Abstract
In the current research, iron oxide nanoparticles were functionalized by acrylic acid polymerization. The Fe3O4/PAA core-shell nanoparticles were utilized for the modification of cation exchange membranes. Ion exchange membranes were prepared by solution casting technique using cation exchange resin powder as functional group agent and tetrahydrofuran as solvent. FTIR analysis proved the formation of PAA on nanoparticles. The SOM images also showed uniform particle distribution for the prepared membrane relatively. The membrane water content was declined from 30 to 17 % by increase of nanoparticle content ratio in membrane matrix. The contact angle measurements showed that membrane surface hydrophilicity was improved by utilizing of nanoparticles in the membrane matrix. The membrane potential, permselectivity, and transport number were improved initially by increase of nanoparticle concentration in the casting solution and then began to decrease by more additive concentration. Membrane ionic flux and permeability were enhanced initially by increase of nanoparticle loading ratio up to 0.5 %wt in membrane matrix and then showed decreasing trend by more increase of nanoparticle concentration from 0.5 to 4 %wt. Membrane areal electrical resistance was decreased sharply by utilization of nanoparticles up to 0.5 %wt in membrane matrix then began to increase by more additive concentration. The prepared membranes exhibited superior selectivity and low ionic flux at neutral condition compared to other acidic and alkaline environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Membrane-based processes are considered as a novel technology in many fields of industry and human life such as food, pharmacy, and downstream processing and water treatment [1–6]. Among them, ion exchange membranes have been used as advanced active separators in electrically driven processes such as electrodialysis for desalting brackish waters, treating industrial effluents containing toxic metals, recovery of valuable metals, desalination of cheese whey solutions, and production of table salt [4, 7–13]. In electrodialysis process, ion interactions with membrane, water, and with each other occur in complex fashions. So, knowledge of the physical and chemical properties of ion exchange membranes is a major contributing factor behind their utilization and efficiency in specific separation processes [10–16].
Preparing cost-effective membranes with specific physical and chemical characteristics such as high ionic permeability and selectivity and low electrical resistance is highly desired [17–19].
A lot of research has already been carried out to improve the physical and chemical properties of ion exchange membranes. Variation of functional group type, selection of different polymeric matrices, polymer blending, using of inorganic additives/filler, alteration of cross-link density, and surface modification are the important techniques to obtain superior ion exchange membranes [17–22].
Utilization of metal oxide nanoparticles into polymeric membranes has been considered extensively in many researches to enhance the mechanical, thermal, and chemical stabilities of membranes in severe conditions and also to modify the separation properties of membranes based on the synergism between the organic–inorganic component properties [23–28].
Among this, iron oxide nanoparticles are considered as cost-effective alternatives for pollutant removal as they have high adsorption capacity, magnetic properties, easy preparation, and low toxicity which make them green and safe options for environmental remediation [29, 30]. Moreover, metal oxide nanoparticles can be modified by the chemicals or be coupled with desired functional groups to obtain superior properties. Reported researches revealed that the potential of metal oxide nanoparticles can be increased considerably if they are covered with organic shells. The organic shells can consist of monomers with functional groups anchoring at the surface of the nanoparticles. Different polymers are utilized as shells for magnetic nanoparticles [31–33].
Regarding to the reported studies, polyacrylic acid can be used as modifier to achieve higher ion uptake onto adsorptive nanoparticles. For the aim, Fe3O4/PAA core-shell nanoparticles were prepared by thermal in situ polymerization technique. The Fe3O4/PAA core-shell nanoparticles were also employed as inorganic filler additive in membrane fabrication in order to improve the membrane physical and electrochemical properties. Currently, no researches considered the utilization of Fe3O4/PAA core-shell nanoparticles in ion exchange membrane, and literature is silent on characteristics and functionality of electrodialysis ion exchange modified by Fe3O4/PAA core-shell nanoparticles. Preparing heterogeneous cation exchange membranes with special adapted physical and chemical properties for the application in electrodialysis processes related to water recovery and water treatment was the primary target of current research.
Mixed matrix polyvinylchloride-co-Fe 3 O 4 /PAA core-shell nanoparticles heterogeneous cation exchange membranes were prepared by solution casting techniques using cation exchange resin powder as functional groups agent. The effect of Fe 3 O 4 /PAA core-shell nanoparticles concentration in the casting solution on membrane properties was studied.
During the experiments, sodium and barium chloride were employed as ionic solutions for membrane characterization. The results are valuable for electromembrane processes especially electrodialysis process for water recovery and water treatment.
Materials and methods
Materials
Polyvinylchloride (PVC, grade S-7054, density 460 g/lit, viscosity number 105 cm3/g) supplied by Bandar Imam Petrochemical Company (BIPC), Iran, was used as membrane matrix. Tetrahydrofuran (THF, molar mass 72.11 g/mol, density 0.89 g/cm3) was employed as solvent. Iron oxide nanoparticle (Fe3O4, nanopowder, MW = 213.53 g/mol, average particle size = 60 nm, SSA > 55 m2/g, purification = 99.2 %, N.R.B. Company, Iran). Cation exchange resin (Ion exchanger Amberlyst® 15, strongly acidic cation exchanger, H+ form more than 1.7 meq/g dry, spec. density 0.6 g/cm3, particle size (0.355–1.18 mm) < 90 %) by Merck Inc., Germany, was used in membrane preparation. Acrylic acid (AA, monomer, Merck Inc.), ethylene glycol (EG, cross-linker, Merck Inc.), and potassium persulfate (KPS, initiator, Merck Inc.) were used in nanoparticle modification. All other chemicals were supplied by Merck. Throughout the experiment, distilled water was used.
Preparation of Fe3O4/PAA core-shell nanoparticles
To prepare the Fe3O4/PAA core-shell nanoparticles (Fig. 1), thermal in situ polymerization of acrylic acid in the aqueous solution was performed using KPS initiator in an air-sealed glass container. As the first step, 20 mg of Fe3O4 nanoparticles was added to 30 ml distillated water containing 2 g of AA monomers, 400 mg EG as cross-linker, and 10 mg KPS as initiator. Then, the mixture was sonicated 30 min for the nanoparticle dispersion. Subsequently, nitrogen gas bubbling was done for 15 min. The glass container was fixed in an oil bath with fixed temperature (90 °C) and stirred vigorously at 400 rpm for 4 h. Finally, the product was washed thoroughly with distillated water and centrifuged several times to remove any nonreacted acrylic acid monomers, unattached PAA, and oligomers. Prepared PAA/Fe3O4 nanoparticles were dried at 50 °C in an oven for 48 h.
Preparation of home-made heterogeneous cation exchange membrane
Heterogeneous cation exchange membranes based on PVC were prepared by solution casting technique. Cation exchange resin powder as functional group agents and tetrahydrofuran as solvent were used in membrane preparation. Steps of preparations are listed as follows:
-
1.
Resin particles were dried in oven (SANEE. V. S. Co.) at 30 ° C for 48 h then pulverized into fine particles in a ball mill (Pulverisette 5, Fritisch Co.) and sieved to the desired mesh size (−300 to +400 mesh).
-
2.
Polymer binder (PVC) was dissolved into solvent (THF/PVC (20:1) (v/w)) in a glass reactor equipped with a mechanical stirrer (model Velp Sientifica Multi 6 stirrer) for more than 4 h.
-
3.
A specific quantity of grinded resin particle (resin/polymer binder (1:1) (w/w)) as functional group agents was dispersed in polymeric solution.
-
4.
Different percentage of PAA/Fe3O4 core-shell nanoparticles (0, 0.5, 1, 2, and 4 % wt.) was added to polymeric solution.
-
5.
To obtain uniform particle distribution in the polymeric solution and break up nanoparticle aggregation, the mixture was mixed vigorously at room temperature for an hour; then, it was sonicated for 30 min using an ultrasonic instrument.
-
6.
After the sonication stage, the mixing process was repeated for another 10 min using the mechanical stirrer.
-
7.
The mixture was casted onto a clean and dry glass plate at 25 °C. The membranes were dried at 25 °C until solvent evaporated and solidification was done totally.
-
8.
Then, polymeric films immersed in distilled water and as the final stage, the membranes were pretreated by immersing in NaCl solution. The composition of casting solution is reported in Table 1.
Table 1 Composition of casting solution used in preparation of mixed matrix cation exchange membranes
Test cell
The electrochemical measurements for the prepared membranes were carried out using the test cell, as reported earlier [14]. The cell consists of two cylindrical compartments (vessel, each 180 cm3) made of Pyrex glass, which are separated by membrane. The membrane was fixed between rubber rings. One side of each vessel was closed by Pt electrode supported with a piece of Teflon, and the other side was equipped with a piece of porous medium to support the membrane. The top of each compartment contained two orifices for feeding and sampling purposes. In order to minimize the effect of boundary layer during experiments and to establish the concentration polarization on the vicinity of membrane’s surface, both sections were stirred vigorously by magnetic stirrers (model Velp Sientifica Multi 6 stirrer).
Nanoparticle characterization
FTIR analysis
FTIR spectra measurements were carried out to provide information about the chemical structure of the unmodified and modified Fe3O4 nanoparticles. FTIR spectra analysis was done using Galaxy series FTIR 5000 spectrometer. Scans were taken between 500 and 4000 cm−1.
Morphological studies
The behavior of prepared membranes is closely related to their structure, especially the spatial distribution of ionic site and particle distribution. The structures of prepared membranes were examined by scanning optical microscopy (SOM Olympus, model IX 70) in transmission mode with light going through the membrane for scanning purposes.
Water content
The water content was measured as the weight difference between the dried and swollen membranes. The wet membrane was weighed (OHAUS, Pioneer™, readability 10−4 g, OHAUS Corp.) and then dried in oven until the constant weight was obtained. The following equation [34] can be used in water content calculations:
Measurements were carried out three times for each sample, and then their average value was reported in order to minimize the experimental errors.
Contact angle measurements
The membrane contact angle measurement was carried out to evaluate the changes in surface hydrophilicity of prepared membranes. Deionized water was used as the probe liquid in all measurements. To minimize the experimental error, the contact angle was measured in five random locations for each sample, and then their average was reported. All experiments were carried out in the ambient conditions.
Membrane potential, transport number, and permselectivity
The membrane potential is algebraic sum of Donnan and diffusion potentials determined by the partition of ions into the pores as well as the mobilities of ions within the membrane phase compared with the external phase [18, 35–37]. This parameter was evaluated for the equilibrated membrane with unequal concentrations of electrolyte solution ((NaCl (0.1 M/0.01 M) or BaCl2 (0.1 M/0.01 M)) at ambient temperature on either sides of membrane using two-cell glassy apparatus. During the experiment, both sections were stirred vigorously to minimize the effect of boundary layers. The developed potential across the membrane was measured by connecting both compartments and using saturated calomel electrode (through KCl bridges) and digital auto multimeter (DEC, model DEC 330FC, Digital Multimeter, China). The measurement was repeated until a constant value was obtained. The membrane potential (EMeasure) is expressed using Nernst equation [38, 39] as follows:
where t i m is the transport number of counter ions in membrane phase, R is the gas constant, T is the temperature, n is the electrovalence of counter-ion, a 1 , a 2 are the solution electrolyte activities in contact membrane surfaces, and F is the faraday constant. The ionic permselectivity of membranes also is quantitatively expressed based on the migration of counter-ion through the IEMs [17, 40–44]:
where t 0 is the transport number of counter-ions in solution [45].
Ionic permeability and flux of ions
Ionic permeability and flux measurements were carried out using the test cell. A 0.1 M (NaCl or BaCl2) solution was placed on one side of the cell and a 0.01 M solution on its other side. A DC electrical potential (Dazheng, DC power supply, model PS-302D) with an optimal constant voltage was applied across the cell with stable platinum electrodes. Cations pass through the membrane to cathodic section. According to anodic and cathodic reactions, the produced hydroxide ions increase the pH of cathodic section.
According to the first Fick’s law, the flux of ions through the membrane can be expressed as follows [11–14, 28]:
where P is the coefficient diffusion of ions, d is the membrane thickness, N is the ionic flux, and C is the cation concentration in the compartments.
where A is the membrane surface area. Integrating Eq. (5) was as follows:
The flux and diffusion coefficient of cations in membrane phase are calculated from pH changes (digital pH-meter, Jenway, model 2710) in cathodic section and Eq. (6), respectively. Also, these parameters can be determined by variations in conductivity as described earlier [11–14].
Electrical resistance
The electrical resistance of equilibrated membrane was measured in NaCl solution with 0.5 M concentration (at 25 °C). Measurement was carried out by an alternating current bridge with 1470-Hz frequency (audio signal generator, Electronic Afzar Azma Co. P.J.S.). The membrane resistance is calculated using the different resistance between the cell (R1) and electrolyte solution (R2) (Rm = R1 − R2) [11–14]. The areal resistance was expressed as follows:
where r is a real resistance and A is the surface area of membrane.
Effect of pH on membrane electrochemical properties
The effect of pH on ionic flux
According to anodic and cathodic reactions, the amount of transported cations through the membrane is equal to the produced hydroxide ions in cathodic section which increase the pH of this region. At higher pH, the Cl2 can react with back diffused hydroxide ions in anodic section to form hypochloric acid or eventually chlorate which can decrease the pH of this compartment and the anodic pH declines by time spontaneously [46]. This pH reached to about 4 after 20 min for prepared membranes. So, to investigate the acidic environment on ionic flux, no adjustment was done. For adjusting pH to neutral (pH = 7) and alkaline (pH = 10), specific amount of NaOH (0.01 M) was added to the ionic solution. The pH measurement was carried out using digital pH-meter, Jenway, model 3510.
The effect of pH on membrane selectivity
The effect of pH on membrane selectivity was investigated at acidic (pH = 4), neutral (pH = 7), and alkaline (pH = 10) conditions. The potential measurements and pH adjustments were followed by the explained process as was mentioned before. During the experiment, both sections were stirred vigorously by magnetic stirrers to minimize the effect of boundary layers on the measurement.
Results and discussion
FTIR analysis
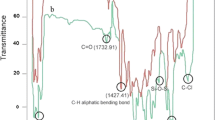
The FTIR analysis of prepared membrane containing Fe3O4 nanoparticles, mixed matrix membrane containing PAA-Fe3O4 composite nanoparticles, and prepared membrane containing PAA are shown in Fig. 2a–c, respectively. The FTIR spectra analysis shows peak at 1740 and 3052 cm−1 which are assigned to PAA grafting on Fe3O4 nanoparticles. These are not visible for bare Fe3O4 nanoparticles. The results approve the PAA polymerization decisively. Moreover, the PAA/Fe3O4 nanoparticles’ SEM image is depicted in Fig. 3. As seen in the image, polymerization was occurred in composite nanoparticles.
Membrane morphological studies
The electrochemical characteristics of prepared membranes are closely related to the spatial distribution of particles in membrane matrix. The membranes SOM images in transmission mode with light going through them are depicted in Fig. 4. The nonconducting area (polymer binder) and conducting areas (resin particles and PAA/Fe3O4 nanoparticles) are clearly seen in the images. As it can be seen, the brightness of images was decreased and dark regions were increased by increase of nanoparticle concentration in the membrane matrix. As it was shown, particles are uniformly distributed in the homemade membranes. It reveals that sonication has a significant effect on distribution of resin particles in prepared membranes and results in formation of more uniform phase. The excessive homogeneity and uniform distribution of particles in the bulk of membrane matrix provide superior conducting regions for the membrane and generate easy flow channels for counter-ion transportation. The presence of more conducting region on the membrane surface can also strengthen the intensity of uniform electrical field around the membrane and decreases the concentration polarization phenomenon [12, 28]. Furthermore, uniform distribution of particle improves the polymer chain relaxation as well as its conformation with particle surfaces and enhances the membrane selectivity. The SOM images also show relatively uniform matrices for the membranes.
Water content
The obtained results (Fig. 5) revealed that membrane water content was decreased from 30.5 to 17 % (±10) by increase of nanoparticle concentration in the membrane matrices. This is in contrast with hydrophilic characteristic of used nanoparticles. By increase of additive concentration, it is possible that free spaces in membrane matrix can be surrounded and occupied by the nanoparticles which result in less water accommodation. The suitable amount of membrane water content can have better control on the pathways of ion traffic and improves the membrane permselectivity. Additionally, high water content can provide more and wider transfer channels for co- and counter-ions transportation and decrease the ion selectivity and leads to a loose structure for the membranes. But, this is not always true and depends on the membrane structure and its other properties. It is worth mentioning that measurements were carried out three times for each sample and then their average value was reported in order to minimize the experimental errors.
Contact angle measurements
The contact angles were measured to evaluate the changes in surface hydrophilicity of the prepared membranes. Results (Table 2, Fig. 6) showed that increase of nanoparticles ratio in the casting solution led to the increase of membrane surface wettability for the prepared membranes. This is attributed to hydrophilic characteristic of nanoparticles on the membrane surface which are hydrated by water molecules and so enhance the membrane surface hydrophilicity.
Membrane potential, transport number, and permselectivity
The obtained results (Figs. 7, 8, and 9) indicated that membrane potential, transport number, and selectivity were increased initially by increase of nanoparticle concentration up to 2 %wt in membrane matrix in both monovalent and bivalent ionic solutions. This may be attributed to the introduction of carboxylic groups throughout the membrane matrix by utilizing of PAA/Fe3O4 composite nanoparticles. The pristine membrane contains sulfonate functional groups only while modified membranes contain sulfonate and carboxylic groups. Actually, the synergism effect between two existing functional groups is responsible for strengthening the Donnan exclusion that results in increment of membrane potential, transport number, and selectivity. Another reason could be the occupation of ionic pathways by the nanoparticles which makes the channels and ionic pathways narrow in membrane matrix. This strengthens the ionic site dominations on ion traffic and improves the membrane potential, transport number, and selectivity.
The membrane potential, transport number, and selectivity were all declined slightly by more increase of nanoparticle loading ratio from 2 to 4 %wt in the casting solution. This may be due to increase of membrane heterogeneity at high additive concentration which makes possible the co-ion percolation through the membrane matrix.
Also, the results showed lower membranes potential, selectivity, and transport number for the membranes in bivalent ionic solution compared to monovalent ones. This different trend might be due to high charge density of bivalent ions which leads to strong bonds with ion exchange functional groups [11, 28]. This phenomenon poisons the membranes and decreases the membrane transport number and selectivity. In fact, bivalent ions have stronger electrostatic attraction with the oppositely fixed charge sites and prevent functional group dissociation. Furthermore, the larger radius of bivalent ions and their hydrated size in comparison with monovalent ones make lower membrane potential, transport number, and permselectivity for them.
Ionic permeability and flux of ions
During the experiment, ions pass through the membrane and reach to the concentrated section. It was found that (Figs. 10 and 11) increase of Fe3O4 /PAA nanoparticle concentration up to 0.5 %wt in the casting solution initially led to increase of ionic flux and permeability for both sodium and barium ions in prepared membranes. This is due to the adsorption characteristic of nanoparticles which increases the cation interaction with membrane surface and so facilitates the ion transportation. Also, the functional group synergism has a significant role to improve ion interaction with membrane surface. Another result might be the hydrophilic nature of PAA/Fe3O4 nanoparticles which enhances the membrane surface hydrophilicity and so improves the ion transportation between solution and membrane phase.
The ionic flux and permeability were decreased again by more increase of additive loading ratio from 0.5 to 4 %wt due to formation of narrow ionic transfer channels in membrane matrix and also lower amount of water content for the prepared membranes at high additive concentration which reduce the ion transportation. Moreover, the occupation of channels and ionic pathways in the membrane matrix by additive particles reduces the accessibility of ion exchange functional groups by their isolation which in turn leads to flux decrease.
The larger radius of barium ions and their hydrated size in comparison with sodium ions reduce the barium mobility through solution and membrane phase.
Electrical resistance
The membrane electrical resistance has practical implications due to its relation with energy consumption in the process. The electrical resistance of prepared membranes (pristine membrane (S1), superior membranes (S2), and S5) was measured in 0.5 M NaCl solution at ambient temperature. Results (Fig. 12) revealed that membrane electrical resistance was decreased by utilizing of 0.5 %wt nanoparticles in the membrane matrix obviously. This may be attributed to the adsorption and hydrophilic characteristic of PAA-Fe3O4 nanoparticles which increase the ion interactions with membrane surface and improves membrane conductivity. The membrane electrical resistance was enhanced again by utilizing of 4 %wt PAA/Fe3O4 nanoparticles in membrane matrix. This might be due to narrower ionic pathways in membrane matrix and also low amount of water content for this membrane which increase the membrane electrical resistance.
The effect of pH on ionic flux
Figure 13 shows the effect of anodic pH on ionic flux. The obtained results exhibited minimum flux for the membranes at neutral environment (pH = 7) compared to alkaline and acidic ones. This can be explained with respect to functional group behavior in different environments. The sulfonate/carboxylic groups have higher dissociation at pH = 7 rather than other pH ranges [18, 47]. This results in more domination of functional groups on ion traffic and makes the counter-ion transportation difficult through the membranes. It is worth mentioning that sulphonic and carboxylic groups in alkaline solutions are more dissociated than acidic ones [18].
The effect of pH on membrane selectivity
The membrane selectivity is highly dependent on the solution nature. The prepared membranes exhibited higher selectivity at pH = 7 compared to other pH values (Fig. 14). This is because of difference in dissociation of membrane functional groups at various pH values which has an important impact on the charge nature of membrane matrix. At neutral environment, more dissociation of functional groups in the membrane matrix strengthens the ionic site domination on ion traffic and improves the membrane selectivity. As carboxylic groups have better performance at pH = 10, membrane selectivity in alkaline solution are higher than the acidic solution [18, 48]. This result indicated the role of carboxylic groups on electrochemical properties of prepared membranes and its synergism with sulphonic groups.
Conclusion
The FTIR spectrum analysis proved the graft polymerization of PAA on Fe3O4 nanoparticles. SOM images showed uniform particle distribution and relatively uniform surface for the membranes. Results revealed that membrane water content was decreased by increase of percentage of nanoparticles in casting solution. Opposite trend was found the membrane surface hydrophilicity. Also, it was found that membrane potential, permselectivity, and transport number were enhanced sharply by increase of additive concentration up to 2 %wt in membrane matrix and then decreased again by more nanoparticle content ratio from 2 to 4 %wt. Membrane ionic flux and permeability were improved initially by increase of nanoparticle concentration in both monovalent and bivalent ionic solutions and then began to decrease. Similar trend was found for the membrane electrical conductivity. Prepared membranes showed lowest ionic flux at pH = 7 compared to other pH values. Also, membranes showed highest selectivity at neutral environment compared to acidic and alkaline conditions markedly.
References
Han G, Zhang S, Li X, Widjojo N, Chung TS (2012) Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem Eng Sci 80:219–231
Nady N, Schroën K, Franssen MCR, Eldin MSM, Zuilhof H, Boom RM (2012) Laccase-catalyzed modification of PES membranes with 4-hydroxybenzoic acid and gallic acid. J Membr Sci 394–395:69–79
Bazinet L, Moalic M (2011) Coupling of porous filtration and ion-exchange membranes in an electrodialysis stackand impact on cation selectivity: a novel approach for sea water demineralization and the production of physiological water. Desalination 277:356–363
Zuo X, Yu S, Xu X, Bao R, Xu J, Qu W (2009) Preparation of organic–inorganic hybrid cation-exchange membranes via blending method and their electrochemical characterization. J Membr Sci 328:23–30
Mc Closkey BD, Park HB, Ju H, Rowe BW, Miller DJ, Freeman BD (2012) A bioinspired fouling-resistant surface modification for water purification Membranes. J Membr Sci 413–414:82–90
Vogel C, Meier-Haack J (2014) Preparation of ion-exchange materials and membranes. Desalination 342:156–174
Nagarale RK, Gohil GS, Shahi VK, Trivedi GS, Rangarajan R (2004) Preparation and electrochemical characterization of cation- and anion-exchange /polyaniline composite membranes. J Colloid Interface Sci 277:162–171
Daraei P, Madaeni SS, Ghaemi N, Salehi E, Khadivi M, Moradian R, Astinchap B (2012) Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J Membr Sci 415–416:250–259
Sivaraman P, Chavan JG, Thakur AP, Hande VR, Samui AB (2007) Electrochemical modification of cation exchange membrane with polyaniline for improvement in permselectivity. Electrochim Acta 52:5046–5052
Hosseini SM, Rahzani B, Asiani H, Khodabakhshi AR, Hamidi AR, Madaeni SS, Moghadassia CAR, Seidypoor A (2014) Surface modification of heterogeneous cation exchange membranes by simultaneous using polymerization of (acrylic acid-co-methyl methacrylate): membrane characterization in desalination process. Desalination 345:13–20
Hosseini SM, Jeddi F, Nemati M, Madaeni SS, Moghadassi AR (2014) Electrodialysis heterogeneous anion exchange membrane modified by PANI/MWCNT composite nanoparticles: preparation, characterization and ionic transport property in desalination. Desalination 341:107–114
Hosseini SM, Madaeni SS, Heidari AR, Khodabakhshi AR (2012) Preparation and characterization of poly (vinyl chloride)-blend-poly (carbonate) heterogeneous cation exchange membrane: investigation of solvent type and ratio effects. Desalination 285:253–262
Hosseinia SM, Madaenia SS, Khodabakhshia AR, Zendehnamb A (2010) Preparation and surface modification of PVC/SBR heterogeneous cation exchange membrane with silver nanoparticles by plasma treatment. J Membr Sci 365:438–446
Hosseini SM, Hamidi AR, Moghadassi AR, Koranian P, Madaeni SS (2015) Fabrication of novel mixed matrix electrodialysis heterogeneous ion exchange membranes modified by ilmenite (FeTiO3): electrochemical and ionic transport characteristics. IONICS 21(2):437–447
Caprarescu S, Purcar V, Vaireanu DI (2012) Separation of copper ions from synthetically prepared electroplating wastewater at different operating conditions using electrodialysis. Sep Sci Technol 47(16):2273–2280
Elattar A, Elmidaoui A, Pismenskaia N, Gavach C, Pourcelly G (1998) Comparison of transport properties of monovalent anions through anion-exchange membranes. J Membr Sci 143:249–261
Hosseini SM, Askari M, Koranian P, Madaeni SS, Moghadassi AR (2014) Fabrication and electrochemical characterization of PVC based electrodialysis heterogeneous ion exchange membranes filled with Fe3O4 nanoparticles. J Ind Eng Chem 20:2510–2520
Nagarale RK, Gohil GS, Shahi VK, Rangarajan R (2004) Preparation and electrochemical characterizations of cation-exchange membranes with different functional groups. Colloid Surface A 251:133–140
Daraei P, Madaeni SS, Ghaemi N, Ahmadi Monfared H, Khadivi M (2013) Fabrication of PES nanofiltration membrane by simultaneous use of multi-walled carbon nanotube and surface graft polymerization method: comparison of MWCNT and PAA modified MWCNT. Sep Purif Technol 104:32–44
Vyas PV, Ray P, Adhikary SK, Shah BG, Rangarajan R (2003) Studies of the effect of variation of blend ratio on permselectivity and heterogeneity of ion-exchange membranes. J Colloid Interface Sci 257:127–134
Volodina E, Pismenskaya N, Nikonenko V, Larchet C, Pourcelly G (2005) Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J Colloid Interface Sci 285:247–258
Zendehnam A, Mokhtari S, Hosseini SM, Rabieyan M (2014) Fabrication of novel heterogeneous cation exchange membrane by use of synthesized carbon nanotubes-co-copper nanolayer composite nanoparticles: characterization, performance in desalination. Desalination 347:86–93
Lin R, Chen BS, Chen GL, Wu JY, Chiu HC, Suen SY (2009) Preparation of porous PMMA/Na+−montmorillonite cation-exchange membranes for cationic dye adsorption. J Membr Sci 326:117–129
Ghaemi N, Madaeni SS, Daraei P, Rajabi H, Zinadini S, Alizadeh A, Heydari R, Beygzadeh M, Ghouzivand S (2015) Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: application of new functionalized Fe3O4 nanoparticles. Chem Eng J 263:101–112
Razmjou A, Mansouri J, Chen V (2011) The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J Membr Sci 378:73–84
Li JF, Xu ZL, Yang H, Yu LY, Liu M (2009) Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl Surf Sci 255:4725–4732
Liang P, Shi T, Li J (2004) Nanometer-size titanium dioxide separation/pre-concentration and FAAS determination of trace Zn and Cd IN water sample. Int J Environ Anal Chem 84(4):315–321
Hosseini SM, Nemati M, Jeddi F, Salehi E, Khodabakhshi AR, Madaeni SS (2015) Fabrication of mixed matrix heterogeneous cation exchange membrane modified by titanium dioxide nanoparticles: mono/bivalent ionic transport property in desalination. Desalination 359:167–175
Ye CZ, Ariya PA, Co-adsorption of gaseous benzene, toluene, ethyl benzene, m-xylene (BTEX) and SO2 on recyclable Fe3O4 nanoparticles at 0–101% relative humidities.
Anbarasu M, Anandan M, Chinnasamy E, Gopinath V, Balamurugan K (2015) Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim Acta A Mol Biomol Spectrosc 135:536–539
Ma W, Xu S, Li J, Guo J, Lin Y, Wang C, Hydrophilic Dual-Responsive Magnetite/PMAA Core/Shell Microspheres with High Magnetic Susceptibility and pH Sensitivity via Distillation-Precipitation Polymerization, Published online 27 April 2011 in Wiley Online Library.
Ceren Atila D, Nuray Y, Nihal A˘g, Alıml AC (2014) A comparative study of Fe3O4nanoparticles modified with differentsilane compounds. Appl Surf Sci 318:297–304
Madaeni SS, Zinadini S, Vatanpour V (2011) A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J Membr Sci 380:155–162
Khan J, Tripathi BP, Saxena A, Shahi VK (2007) Electrochemical membrane reactor: in situ separation and recovery of chromic acid and metal ions. Electrochim Acta 52:6719–6727
Nagarale RK, Shahi VK, Schubert R, Rangarajan R, Mehnert R (2004) Development of urethane acrylate composite ion-exchange membranes and their electrochemical characterization. J Colloid Interface Sci 270:446–454
Barragaan VM, Bauza CR (1999) Membrane potentials and electrolyte permeation in a cation-exchange membrane. J Membr Sci 154:261–272
Sata T (2004) Ion exchange membranes: preparation, characterization, modification and application. The Royal Society of Chemistry, Cambridge, United Kingdom
Nagarale RK, Gohil GS, Shahi VK, Rangarajan R (2004) Preparation and electrochemical characterizations of cation-exchange membranes with different functional groups. Colloids Surf A Physicochem Eng Asp 251:133–140
Li X, Wang Z, Lu H, Zhao C, Na H, Zhao C (2005) Electrochemical properties of sulphonated PEEK used for ion exchange membranes. J Membr Sci 254:147–155
Tanaka Y (2007) Ion exchange membranes: fundamentals and applications, membrane science and technology series, vol 12. Elsevier, Netherlands
Nagarale RK, Shahi VK, Thampy SK, Rangarajan R (2004) Studies on electrochemical characterization of polycarbonate and polysulfone based heterogeneous cationexchange membranes. React Funct Polym 61:131–138
Gohil GS, Binsu VV, Shahi VK (2006) Preparation and characterization of monovalent ion selective polypyrrole composite ion-exchange membranes. J Membr Sci 280:210–218
Kerres J, Cui W, Disson R, Neubrand W (1998) Development and characterization of crosslinked ionomer membranes based upon sulfinated and sulfonated PSU crosslinked PSU blend membranes by disproportionation of sulfinic acid groups. J Membr Sci 139:211–225
Shahi VK, Thampy SK, Rangarajan R (1999) Studies on transport properties of surfactant immobilized anion-exchange membrane. J Membr Sci 158:77–83
Nagarale RK, Shahi VK, Rangarajan R (2005) Preparation of polyvinylalcohol-silica hybrid heterogeneous anion-exchange membranes by sol–gel method and their characterization. J Membr Sci 248:37–44
Lide DR, (2006–2007) CRC Handbook of Chemistry and Physics, 87th ed. CRC Press
Balster J, Stamatialis DF, Wessling M (2004) Electro-catalyticmembrane reactors and the development of bipolar membrane technology. Chem Eng Process 43:1115–1127
Hosseini SM, Madaeni SS, Khodabakhshi AR (2012) The electrochemical characterization of ion exchange membranes in different electrolytic environments: investigation of concentration and pH effects. Sep Sci Technol 47:455–462
Acknowledgments
The authors gratefully acknowledge Arak University for the financial support during this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nemati, M., Hosseini, S.M. Fabrication and electrochemical property modification of mixed matrix heterogeneous cation exchange membranes filled with Fe3O4/PAA core-shell nanoparticles. Ionics 22, 899–909 (2016). https://doi.org/10.1007/s11581-015-1603-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1603-z