Abstract
Gel polymer electrolytes containing various concentrations of an ionic liquid (IL), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide in an optimized composition of poly(ε-caprolactone) (PCL), and zinc triflate (ZnTr) were prepared by solution casting technique and characterized using Fourier transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC), and AC impedance spectroscopic techniques. The occurrence of complexation of polymer host with dopant salt zinc triflate as well as ionic liquid has been confirmed from FTIR results whereas from the present DSC data, it is quite evident that with an increase in the content of ionic liquid, the degree of crystallinity decreases. Complex impedance studies have revealed the occurrence of a maximum electrical conductivity of 1.1 × 10−4 S cm−1 at 25 °C in the case of the sample with a composition of 0.375 g PCL:0.125 g ZnTr:0.5 g IL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the realm of material science, polymers have long been generally considered and treated as insulating materials prior to the invention of conjugated polymers and ionically conducting polymers. Wright in the 1970s invented the first ionically conducting polymer also known as polymer electrolyte (PE) [1], formed by complexing a suitable dopant salt with a host polymer containing polar groups such as O, N, S, and F. The dopant salt provides required ionic species to the PE whereas the polymer essentially provides the medium for the ion transport to occur.
Several polymers including poly(ethylene oxide) (PEO), poly(vinylidene fluoride) (PVdF), poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP), poly(methylmethacrylate) (PMMA), poly(vinyl chloride) (PVC), poly(acrylonitrile) (PAN), poly(vinyl alcohol) (PVA), and similar systems were effectively utilized for the preparation of a variety of polymer electrolytes over the years. In the present case, a biodegradable polymer, poly (ε-caprolactone) (PCL), has been chosen to be the host polymer in view of the fact that PCL is known to exhibit a low glass transition temperature (T g) of around −60 °C and a melting temperature (T m) in the range 59–60 °C [2].
Owing to the highly reactive nature of lithium and safety concerns associated with lithium-based devices, many research attempts have been persuaded worldwide in order to find alternative materials to replace lithium. In this context, zinc triflate (ZnTr) has been chosen to be the dopant salt, since zinc is found to be less toxic, while it is quite abundant, cheap and stable, and having high specific energy values [3–5]. In our previous work, it was realized that the typical polymer electrolyte based on PCL with varying concentrations of ZnTr would show a maximum electrical conductivity of 8.8 × 10−6 S cm−1 in the case of the composition having 75:25 wt% ratio of polymer and salt at room temperature (25 °C) [6].
The incorporation of appropriate plasticizers into a polymer electrolyte is considered as a general practice in order to enhance the conductivity of polymer electrolyte systems thus leading to the development of a class of electrolytes designated as gel polymer electrolytes (GPEs) in which a large quantity of liquid electrolyte is usually entrapped within the polymer network. GPEs are known to possess appreciably high room temperature ionic conductivity, good cationic transport number with acceptable mechanical and dimensional stability [7]. Nowadays, many researchers are focusing on the utilization of ionic liquids (IL) as an alternative group of plasticizers [8–12] instead of traditional organic liquids such as ethylene carbonate (EC), propylene carbonate (PC), diethyl carbonate (DEC), dimethyl carbonate (DMC), and many other, as these ILs are thermally stable, non-flammable, and exhibit a wide electrochemical stability window up to 6 V [13]. Moreover, ILs are found to be less volatile and more safer than organic liquids to be employed in energy storage applications [14].
Therefore, in the present work, the optimized composition of 75:25 wt% of polymer and salt has been utilized to prepare a series of GPEs by the incorporation of varying concentrations of ionic liquid namely, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [emim][TF2N]. Structural characterization of all these samples was done using Fourier transform infrared (FTIR), and thermal behavior was examined using differential scanning calorimetry (DSC). The electrical conductivity was studied by means of AC impedance spectroscopy.
Experimental
Materials
PCL (M n = 80 kDa), ZnTr, and [emim][TF2N] were chosen to be the host polymer, dopant salt, and ionic liquid during the course of the present study and were procured from Sigma-Aldrich, USA. ZnTr was dried at 100 °C for an hour prior to use as the dopant salt, while PCL and [emim][TF2N] were used, as received. The structural formula for PCL, ZnTr, and [emim][TF2N] are given in Fig. 1.
Preparation of GPEs
Initially, the optimized amounts of polymer and salt were considered to be 0.375 and 0.125 g (designated as IL0), respectively, as this particular composition had yielded mechanically stable and highly conducting films. Consequently, a new series of GPE systems having the typical configuration PCL/ZnTr/IL have been prepared and designated as IL1, IL3, IL5, IL7, and IL10 corresponding to five different compositions of 0.375 g:0.125 g:0.05 g, 0.375 g:0.125 g:0.15 g, 0.375 g:0.125 g:0.25 g, 0.375 g:0.125 g:0.35 g, and 0.375 g:0.125 g:0.5 g, respectively, using solution casting technique by employing tetrahydrofuran (THF) as the common solvent. Appropriate amounts of IL were added into the solution containing the optimized composition of polymer (PCL) and salt (ZnTr). The mixture was stirred continuously for several hours in order to obtain a homogenous viscous solution which was then solvent cast onto glass petri dishes and maintained at 45 °C for THF to evaporate and then vacuum dried at 45 °C for 15 h. The self-standing translucent thick films thus obtained were further dried slowly at room temperature inside a desiccator for 2 days so as to remove any trace of the solvent. A representative picture of the prepared GPE IL10 is shown in Fig. 2.
Characterization techniques
FTIR spectra of GPEs were recorded at room temperature for thin films of GPE cast on KBr pellets by depositing a drop of solution and then evaporating the solvent. PerkinElmer RX1 spectrophotometer was utilized to perform FTIR studies with a wavenumber resolution of 4 cm−1 over the wavenumber range 400–4000 cm−1.
Thermal behavior of the GPEs was analyzed using a differential scanning calorimetry (DSC) by using NETZSCH DSC 204. The samples were heated in closed aluminum pans under nitrogen atmosphere to 100 °C, cooled to −100 °C and then heated to 100 °C at a heating rate of 10 °C/min. The set of thermograms corresponding to second heating were used for detailed analysis. The degree of crystallinity, χ c was calculated using the formula,
where ΔH m represents the melting enthalpy of the sample and ΔH 0PCL represents the melting enthalpy of 100 % crystalline PCL.
AC impedance measurements were carried out in the frequency range 1–20 Hz with an excitation signal of 50 mV using a computer-controlled Hewlett-Packard Model HP 4284A Precision LCR Meter at room temperature (25 °C). The GPE film was sandwiched between two polished stainless steel (SS) disks and such symmetrical cells involving blocking interfaces having the configuration—[SS/GPE/SS] were used for impedance measurements.
Results and discussion
Fourier transform infrared spectroscopy
Figure 3a shows the Fourier transform infrared (FTIR) spectra of pure PCL, optimized composition of PCL-ZnTr complex, and ionic liquid [emim][TF2N] at room temperature. The peaks identified along with their assignments are summarized in Table 1.
It is evident from Table 1 that pure PCL has a strong absorption band in the region 1800–1650 cm−1 corresponding to the carbonyl (C = O) stretching mode of vibration. Since the carbonyl group is the strong electron donor group of the polymer chain of PCL, zinc ion tends to coordinate with the oxygen atom of the carbonyl group [22]. As this carbonyl stretching band is highly sensitive to ionic interaction, a shoulder peak at 1650 cm−1 appears with the addition of salt in the case of the polymer-salt complex of PCL and ZnTr. This feature confirms the interaction of the salt and polymer and hence the successful doping of the polymer with the dopant salt, ZnTr as well. Certain new peaks at 1033, 1274, and 640 cm−1 also appear in the polymer-salt complex corresponding to the presence of triflate ion.
In the case of the FTIR spectrum obtained for pure IL [emim][TF2N], those bands observed between 700–900 cm−1 are mainly due to the ring bending modes of the imidazolium cation, and those bands in the range 1000–1400 cm−1 are dominated by the vibrations of [TF2N]− anion.
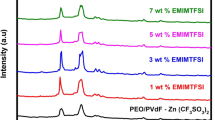
Figure 3b shows the FTIR spectra of GPE with varying concentrations of IL.
With the incorporation of IL into the optimized composition 75:25 wt% of PCL/ZnTr, certain new peaks appear due to the complexation of IL with polymer and salt. Upon addition of IL, the [emim]+ cation interacts with the carbonyl group of the polymer as is evident from the change in the observed position of the shoulder peak at 1650 cm−1 corresponding to the interaction of the carbonyl group with Zn2+ ions. This shoulder peak shifts towards lower wavenumber. New peaks at 1055 and 1348 cm−1 appear corresponding to the asymmetric S-N-S and C-SO2-N stretching modes of ionic liquid, in the spectra of IL1 and become more pronounced upon increasing the concentration of the ionic liquid which may be ascribed to the increased number of [TF2N]− anions.
It is also obvious from Fig. 3b that the free triflate peak noticed at 1033 cm−1 tends to increase in intensity with the increase in the concentration of ionic liquid. This aspect may be due to the enhanced dissociation of salt produced by the plasticizing effect offered by the ionic liquid [23]. The increase in the number of free triflate is an evidence of the increase in the number of Zn2+ ions in the case of the GPE, IL10.
The peak at 1293 cm−1 corresponds to the C-O and C-C stretching in the crystalline phase. With the addition of ionic liquid, the intensity of this peak decreases apparently due to the transformation of crystalline phases into an amorphous one. With further increase in the concentration of IL, the peak tends to become softened completely which shows that the sample IL10 is more amorphous among the series of GPEs prepared.
Thermal analysis
Figure 4a shows the DSC curves observed in the case of pure polymer PCL and the optimized composition with 75:25 wt% of PCL/ZnTr. Pure polymer shows a sharp endothermic peak at 55.3 °C corresponding to the melting of crystalline phases of the semi-crystalline polymer and T g at −63.3 °C. As can be seen from Table 2, the melting peak shifts towards a lower temperature upon loading of 25 wt% of salt corresponding to the complexation of polymer with salt and whereas the glass transition temperature (T g) of the electrolyte increases due to the effective intermolecular interaction of carbonyl oxygen and Zn2+ ions. The degree of crystallinity was calculated according to Eq. (1) by considering the value of enthalpy of fusion of hypothetical crystal of PCL, ΔH 0PCL as 136 J/g [24]. The degree of crystallinity is found to decrease from 45.2 to 31 % with the addition of 25 wt% of ZnTr salt which may be attributed to the transformation of crystalline into an amorphous phase. The exothermic peaks observed in both the curves of Fig. 4a correspond to the recrystallisation of the polymer chains.
Figure 4b shows the series of DSC curves obtained in the case of GPEs with varying concentrations of IL. With the addition of IL, the melting peak decreases from 50.6 °C to a minimum of 44.3 °C which is due to the increase in the complexation of [emim]+ cation with the carbonyl group of the polymer as evidenced from FTIR studies. Exothermic peaks were absent in the case of all GPE which implies the suppression of recrystallisation process with the addition of IL. The value of degree of crystallinity attains a minimum value of 16.4 % for the sample IL10 as revealed from the present FTIR studies which may be viewed as the presence of IL disrupts the alignment of polymer chains thereby preventing the process of crystallization.
AC impedance measurements
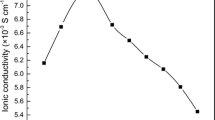
Figure 5 shows the variation of ionic conductivity of GPEs as a function of IL concentration at room temperature (25 °C) whereas the inset shows the Nyquist plot for a representative sample of IL10 which possesses a high frequency semicircle and a low frequency spike corresponding to the bulk relaxation and electrode/electrolyte interfacial phenomena, respectively. As seen from Fig. 5, the ionic conductivity increases with the amount of [emim][TF2N] and reaches a maximum value of 1.1 × 10−4 S cm−1 for the sample IL10.
The increase in the value of ionic conductivity with an increase of IL concentration may be ascribed to the increase in the number density of charge carriers as the ionic liquid enhances salt dissociation. Moreover, the presence of IL also reduces the interaction of mobile Zn2+ ions with the carbonyl group of the polymer that may promote the migration of Zn2+ ions. Also, the ionic liquid [emim][TF2N] is less viscous, having a viscosity of 34 cP (at 25 °C) [25] that may be effectively responsible for the enhancement in the flexibility of the polymer backbone which would in turn facilitate the segmental motion of the polymer and hence support the ion hopping mechanism.
Conclusions
GPEs containing ionic liquid [emim][TF2N] within the matrix of poly(ε-caprolactone) and zinc triflate were prepared by solution casting technique and the complexation of polymer, salt, and ionic liquid has been confirmed by FTIR analysis whereas DSC results have revealed an increase in the amorphous nature of these GPEs with increasing IL content. A maximum ionic conductivity of 1.1 × 10−4 S cm−1 has been obtained at room temperature.
References
Wright PV (1975) Br Polym J 7:319
Khatiwala VK, Shekhar N, Aggarwal S, Mandal UK (2008) J Polym Environ 16:61
Ikeda S, Mori Y, Furuhashi Y, Masuda H (1999) Solid State Ion 121:329
Kumar GG, Sampath S (2004) Polymer 45:2889
Kumar GG, Sampath S (2005) Solid State Ion 176:773
Sownthari K, Suthanthiraraj SA (2013) Express Polym Lett 7:495
Periasamy P, Tatsumi K, Kalaiselvi N, Shikano M, Fiyieda T, Saito Y, Sakai T, Mizukata M, Kajinami A, Deki S (2002) Ionics 8:453
Suleman M, Kumar Y, Hashmi SA (2013) J Phys Chem B 117:7436
Pandey GP, Hashmi SA (2009) J Power Sources 187:627
Kim J-K, Niedzicki L, Scheers J, Shin C-R, Lim D-H, Wieczorek W, Johansson P, Ahn J-H, Matic A, Jacobsson P (2013) J Power Sources 224:93
Libo L, Jiajia W, Peixia Y, Shaowen G, Heng W, Xiuchun Y, Xuwei M, Shuo Y, Baohua W (2013) Electrochim Acta 88:147
Cheng H, Zhu C, Huang B, Lu M, Yang Y (2007) Electrochim Acta 52:5789
Xu JJ, Ye H, Huang J (2005) Electrochem Commun 7:1309
Pandey GP, Kumar Y, Hashmi SA (2010) Indian J Chem 49A:743
Ravi M, Bhavani S, Pavani Y, Narasimha Rao VVR (2013) Indian J Pure Appl Phys 51:362–366
Kumar D, Hashmi SA (2010) Solid State Ion 181:416
Noack K, Schulz PS, Paape N, Kiefer J, Wassercheid P, Leipertz A (2010) Phys Chem Chem Phys 12:14153
Höfft O, Bahr S, Kempter V (2008) Langmuir 24:11562
Yokozeki A, Kasprzak DJ, Shiflett MB (2007) Phys Chem Chem Phys 9:5018
Woo HJ, Majid SR, Arof AK (2011) Solid State Ion 199–200:14
Elzein T, Nasser-Eddine M, Delaite C, Bistac S, Dumas P (2004) J Colloid Interface Sci 273:381
Elubair A, Elias CN, Suarez JCM, Lopes HP, Vieira MVB (2006) J Dent 34:784
Wu I-D, Chang F-C (2007) Polymer 48:989
Ramesh S, Liew C-W, Arof AK (2011) J Non-Cryst Solids 357:3654
Avella M, Errico ME, Laurienzo P, Martuscelli E, Raimo M, Rimedio R (2000) Polymer 41:3875
Acknowledgment
The financial support received in the form of the INSPIRE-SRF programme from the Department of Science and Technology (DST), Government of India, New Delhi is gratefully acknowledged by one of the authors (K.S).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sownthari, K., Suthanthiraraj, S.A. Structural, thermal, and electrical studies on gel polymer electrolytes containing 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. Ionics 21, 1649–1654 (2015). https://doi.org/10.1007/s11581-014-1324-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1324-8