Abstract
Novel mixed matrix polyvinylchloride-based electrodialysis heterogeneous cation-exchange membranes were prepared by casting technique. Ilmenite (FeTiO3) was employed as additive in membrane fabrication. The effect of additive concentration on electrochemical properties of membranes was studied. Water content was decreased slightly by increase of FeTiO3 concentration. Ion-exchange capacity was improved initially by increase in additive content to 16 wt.% and then showed decreasing trend by more additive loading. Membrane potential, transport number and selectivity were enhanced initially in NaCl solution by increase of FeTiO3 content up to 16 wt.% and then decreased by more additive loading. Increment of FeTiO3 concentration led to decrease of selectivity and transport number in BaCl2 solution. Permeability and flux were declined slightly by increase in additive content up to 8 wt.% and then began to increase sharply by more additive content from 8 to 16 wt.%. Permeability and flux were decreased again by more additive concentration from 16 to 32 wt.%. Electrodialysis experiment results in laboratory scale showed higher dialytic rate for modified membrane compared to pristine one. Membrane areal electrical resistance was declined by increase of additive concentration. Membranes exhibited higher selectivity and flux for monovalent ions compared to bivalent ones.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, membranes gain much attention in the diverse industries and also humans’ life [1–4]. Among the various types of membranes, ion-exchange membranes have been widely studied and utilized as active separators in divers electrically driven processes such as electrodialysis for desalting brackish waters, reconcentrating brine from seawater and production of table salt. Additionally, ion-exchange membranes (IEMs) are efficient tools in resource recovery and food and pharmacy processing as well as manufacturing of basic chemical products. Ion-exchange membranes also can be employed in environmental protection such as treating industrial and biological effluents and many more processes [1, 2, 5–14].
In ion-exchange membranes, charged groups attached to polymer backbone are freely permeable to opposite sign ions under an electrical field influence [15]. In such processes, ion interactions with membrane, water, and with each other occur in complex fashions. Knowledge of the electrokinetic properties of ion-exchange membranes is a major contributing factor behind decisions about their applicability in specific separation processes and energy storage devices [4, 12, 16–18].
At the present time, demands for the superior ion-exchange membranes are increased for separation of pollutants soluble in water especially metals. Preparing inexpensive membranes with special adapted physico/chemical characteristics may be as vital step in future chemical and treatment application [2, 3, 12, 19, 20]. A lot of research has already been performed to improve the IEM physicochemical properties which resulted in various modification techniques. Variation of functional group type, selection of different polymeric matrices, polymer blending, additive loading, alteration of cross-link density, and surface modification are the important techniques to obtain superior IEMs [4–6, 10, 12, 21–29]. Many studies describe adsorptive membranes preparation by polymer blending and additive loading techniques using adsorptive polymer like chitosan and materials like metal oxide to enhance membrane performance especially for pollutant elimination from water [21, 25, 30–35].
Preparing novel heterogeneous cation-exchange membranes with appropriate physicochemical properties for the application in electrodialysis processes related to water recovery and treatment was the primary target of current research. For the purpose, mixed matrix polyvinylchloride-based heterogeneous cation-exchange membranes were prepared by solution casting techniques using cation-exchange resin powder as functional group agent and tetrahydrofuran as solvent. PVC is a flexible and durable polymer with suitable biological and chemical resistance [36–38]. Ilmenite (FeTiO3) particle was also employed as inorganic filler additive in membrane fabrication in order to improve the IEM physicochemical properties. Ilmenite is a new class of advanced materials with very interesting features and capacity such as antiferromagnetic insulators and adsorption characteristics because of valence states of cations (Ti, Fe) in FeTiO3 which provides unique physical and chemical properties. The nominal valence state of Ti ions in FeTiO3 has been considered 4+ which confirms formation of Fe2+. The charge transfer of Fe2+ also can be observed at high pressure. So, the amount of Fe3+ is little compared to Fe2+ in this study. Paramagnetic Fe2+ ions also had large polarization under the magnetic field. The researches reported the ratio of Fe3+/Fe2+ less than 10 % [39, 40]. Utilizing of inorganic particles or fillers especially metal oxide into polymeric materials has been examined in many applications to enhance the physicochemical characteristics and separation properties of polymeric matrixes based on the synergism between the organic–inorganic components properties [11, 21, 30, 34, 35]. Currently, no reports have considered incorporating ilmenite (FeTiO3) particles into ion-exchange membranes, and the literature is silent on characteristics and functionality of electrodialysis IEMs prepared using FeTiO3. The effects of ilmenite (FeTiO3) concentration in the casting solution on physicochemical properties of heterogeneous cation-exchange membranes were investigated. During the experiments, sodium chloride (NaCl) and barium chloride (BaCl2) were employed ionic solutions for the membranes characterization. Electrodialysis experiment was also carried out in a laboratory scale unit to evaluate the electrodialytic performance of modified membranes in ion removal from waste water. The results are valuable for electromembrane processes especially electrodialysis process for water recovery and treatment.
Materials and methods
Materials
Polyvinylchloride (PVC, grade 7054, bulk density 490 gr/l, viscosity 105 cm3/gr) was used as binders. Tetrahydrofuran (THF) was employed as solvent. Ilmenite particles (FeTiO3, powder, average particle size <37 μm) as inorganic filler additive and cation-exchange resin (Ion exchanger Amberlyst® 15, strongly acidic cation exchanger, H+ form—more than 1.7 meq/gr dry, density 0.6 gr/cm3, particle size (0.355–1.18 mm) ≥90 %) by Merck Inc., Germany were also used in membrane fabrication. All other chemicals were supplied by Merck. Throughout the experiment, distilled water was used.
Fabrication of homemade membranes
In order to undertake the membrane preparation, resin particles were dried in oven (SANEE. V. S. Co) at 30 °C for 48 h and then pulverized into fine particles in a ball mill (Pulverisette 5, Fritsch Co.) and sieved to the desired mesh size. The ion-exchange resin with desired particles size (−325 +400 mesh) was used in membrane fabrication. The preparation proceeded by dissolving the polymer binder into THF solvent in a glass reactor equipped with a mechanical stirrer (model: Velp Scientifica Multi 6 stirrer) for more than 5 h. This was followed by dispersing a specific quantity of grinded resin particle as functional group agents and FeTiO3 particle as additive in polymeric solution, respectively. The mixture was mixed vigorously at room temperature to obtain uniform particle distribution in the polymeric solution. In addition, for better dispersion of particles and breaking up their aggregates, the solution was sonicated for 1 h using an ultrasonic instrument. The excessive homogeneity and uniform distribution of functional groups on the surface and in the bulk of membrane matrix provide superior conducting regions in the membrane and generate easy flow channels for the counterion transportation and so improve the membrane electrochemical properties. Moreover, uniform distribution of particles increases the viscosity of solution and reduces the evaporation rate of casting solvent and so improves the polymer chain relaxation as well as its conformation with particle surfaces which makes better membrane selectivity [25, 28, 41]. Then, the mixing process was repeated for another 30 min using the mechanical stirrer. The mixture was then cast onto a clean and dry glass plate at 25 °C. The membranes were dried at ambient temperature and immersed in distilled water. As the final stage, the membranes were pretreated by immersing in NaCl solution. The membrane thickness was measured by a digital caliper device (Electronic outside Micrometer, IP54 model OLR) around 70–90 μm. The composition of casting solution is depicted in Table 1.
Test cell
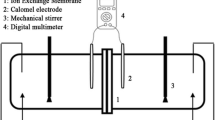
The electrochemical properties measurements for the membranes were carried out using the test cell (Fig. 1) as described elsewhere [21, 28]. The cell consists of two cylindrical compartments made of Pyrex glass which are separated by membrane. One side of each vessel was closed by Pt electrode supported with a piece of Teflon, and the other side was equipped with membrane. The membrane area was 19.63 cm2.
Membrane characterization
Water content
The water content was measured as the weight difference between the dried and swollen membranes. The wet membrane was weighed (OHAUS, Pioneer™, readability 10−4 gr, OHAUS Corp.) and then dried in oven until the constant weight was obtained. The following equation [9, 20, 24, 42] can be used in water content calculations:
Ion-exchange capacity
The ion-exchange capacity (IEC) determination was performed using titration method. For the IEC measurements, the membranes in acid form (H+) was converted to Na+ form by immersing in 1 M NaCl solution to liberate the H+ ions. The H+ ions in solution were then titrated with 0.01 M NaOH and phenolphthalein indicator. The IEC can be calculated from the following equation [8, 20, 23, 24, 42]:
where a is the milliequivalent of ion-exchange group in membrane and W dry is the weight of dry membrane (g).
Membrane potential, transport number, and permselectivity
The membrane potential is algebraic sum of Donnan and diffusion potentials determined by the partition of ions into the pores as well as the mobilities of ions within the membrane phase compared with the external phase [5, 29, 43]. This parameter was evaluated for the equilibrated membrane with unequal concentrations (C 1 = 0.1 M/C 2 = 0.01 M) of electrolyte solution (NaCl, BaCl2) at ambient temperature on either sides of membrane using two-cell glassy cell. During the experiment, both sections were stirred vigorously to minimize the effect of boundary layers. The developed potential across the membrane was measured by connecting both compartments and using saturated calomel electrode (through KCl bridges) and digital auto multimeter (DEC, model: DEC 330FC, Digital Multimeter, China). The measurement was repeated until a constant value was obtained. The membrane potential (E Measure) is expressed using Nernst equation [4, 5, 20, 42–44] as follows:
where t i m is transport number of counterions in membrane phase, R is gas constant, T is the temperature, n is the electrovalence of counterion, and a 1 and a 2 are solutions of electrolyte activities in contact membrane surfaces. The ionic permselectivity of membranes also is quantitatively expressed based on the migration of counterion through the IEMs [4, 5, 43, 44]:
where t 0 is the transport number of counterions in solution [45].
Ionic permeability and flux of ions
The measurements of ionic permeability and flux were carried out using the test cell (Fig. 1). A 0.1 M solution (NaCl or BaCl2) was placed on one side of the cell and a 0.01 M solution on its other side. A DC electrical potential (Dazheng, DC power supply, model: PS-302D) with an optimal constant voltage was applied across the cell with stable platinum electrodes. During the experiment, both sections were recirculated and stirred vigorously to minimize the effect of boundary layers. The cations pass through the membrane to cathodic section. Also, according to anodic and cathodic reactions, the produced hydroxide ions remain in cathodic section and increase the pH of this region.
According to the first Fick’s law, the flux of ions through the membrane can be expressed as follows [19, 20]:
where P is coefficient diffusion of ions, d is membrane thickness, N is ionic flux, and C is the cations concentration in the compartments.
where A is the membrane surface area. Integrating of Eq. (7) was as follows:
The diffusion coefficient and flux of cations in membrane phase are calculated from Eq. (8) considering pH changes measurements (Digital pH-meter, Jenway, model: 3510) in cathodic section. Also, these parameters can be determined by variations in conductivity in cathodic compartment as described earlier [21, 23, 25, 26].
Electrodialysis for ion removal
The electrodialysis experiment was also carried out in a laboratory scale electrodialysis unit containing one cell pair of homemade cation-exchange membrane and commercial anion-exchange membrane with 19.63 cm2 effective area to evaluate the electrodialytic performance of the modified membranes for water treating in ion removal. Commercial heterogeneous anion-exchange membrane (Ralex® AMH-PES), made by MEGA a.s., Czech Republic, was used in this study. The anionic membrane contains fixed quaternary ammonium functional groups. Water content of the commercial membrane was measured as 63 % (gr absorbed water/gr dry membrane). The ion-exchange capacity (IEC) was also obtained as 1.85 (meq/gr dry membrane). Moreover, detailed membranes characteristics can be found elsewhere [46]. The volume of electrolyte solution was 180 cm3. The variation in concentration can be determined by considering conductivity changes in treated compartment or pH changes in cathodic region.
Electrical resistance
The electrical resistance of equilibrated membrane was measured in NaCl solution with 0.5 M concentration (at 25 °C). Measurement was carried out by an alternating current bridge with 1,500 Hz frequency (audio signal generator, Electronic Afzar Azma Co. P.J.S). The membrane resistance is calculated using the different resistance between the cell (R 1) and electrolyte solution (R 2) (R m = R 1 − R 2) [24, 42]. The areal resistance was expressed as follows:
where r is areal resistance and A is the surface area of membrane.
Results and discussion
Membrane water content and ion-exchange capacity
Obtained results (Fig. 2) revealed that membrane water content was decreased slightly by increase of FeTiO3 concentration in the casting solution. This may be attributed to pore (voids/cavities)-filling phenomenon by the additive particles which declines the amount of water accommodation in membrane matrix. The swelling in prepared membranes was less than 5 % in thickness. Moreover, this was negligible in length and width. This indicates that solvation does not change membrane dimensions manifestly which confirms water content results.
IEC results (Fig. 2) indicated that increment of FeTiO3 concentration up to 16 wt.% in the casting solution initially led to an improvement in ion-exchange capacity from 2.2 (meq/gr) to 2.8 (meq/gr) in prepared membranes. This may be due to adsorption characteristic of ilmenite (FeTiO3) which makes superior interaction between ions and membrane surface and so facilitates the ion transportation between the solution and membrane phase. This improves membrane ion-exchange possibilities. Membrane ion-exchange capacity was decreased again from 2.8 (meq/gr) to 1.5 (meq/gr) with more increase in additive concentration from 16 to 32 wt.%. This is because of decrease in accessibility of ion-exchange functional groups in membrane matrix at high additive loading ratio which occupies the spaces around the resin particles and reduces the accessibility of ion-exchange functional groups by their surrounding/isolation.
Membrane potential, permselectivity, and transport number
Characterization in monovalent ionic solution (NaCl)
The membrane potential, transport number, and permselectivity (Figs. 3, 4, and 5) were improved initially in NaCl ionic solution by increase of FeTiO3 loading ratio up to 16 wt.% in the membranes. This may be attributed to increase of membrane’s ion-exchange capacity by increase of additive content (Fig. 2) which generates suitable flow channels with improved control of pathways for the counterions passage. Moreover, by increase of additive concentration in membrane matrix, the ionic channels are occupied by the additive particles and so narrowed by them as space-limiting factors. This strengthens the ionic site domination on ion traffic and improves Donnan exclusion that is responsible for the increment of membrane potential, transport number, and selectivity [4, 5, 43]. Membrane potential, transport number, and permselectivity were declined again by more increase in additive concentration from 16 to 32 wt.% in prepared membranes because of decrease in membrane ionic concentration which facilitates co-ion percolation through the membrane. Moreover, adsorptive affinity of FeTiO3 at high additive concentration makes superior interaction between the ions and membrane surface and strengthens the possibility of co-ion percolation due to development of concentration gradient. In addition, at higher additive loading, discontinuity of polymer chain binder due to enhancement of particle density can reduce the membrane transport number and selectivity.
The modified mixed matrix membranes containing ilmenite (FeTiO3) exhibited better selectivity and transport number in sodium chloride solution compared to pristine one without additives.
Characterization in bivalent ionic solution (BaCl2)
The obtained results revealed (Figs. 3, 4, and 5) that the increment of FeTiO3 concentration in the casting solution led to decrease of membrane potential, transport number, and selectivity in BaCl2 ionic solution which is different from membrane behavior in NaCl ionic solution. This may be attributed to different adsorptive characteristic of FeTiO3 for bivalent ions which is responsible for hindered transport of them through the membrane. Moreover, prepared membranes exhibited lower potential, selectivity, and transport number for the bivalent ions in comparison with monovalent type. These lower electrochemical properties of membranes for bivalent ions compared to monovalent type can be explained by the stronger bonds of bivalent cations with ion-exchange functional groups [29, 47] and adsorptive sites which poison the membranes and decrease the membranes transport number and selectivity. Furthermore, the larger radius of barium and their hydrated size in comparison with sodium ions make lower electrochemical properties for the membrane in bivalent ionic solution [21, 29, 47].
Ionic permeability and flux
During the experiment, ions pass through the membrane and reach to concentrated section. According to anodic and cathodic reactions, the amount of transported sodium ions through the membrane is equal to the produced hydroxide ions in cathodic section. As shown in Figs. 6 and 7, increase of FeTiO3 loading ratio up to 8 wt.% in the casting solution slightly led to decrease in ionic permeability and flux for sodium ions in prepared membranes. This is because of narrower ionic pathways’ formation and pore-filling phenomenon in membrane matrix by the additive particles and also lower amount of membrane water content which makes difficult ion traffic and so declines the flux. The sodium permeability and flux increased sharply by more increase in FeTiO3 content from 8 to 16 wt.%. This may be attributed to high adsorption characteristic of ilmenite at high additive concentration which can be prevailed upon the negative effects of membrane channels filling and improves the ionic permeability and flux by increase of ion interaction with membrane surface. Sodium permeability and flux were declined again by more increase in FeTiO3 concentration from 16 to 32 wt.%. This is because of decrease in accessibility of ion-exchange functional groups in membrane matrix (as was mentioned before) by FeTiO3 particles which occupy the spaces around the resin particles and so reduces the accessibility of ion-exchange functional groups by their isolation. The prepared membranes also showed similar behavior for bivalent ions (Figs. 6 and 7) in barium chloride ionic solution. Also, prepared membranes showed higher ionic flux and permeability for sodium ions compared to barium ones. This may be attributed to smaller radius of sodium ions and also formation of weaker bond of monovalent ions with membrane’s functional groups and FeTiO3 particles that can facilitate the ion transportation. The potential and strong adsorptive affinity of ilmenite particles toward attraction of bivalent ions makes the release of bivalent ions difficult and leads to barium permeability and flux decreasing compared to sodium ones.
Electrodialysis for ion removal
Electrodialysis experiment was carried out in a laboratory scale unit to evaluate the electrodialytic performance of the modified membranes for ion removal. The ionic flux/dialytic rate of pristine membrane and also modified membrane containing 16 wt.% FeTiO3 are shown in Fig. 8. Results showed that dialytic rate was increased sharply for ion removal by using ilmenite FeTiO3 in membrane matrix which is assigned to the superior and unique adsorptive characteristic of FeTiO3 in cation adsorption which enhances the ionic flux obviously in water treatment.
Electrical resistance
The membrane areal electrical resistance (Fig. 9) was declined sharply by increase of FeTiO3 concentration in casting solution. This may be due to adsorption property of ilmenite FeTiO3 which improves membrane conductivity. In addition, in general, less selective membranes have lower membrane resistances but this is not always true and depends on the membrane structure and its properties [46].
A comparison between electrochemical properties of prepared membrane in this research and some commercial membranes is given in Table 2. Results show that modified membrane in this present study is comparable with that of other commercial ones.
Conclusion
Membrane water content was decreased slightly by increase of FeTiO3 concentration in the casting solution. Results indicated that membrane IEC was increased initially by increase in additive content up to 16 wt.% in casting solution and then showed decreasing by more increase in additive concentration from 16 to 32 wt.%. Also, membrane potential, transport number, and selectivity were improved initially in NaCl ionic solution by increase of FeTiO3 loading ratio up to 16 wt.% and then decreased again by more additive loading. It was found that increment of FeTiO3 concentration in prepared membranes led to decrease of membrane potential, transport number, and selectivity in BaCl2 ionic solution. Moreover, prepared membranes exhibited lower potential, selectivity, and transport number for the bivalent ions in comparison with monovalent type. Increase of FeTiO3 content up to 8 wt.% in the casting solution slightly led to decrease in membrane ionic permeability and flux. The ionic permeability and flux increased sharply by more increase in FeTiO3 content from 8 to 16 wt.% and then showed decreasing trend by more increase in additive concentration from 16 to 32 wt.%. Also, prepared membranes showed higher ionic flux and permeability for sodium ions compared to barium ones. The electrodialysis experiment results in a laboratory scale unit showed that dialytic rate was increased sharply for ion removal by using ilmenite FeTiO3 in membrane matrix. The membrane areal electrical resistance was also declined by increase of FeTiO3 concentration in casting solution.
References
Nagarale RK, Gohil GS, Shahi VK, Trivedi GS, Rangarajan R (2004) Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J Colloid Interface Sci 277:162–171
Kariduraganavar MY, Nagarale RK, Kittur AA, Kulkarni SS (2006) Ion-exchange membranes: preparative methods for electro-dialysis and fuel cell application. Desalination 197:225–246
Nagarale RK, Gohil GS, Shahi VK (2006) Recent developments on ion-exchange membranes and electro-membrane processes. Adv Colloid Interf Sci 119:97–130
Gohil GS, Binsu VV, Shahi VK (2006) Preparation and characterization of mono-valent ion selective polypyrrole composite ion-exchange membranes. J Membr Sci 280:210–218
Shahi VK, Thampy SK, Rangarajan R (1999) Studies on transport properties of surfactant immobilized anion-exchange membrane. J Membr Sci 158:77–83
Vyas PV, Ray P, Adhikary SK, Shah BG, Rangarajan R (2003) Studies of the effect of variation of blend ratio on permselectivity and heterogeneity of ion-exchange membranes. J Colloid Interface Sci 257:127–134
Volodina E, Pismenskaya N, Nikonenko V, Larchet C, Pourcelly G (2005) Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J Colloid Interface Sci 285:247–258
Nagarale RK, Shahi VK, Schubert R, Rangarajan R, Mehnert R (2004) Development of urethane acrylate composite ion-exchange membranes and their electrochemical characterization. J Colloid Interface Sci 270:446–454
Hwang GJ, Ohya H, Nagai T (1999) Ion exchange membrane based on block copolymers. Part III: preparation of cation exchange membrane. J Membr Sci 156:61–65
M' Bareck CO, Nguyen QT, Alexandre S, Zimmerlin I (2006) Fabrication of ion-exchange ultrafiltration membranes for water treatment I. Semi-interpenetrating polymer networks of polysulfone and poly (acrylic acid). J Membr Sci 278:10–18
Xu T (2005) Ion exchange membrane: state of their development and perspective. J Membr Sci 263:1–29
Elattar A, Elmidaoui A, Pismenskaia N, Gavach C, Pourcelly G (1998) Comparison of transport properties of monovalent anions through anion-exchange membranes. J Membr Sci 143:249–261
Schauer J, Brozova L (2005) Heterogeneous ion-exchange membranes based on sulfonated poly (1, 4-phenylene sulfide) and linear polyethylene: preparation, oxidation stability, methanol permeability and electrochemical properties. J Membr Sci 250:151–157
Koter S, Warszawski A (2000) Electro-membrane processes in environment protection. Pol J Environ Stud 9(1):45–56
Baker RW (2004) Membrane technology and applications, 2nd edn. John Wiley & Sons Ltd, England
Shahi VK, Trivedi GS, Thampy SK, Rangarajan R (2003) Studies on the electrochemical and permeation characteristic of asymmetric charged porous membranes. J Colloid Interface Sci 262:566–573
Gohil GS, Shahi VK, Rangarajan R (2004) Comparative studies on electrochemical characterization of homogeneous and heterogeneous type of ion-exchange membranes. J Membr Sci 240:211–219
Dlugolecki P, Anet B, Metz SJ, Nijmeijer K, Wessling M (2010) Transport limitations in ion exchange membranes at low salt concentrations. J Membr Sci 346:163–171
Kerres J, Cui W, Disson R, Neubrand W (1998) Development and characterization of crosslinked ionomer membranes based upon sulfinated and sulfonated PSU Crosslinked PSU blend membranes by disproportionation of sulfinic acid groups. J Membr Sci 139:211–225
Li X, Wang Z, Lu H, Zhao C, Na H, Zhao C (2005) Electrochemical properties of sulfonated PEEK used for ion exchange membranes. J Membr Sci 254:147–155
Hosseini SM, Madaeni SS, Heidari AR, Amirimehr A (2012) Preparation and characterization of ion-selective polyvinyl chloride based heterogeneous cation exchange membrane modified by magnetic iron-nickel oxide nanoparticles. Desalination 284:191–199
Balster J, Krupenko O, Punt I, Stamatialis DF, Wessling M (2005) Preparation and characterisation of monovalent ion selective cation exchange membranes based on sulphonated poly (ether ether keton). J Membr Sci 263:137–145
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Preparation and characterization of ABS/HIPS heterogeneous cation exchange membranes with various blend ratios of polymer binder. J Membr Sci 351:178–188
Sata T (2004) Ion exchange membranes: preparation, characterization, modification and application. The Royal Society of Chemistry, Cambridge, United Kingdom
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Preparation and characterization of ABS/HIPS heterogeneous anion exchange membrane filled with activated carbon. J Appl Polym Sci 118:3371–3383
Hosseini SM, Madaeni SS, Zendehnam A, Moghadassi AR, Khodabakhshi AR, Sanaeepur H (2013) Preparation and characterization of PVC based heterogeneous ion exchange membrane coated with Ag nanoparticles by (thermal-plasma) treatment assisted surface modification. J Ind Eng Chem 19:854–862
Sata T, Yang W (2002) Studies on cation-exchange membranes having permselectivity between cations in electro dialysis. J Membr Sci 206:31–60
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Preparation and characterization of PC/SBR heterogeneous cation exchange membrane filled with carbon nano-tubes. J Membr Sci 362:550–559
Nagarale RK, Gohil GS, Shahi VK, Rangarajan R (2004) Preparation and electrochemical characterization of cation-exchange membranes with different functional groups. Colloid Surf A 251:133–140
P. Daraei, S. S. Madaeni, N. Ghaemi, E. Salehi, M. Khadivi, R. Moradian, B. Astinchap, Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water, Journal of Membrane Science, Article in press, Corrected proof, Available online 18 May 2012
Ghaee A, Shariaty-Niassar M, Barzin J, Matsuura T (2010) Effects of chitosan membrane morphology on copper ion adsorption. Chem Eng J 165:46–55
Boricha AG, Murthy ZVP (2009) Acrylonitrile butadiene styrene/chitosan blend membranes: preparation, characterization and performance for the separation of heavy metals. J Membr Sci 339:239–249
Liu C, Bai R (2006) Adsorptive removal of copper ions with highly porous chitosan/ cellulose acetate blend hollow fiber membranes. J Membr Sci 284:313–322
Ng LY, Mohammad AW, Leo CP, Hilal N (2013) Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308:15–33
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie GX, Liu ZF (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Wiks ES (2001) Industrial polymers handbook: products, processes, application. WILEY-VCH press, Germany
James E (1999) MARK, polymer data handbook. Oxford University Press, Inc., New York
Harper CA (1975) Handbook of plastic and elastomers. McGraw-Hill, New York
Fujii T, Yamashita M, Fujimori S, Saitoh Y, Nakamura T, Kobayashi K, Takada J (2007) Large magnetic polarization of Ti4+ ions in FeTiO3. J Magn Magn Mater 310:e555–e557
Raghavender AT, Hong NH, Lee KJ, Jung MH, Skoko Z, Vasilevskiy M, Cerqueira MF, Samantilleke AP (2013) Nano-ilmenite FeTiO3: synthesis and characterization. J Magn Magn Mater 331:129–132
Powell CE, Qiao GG (2006) Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J Membr Sci 279:1–49
Tanaka Y (2007) Ion exchange membranes: fundamentals and applications, membrane science and technology series, 12. Elsevier, Netherlands
Nagarale RK, Shahi VK, Thampy SK, Rangarajan R (2004) Studies on electrochemical characterization of polycarbonate and polysulfone based heterogeneous cation-exchange membranes. React Funct Polym 61:131–138
Nagarale RK, Shahi VK, Rangarajan R (2005) Preparation of polyvinyl alcohol-silica hybrid heterogeneous anion-exchange membranes by sol–gel method and their characterization. J Membr Sci 248:37–44
D.R. Lide, CRC Handbook of Chemistry and Physics, CRC press, 87th edition, 2006–2007
Długolecki P, Nymeijer K, Metz S, Wessling M (2008) Current status of ion exchange membranes for power generation from salinity gradients. J Membr Sci 319:214–222
Hosseini SM, Madaeni SS, Khodabakhshi AR (2010) Heterogeneous cation exchange membrane: preparation, characterization and comparison of transport properties of mono and bivalent cations. Sep Sci Technol 45:2308–2321
Acknowledgments
The authors gratefully acknowledge Arak University for the financial support during this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, S.M., Hamidi, A.R., Moghadassi, A.R. et al. Fabrication of novel mixed matrix electrodialysis heterogeneous ion-exchange membranes modified by ilmenite (FeTiO3): electrochemical and ionic transport characteristics. Ionics 21, 437–447 (2015). https://doi.org/10.1007/s11581-014-1186-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1186-0