PPCE as a solid electrolyte for LIBs, the charge/discharge performance and cyclic ability of the assembled Li|PPCE-7.5%|LiFePO4 cells was evaluated at 0.2 C in a voltage range of 2.0–4.2 V. The LFP cathode was soaked in the PCE containing succinonitrile. The battery of LiFePO4|1M LiPF6/(EC + EMC + DMC, 1:1:1 in volume) |Li) was also assembled for comparison. As shown in Fig. 4a, the initial discharge capacity of PPCE-7.5% is 145.5 mAh g−1, slightly lower than that of the liquid electrolyte (160.2 mAh g−1). It is mainly because of the slightly suppressed ion transport in the solid electrolyte. Besides, there are no substantial decrease in the capacity for both of the commercial liquid electrolyte (163.3 mAh g−1) and PPCE-7.5% (143.4 mAh g−1) after 25 cycles [16, 20]. Consistent with the previously reported LiFePO4 cathodes, typical voltage profiles are observed around 3.3–3.5 V in the case of the liquid electrolyte (Fig. 4b). The platform voltage difference of PPCE-7.5% is slightly larger than the liquid electrolyte, indicating the lower degree of polarization in the electrodes during the electrochemical reaction. The voltage platform is also flat in the PPCE-7.5%, similar to the liquid electrolyte of 1M LiPF6/(EC + EMC + DMC, 1:1:1 in volume). This suggests that the redox reaction is stable in the charge/discharge process which attributes to the excellent electrochemical stability of the PPCE. Finally, Fig. 4b inset demonstrates that the charged battery containing the LiFePO4 cathode, the PPCE-7.5% membrane, and the lithium anode could light up the 3.3 V small bulb, suggesting the decent operation of the battery system and the applicability of the PPCE in various battery systems.
Cycle performance (a) and the initial charge/discharge curves (b) at a constant charge/discharge current density (0.2 C/0.2 C) for LiFePO4 | PPCE-7.5% | Li and LiFePO4 | 1M LiPF6/(EC + EMC + DMC, 1:1:1 in volume) | Li cells at 25 °C, inset is the LED bulb lighted by all-solid-state Lithium ion batteries
Conclusion
In summary, a free-standing, thermostable, flexible polymer/plastic crystal electrolyte (PPCE) was fabricated as a promising solid electrolyte for LIBs. PPCE possesses a crosslinked network and the plastic crystal behavior is not impacted during the polymerization process. The electrolyte possesses high ionic conductivity, stable electrochemical window, and excellent component compatibility. In the cell assembled with the LiFePO4 cathode, the battery shows high capacity and high cycling stability at room temperature. These results suggest that this polymer/plastic crystal electrolyte could be a potential candidate for solid-state LIBs.
References
Wen L, Li F, Cheng H-M (2016) Carbon nanotubes and graphene for flexible electrochemical energy storage: from materials to devices. Adv Mater 28:4306–4337
Arico AS, Bruce P, Scrosati B, Tarascon JM, Schalkwijk WV (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Quartarone E, Mustarelli P (2011) Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev 40:2525–2540
Goodenough JB, Kim Y (2009) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Zhang J, Zhao N, Zhang M, Li Y, Chu PK, Guo X, Di Z, Wang X, Li H (2016) Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 28:447–454
Christina Nancy A, Austin Suthanthiraraj S (2016) Preparation and characterization of a new PEO-PEG blend polymer electrolyte system. Ionics 22:2399–2408
Palacin MR (2009) Recent advances in rechargeable battery materials: a chemist’s perspective. Chem Soc Rev 38:2565–2575
Long S, MacFarlane DR, Forsyth M (2003) Fast ion conduction in molecular plastic crystals. Solid State Ionics 161:105–112
MacFarlane DR, Huang J, Forsyth M (1999) Lithium-doped plastic crystal electrolytes exhibiting fast ion conduction for secondary batteries. Nature 402:792–794
Liu K, Ding F, Lu Q, Liu J, Zhang Q, Liu X, Xu Q (2016) A novel plastic crystal composite polymer electrolyte with excellent mechanical bendability and electrochemical performance for flexible lithium-ion batteries. Solid State Ionics 289:1–8
Jin LY, Howlett PC, Pringle JM, Janikowski J, Armand M, MacFarlane DR, Forsyth M (2014) An organic ionic plastic crystal electrolyte for rate capability and stability of ambient temperature lithium batteries. Energy Environ Sci 7:3352–3361
Ha HJ, Kil EH, Kwon YH, Kim JY, Lee CK, Lee SY (2012) UV-curable semi-interpenetrating polymer network-integrated, highly bendable plastic crystal composite electrolytes for shape-conformable all-solid-state lithium ion batteries. Energy Environ Sci 5:6491–6499
Alarco PJ, Abu-Lebdeh Y, Abouimrane A, Armand M (2004) The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat Mater 3:476–481
Fan LZ, Hu YS, Bhattacharyya AJ, Maier J (2007) Succinonitrile as a versatile additive for polymer electrolytes. Adv Funct Mater 17:2800–2807
Lu QW, Fang JH, Yang J, Yan GW, Liu SS, Wang JL (2013) A novel solid composite polymer electrolyte based on poly(ethylene oxide) segmented polysulfone copolymers for rechargeable lithium batteries. J Membr Sci 425:105–112
Li YH, Wu XL, Kim JH, Xin S, Su J, Yan Y, Lee JS, Guo YG (2013) A novel polymer electrolyte with improved high-temperature-tolerance up to 170 °C for high-temperature lithium-ion batteries. J Power Sources 244:234–239
Fan LZ, Maier J (2006) Composite effects in poly (ethylene oxide)-succinonitrile based all-solid electrolytes. Electrochem Commun 8:1753–1756
Abu-Lebdeh Y, Abouimrane A, Alarco PJ, Hammami A, Ionescu-Vasii L, Armand M (2004) Ambient temperature proton conducting plastic crystal electrolytes. Electrochem Commun 6:432–437
Patel M, Bhattacharyya AJ (2011) A crosslinked “polymer-gel” rechargeable lithium-ion battery electrolyte from free radical polymerization using nonionic plastic crystalline electrolyte medium. Energy Environ Sci 4:429–432
Fan LZ, Wang XL, Long F (2009) All-solid-state polymer electrolyte with plastic crystal materials for rechargeable lithium-ion battery. J Power Sources 189:775–778
Ha HJ, Kwon YH, Kim JY, Lee SY (2011) A self-standing, UV-cured polymer networks-reinforced plastic crystal composite electrolyte for a lithium-ion battery. Electrochim Acta 57:40–45
Wang QJ, Fan HH, Fan LZ, Shi Q (2013) Preparation and performance of a non-ionic plastic crystal electrolyte with the addition of polymer for lithium ion batteries. Electrochim Acta 114:720–725
Choi KH, Kim SH, Ha HJ, Lil EH, Lee CK, Lee SB, Shim JK, Lee SY (2013) Compliant polymer network-mediated fabrication of a bendable plastic crystal polymer electrolyte for flexible lithium-ion batteries. J Mater Chem A 1:5224–5231
Ding YH, Zhang P, Long ZL, Jiang Y, Xua F, Di W (2009) The ionic conductivity and mechanical property of electrospun P(VdF-HFP)/PMMA membranes for lithium ion batteries. J Membr Sci 329:56–59
Prasanth R, Aravindan V, Srinivasan M (2012) Novel polymer electrolyte based on cob-web electrospun multi component polymer blend of polyacrylonitrile/poly (methyl methacrylate)/polystyrene for lithium ion batteries-preparation and electrochemical characterization. J Power Sources 202:299–307
Michael MS, Prabaharan SRS (2004) Rechargeable lithium battery employing a new ambient temperature hybrid polymer electrolyte based on PVK+PVdF–HFP (copolymer). J Power Sources 136:408–415
Fan HH, Li HX, Fan LZ, Shi Q (2014) Preparation and electrochemical properties of gel polymer electrolytes using triethylene glycol diacetate-2-propenoic acid butyl ester copolymer for high energy density lithium-ion batteries. J Power Sources 249:392–396
Wang XL, Fan LZ, Xu ZH, Lin YH, Nan CW (2008) Temperature dependent ionic transport properties in composite solid polymer electrolytes. Solid State Ionics 179:1310–1313
Acknowledgements
Financial supports from NSF of China (51532002, 51372022, 51575030) and 973 Project (2015CB932500) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Y., Ding, T. & Fan, LZ. A free-standing and thermostable polymer/plastic crystal electrolyte for all-solid-state lithium batteries. Ionics 23, 3339–3345 (2017). https://doi.org/10.1007/s11581-017-2152-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2152-4