Abstract
Thin films of pure and molybdenum (Mo)-doped tungsten trioxide (WO3) were deposited on indium tin oxide (ITO)-coated glass and Corning glass substrates by RF magnetron sputtering technique. The effect of Mo doping on the structural, morphological, optical and electrochromic properties of WO3 films was studied systematically. The energy dispersive X-ray analysis (EDAX) revealed that the films consist of molybdenum concentrations from 0 to 2 at.%. X-ray diffraction (XRD) studies indicated that with the increase of Mo concentration the structural phase transformation takes place from polycrystalline to amorphous phase. The crystallite size of the films decreased from 24 to 12 nm with increase of doping concentration of Mo in WO3. Scanning electron microscope (SEM) analysis revealed that Mo dopant led to significant changes in the surface morphology of the films. The electrochemical and electrochromic performance of the pure and Mo-doped WO3 were studied. The WO3 films formed with 1.3 at.% Mo dopant concentration exhibited high optical modulation of 44.3 % and coloration efficiency of 42.5 cm2/C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal oxides such as tungsten trioxide, titanium dioxide, nickel oxide, iridium oxide, vanadium oxide and molybdenum oxide have shown considerable application in the areas of electrochromic, gas sensors and lithium ion batteries [1–6]. Zheng et al. [7] synthesized nanostructured WO3 films using different methods for traditional applications like electrochromic, gas sensors and photocatalysis. In addition, these films are also used in dye-sensitized solar cells, optical data storage, field-emission displays and high-T c superconductors. Among these, tungsten trioxide (WO3) has been exhibited good electrochromic properties. The electrochromic effect observed in these materials has led to their use in the development of display devices and smart windows. The development of new and improved electrochromic devices depends on enhancement in the color efficiency. However, the color efficiency of the electrochromic device can also be modified by doping suitable metal ions with lower oxidizing capacity than the host materials. Faughnan and Crandall [8] reported that the higher electrochromic efficiencies are expected as a result of enhanced electron intervalence transfer between Mo5+ and W6+ states, in addition Mo5+ to Mo6+ and W5+ to W6+ transitions. The high color efficiency depends on the rate of intercalation that is coloration under applied electric field and controlled by the charge transfer at the WO3/electrolyte interface, which critically depend on the surface texture, defect structure and dopant content of the films. There are several reports on the titanium [8], niobium and vanadium [9] and nickel [10] doped WO3 films deposited by different techniques. Penin et al. [11] prepared Mo x W1−x O3 (x = 0–1) films by cathodic electrodeposition technique, and they reported the structure, composition and electrochemical associated lithium-insertion properties of mixed molybdenum and tungsten oxide films. Kondrachova et al. [12] have reported that the mixed MoO3-WO3 films exhibited better electrochromic properties compared to binary oxide films. Gesheva et al. [13] deposited Mo-doped (0–10 at.%) WO3 films by spray pyrolysis and studied their electrochromic response. Rueda de Leon et al. [14] and Kalidindi et al. [15, 16] studied the effect of substrate temperature on crystallite structure, phase and electrical conductivity of Ti-doped WO3 films deposited RF magnetron sputtering technique. In this investigation, we report the influence of Mo dopant concentration in WO3 films prepared by RF magnetron sputtering technique on the structural and morphological, optical and electrochromic properties.
Experimental
Pure and Mo-doped WO3 thin films were deposited on glass and ITO-coated glass substrates by RF magnetron sputtering technique. The vacuum pumping system was employed for sputter deposition uses a combination of diffusion and rotary pumps to achieve an ultimate pressure of 4×10−4 Pa. Pure oxygen and argon were used as reactive and sputter gases, respectively. Required quantities of oxygen and argon gases were admitted into the sputter chamber through fine controlled needle valves and their flow rates were monitored individually employing Aalborg mass flow controllers. Digital Pirani and Penning gauges were used to measure the pressure in the sputter chamber. The target to substrate distance was kept at 5 cm. The films were deposited at a fixed substrate temperature of 473 K, oxygen partial pressure of 6×10−2 Pa and sputter pressure of 4 Pa. The RF power applied to the sputter target was 150 W, and the deposition time was 120 min. Molybdenum doping was achieved by selectively masking of an erosion portion of the tungsten target by pure molybdenum strips of different sizes. The chemical composition of the films estimated by using energy dispersive X-ray analyser (Philips XL 308) attached to scanning electron microscope. Structural properties was investigated by X-ray diffraction (XRD) technique (Siefent model 3003TT) with Cu Kα radiation source (λ = 0.15406 nm). Surface morphology was studied by scanning electron microscopy (Hitachi SEM model S-400). The optical transmittance of the films was recorded by UV–Vis–NIR spectrophotometer (Perkin Elmer model Lambda 950) in the wavelength range 300–1500 nm.
The electrochromic and electrochemical properties of the pure and Mo-doped WO3 films were studied by three-electrode cell configuration with platinum as a counter electrode and calomel electrode as a reference electrode and pure and doped WO3 films formed on ITO-coated glass as a working electrode. The cyclic voltammetry experiments were performed by varying the potential in the range from +1 to −1 V in the electrolyte solution of 1 M Li2SO4 and at a scan rate of 50 mV/s. The colored and bleached states of the films were recorded using the spectrophotometer.
Results and discussions
Chemical composition
The chemical composition of the pure and Mo-doped WO3 films deposited on glass substrates were determined by using EDAX analysis. Figure 1 shows the EDAX spectra of pure and Mo-doped WO3 films formed with different concentrations. Figure 1a shows the characteristics peaks of tungsten and oxygen. The chemical component present in the films calculated from the intensity of the peaks was 25.9 at.% of tungsten and 74.1 at.% of oxygen and with the ratio of oxygen to tungsten found to be 2.96, which indicated the grown the WO3 films of nearly stoichiometric. Figure 1b, c and d clearly shows that the additional peaks were related to the molybdenum along with tungsten and oxygen. It was found that the intensity of molybdenum peak increased with the increase in the area of the molybdenum strips fixed on the sputter target of tungsten. The atomic percent of oxygen, tungsten and molybdenum present in the films are listed in Table 1. The estimated molybdenum atomic percentages present in the WO3 films were found to be 0.9, 1.3 and 2.0 at.%.
Structural properties
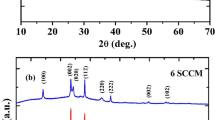
Figure 2 shows the XRD patterns of pure and Mo-doped WO3 films deposited on ITO-coated glasses substrates formed at the substrate temperature of 473 K. From the XRD patterns, it was observed that the pure WO3 films showed a weak diffraction peak at 2θ = 23.5o related to the (020) reflection of orthorhombic phase of WO3 (JCPDS Card No. 89-4480) in the amorphous matrix. For the films formed with 0.9 at.% of Mo doping, the intensity of the (020) reflection peak was increased and a new weak diffraction peak seen at 2θ = 22.8° which related to the (002) reflection of WO3. For films doped with Mo 1.3 at.%, the intensity of the (002) peak was increased where as the intensity of (020) reflection was found to decreased. The presence of two additional peaks observed at 26.7° and 35.2° related to the (120) and (122) reflections of WO3 which indicated that the grown films were of polycrystalline in nature. Such orthorhombic WO3 phase was also achieved in titanium-doped (1–10 wt.%) WO3 films deposited by pulsed laser deposition technique [17] and nitrogen-doped WO3 films formed by pulsed laser deposition [18]. At further increase of Mo dopant content to 2 at.%, the films exhibited an amorphous nature. In all films, there was no diffraction peaks related to either metallic molybdenum or MoO3, which clearly indicated that the molybdenum substituted in the tungsten sites in WO3 due to the same size of their ionic radii (73 pm for Mo+6 and 74 pm for W+6). Bathe and Patil [9] also reported the polycrystalline to amorphous phase transformation with increase of titanium doping from 2 at.% to 6 at.% in WO3 films formed by pulsed spray pyrolysis technique. The crystallite size (L) of the films was calculated by using Debye–Scherrer’s relation [19],
where λ is the wavelength of the X-rays, β is the full width at half-maximum of diffraction intensity in radians and θ is the diffraction angle. The crystallite size of the films formed with 0.9 at.% of Mo was 24 nm, and it decreased to 12 nm for the films doped with 1.3 at.% of Mo. The incorporated molybdenum inhibits the grain growth in WO3 matrix. Lethy et al. [17] also observed the suppression of the grain growth in titanium-doped WO3 films by incorporation of titanium ions which interfering into the intergranules and thereby introducing sufficient pinning force. The incorporated titanium ions can inhibit the grain boundary mobility or modify the interfacial tension/surface energy leading to a decrease in grain growth velocity or nucleation energy barrier, resulting in reduction in grain size. The similar nature of decrement in the crystallite size was observed in lithium-doped WO3 films [20].
It is to be noted that the value of 2θ shifted to higher angle side with the increase of Mo doping which modifies the lattice parameters. The lattice constants a, b, and c of the films are calculated using the formula
where d is the interplanar distance, and h, k, l are Miller indices. The lattice parameters are calculated from the position of diffraction peaks are found to be a = 7.409 Å, b = 7.4714 Å and c = 7.788 Å for the films doped with 1.3 at.% Mo. For WO3 films the standard values of lattice parameters are a = 7.3902, b = 7.5655 and c = 7.7875 (JCPDS Card number 89-4480). It is observed that the small change in lattice parameters can be attributed to the increase of cation size in the octahedral site with the substitution of molybdenum ions into the tungsten ion lattice.
The strain developed in the films was calculated using the relation.
where β is the full width at half-maximum and θ is the diffraction angle. The strain of the films increased from 1×10−1 to 1.6×10−1 with increase of Mo concentration from 0.9 to 1.3 at.%, respectively. There is not much variation in the lattice strain due to the same atomic radii of tungsten and molybdenum.
Surface morphology
The surface morphology of the pure and Mo-doped WO3 films deposited on glass substrates were examined using SEM. Figure 3a–d shows the scanning electron microscope images of pure and Mo-doped WO3 films. Figure 3a confirms the spherical grain growth in pure WO3 films, and the size of the grains was around 70 nm. For Mo-doped (0.9 at.%) WO3 films, the agglomeration of grains observed and the average grain size was 45 nm as shown in Fig. 3b. The leaf-like grain structures was observed at 1.3 at.% of Mo-doped films with grain size of 60 nm (Fig. 3c). The films formed with 2 at.% of Mo exhibited completely different morphology in which agglomerated grain structure disappeared as shown in Fig. 3d.
Optical properties
Optical transmittance spectra of pure and Mo-doped WO3 films deposited on glass substrates in the wavelength region of 300–1500 nm are shown in Fig. 4. All the films showed high transmittance in the visible range with an average transmittance varied between 80 % and 75 %. Papadimitropoulos et al. [21] reported the high optical transmittance of 90 % for pure WO3 films prepared by hotwire deposition technique. The absorption edge shifted towards the lower wavelength with increase of Mo dopant, indicating the increase in the optical band gap with dopant concentration of molybdenum. The optical absorption coefficient (α) of the films was calculated from the optical transmittance (T) data using the relation,
where t is the film thickness. The optical absorption in the films was fitted to the Tauc’s relation assuming that the direct transitions were takes place from the top of the valance band and bottom of the conduction band as given by the relation,
Figure 5 shows the plots of (αhυ)2 versus photon energy (hυ) of pure and Mo dopant of WO3 films. It is observed that the optical band gap of the films was found to increased from 3.21 to 3.33 eV with the increase of dopant concentration of Mo from 0 to 2 at.%, respectively. Gullapalli et al. [22] reported the optical band gap value 3.17 eV for pure WO3 films deposited at substrate temperature of 473 K. The increase in the optical band gap with Mo dopant concentration is due to the Moss–Burstein shift. Gaury et al. [23] also noticed such increase in the band gap in Nb-doped WO3 films. The refractive index (n) of the films was determined from the optical transmittance interference data employing Swanepoel’s envelope method [24] using the relation
and
where n 0 and n 1 are the refractive indices of air and substrate, and T max and T min are the successive optical transmittance maxima and minima, respectively. Figure 6 shows the wavelength dependence of refractive index of pure and Mo-doped WO3 films. In all films, the refractive index of the films decreased with the increase of wavelength and remain almost constant at higher wave length region. At a fixed wavelength of 550 nm, the refractive index of the films increased from 2.12 to 2.25 with increase of molybdenum dopant concentration from 0 to 2 at.%, respectively.
Electrochemical properties
Figure 7 shows the cyclic voltammograms of pure and Mo-doped WO3 films recorded at the scan rate of 50 mV/s in a 1 M Li2SO4 electrolyte solution. From the figure, it is observed that as the potential decreased from +1 to −1 V a progressive increase in the cathodic current peaks associated with the reduction of W6+ → W5+ due to the Li+ ion insertion in the films. These processes lead to the coloration in the films. Reversing the potential from −1 to +1, the anodic current peaks are observed and the colored films transformed to transparent due to the oxidation of W5+ → W6+. Figure 7 shows that anodic potential shifted towards positive potential from −0.05 to 0.15 V with increase of Mo dopant in WO3 films. A shift in anodic peak potential indicated that the energy required for extracting the intercalated Li+ ions from the film decreased with increase of Mo dopant in WO3. The magnitude of CV curves was increased with the increase of Mo dopant up to 1.3 at.% due to the intercalation of a greater number of Li+ ions in the films. The enhancement lies in the rearrangement of WO3 structure by substitution of tungsten with molybdenum. Patil et al. [25] also noticed an increase in the area of CV plots with increase of Mo-doped V2O5 films due to easy way to diffusion and charge transfer process of ions. Mahajan et al. [26] also reported such an improvement in the CV performance in Ti-doped MoO3 films may be due to the Ti4+ supported the Mo6+ to Mo5+ reaction. The anodic and cathodic current densities as a function of Mo dopant in WO3 films are shown in Fig. 8. It is clearly observed that the anodic current density increased from 0.29 to 0.49 mA/cm2 for pure to 1.3 at.% Mo-doped WO3 films thereafter it decreased to 0.37 mA/cm2 with further increase of Mo concentration to 2 at.% due to the fewer Li+ ions participated in the reaction. The insertion of Li+ ions depends on the diffusion coefficient and density of the films. The diffusion coefficient (D) of Li+ ions during intercalation and de-intercalation process was calculated using Randles–Servik equation [27],
where i p is the anodic peak current density, n the number of electrons transferred in the redox reaction (which is assumed to be unity), C o is the concentration of active ions in the solution, ν is the scan rate and A is the area of the film.
Figure 9 shows the dependence of diffusion coefficient on the content of molybdenum doping concentration in the WO3 films. It is seen from the figure that the diffusion coefficient increased from 1.85×10−11 to 8.5×10×11 cm2/s with the increase of Mo dopant concentration from 0 to 1.3 at.%; thereafter, it decreased to 5.6×10−11 cm2/s with the further increase of Mo dopant concentration to 2.0 at.%. This could be due to the variation in the mobility of Li+ ions in the redox reaction. Leon et al. [14] observed that the diffusion coefficient increased from 4.07×10−12 to 6.30×10−11 cm2/s with the increase of Mo dopant concentration.
Electrochromic properties
Figure 10 shows the optical transmittance spectra of pure and Mo-doped WO3 films deposited on ITO-coated glass substrates in virgin and colored conditions. The optical modulation is one of the important parameter to estimate the electrochromic quality of the electrode. The change in the optical modulation was estimated by ΔT = T b − T c, where T b and T c are the transmittance for virgin and colored states at a wavelength of 550 nm. Figure 11 shows the variation of optical modulation with doping content of Mo in the WO3 films. It is observed that the optical modulation increased from 40 % to 44.3 % with the increase of Mo content from 0 to 1.3 at.% and it decreased to 41.5 % at Mo dopant of 2.0 at.%. The coloration efficiency (η) is defined as the change in the optical density which changes intercalated per unit area of electrode. The coloration efficiency of the films was calculated by using the relation
where ΔOD is the optical density, Q the charge inserted into the films and A the area of the films. Figure 11 shows the dependence of coloration efficiency on the Mo dopant content in the WO3 films. It is observed that the coloration efficiency increased from 33.8 to 42.5 cm2/C with the increase of Mo concentration from 0 to 1.3 at.%, respectively. The enhanced intercalation properties of Mo-doped WO3 films was due to the rearrangement of atoms in the WO3 crystal structure caused by the substitution of Mo ions at the W sites. Chen et al. [28] reported the coloration efficiency 25 cm2/C in pure WO3 films deposited by RF magnetron sputtering. Faughnan and Crandall [8] reported that the enhancement of electrochromic properties in mixed oxide films due to the inter-valance charge transfer between Mo5+ and W6+ sites. At further increase of Mo concentration to 2 at.%, the coloration efficiency decreased to 38 cm2/C. This indicated that an appropriate doping concentration of molybdenum in WO3 concentration play an important role to improve the electrochromic properties. Kondrachova et al. [12] reported that the coloration efficiency was increased from 29 to 34 cm2/C with increase in Mo concentration. Hence, it is clearly indicated that the 1.3 at.% Mo-doped WO3 films exhibit better electrochromic properties.
Conclusions
Thin films of Mo-doped WO3 were deposited on ITO and glass substrates held at temperature of 473 K by RF magnetron sputtering technique. The deposited films were characterized for their chemical composition, crystalline structure, surface morphology, optical and electrochromic properties. The XRD studies on pure WO3 films indicated that microcrystallites with orthorhombic structure were in the amorphous matrix. As the Mo dopant concentration increased the films undergo transformation from polycrystalline to amorphous structure. The crystallite size of 0.9 at.% Mo-doped WO3 film was 24 nm and it was decreased with increase of dopant concentration. The scanning electron microscopy results showed different morphologies as the Mo doping concentration increased. The WO3 films composed of grain-like structure which transformed to leaf-like structures with increase of doping of molybdenum dopant concentration from 0 to 2.0 at.%, respectively. The optical band gap and refractive index were increased with increase of Mo dopant concentration in WO3 films. The electrochemical studied showed high anodic current density of 0.49 mA/cm2 and diffusion coefficient of 8.5×10−11 cm2/s in 1.3 at.% Mo-doped WO3 films. The better electrochromic performance with high coloration efficiency of about 42.5 cm2/C was obtained at 1.3 at.% doping of Mo in WO3 films.
References
Granqvist CG (1995) Handbook of inorganic elecrochromic materials. Elsevier, Amsterdam
Cai GF, Zhou D, Xiong QQ, Zhang JH, Wang XL, Gu CD, Tu JP (2013) Sol Energy Mater Sol Cells 117:231
Noerochim L, Wang JZ, Wexler D, Chao Z, Liu HK (2013) J Power Sources 228:198
Qin Y, Fan G, Liu K, Hu M (2014) Sensors Actuators B 190:141
Hari Krishna K, Hussain OM, Julien CM (2010) Appl Phys A Mater Sci Process 99:921
Madhavi V, Kondaiah P, Subba Rayudu S, Hussain OM, Uthanna S (2013) Mater Express 5:135
Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A, Kalantar-zadeh K (2011) Adv Funct Mater 21:2175
Faughnan BW, Crandall RS (1977) Appl Phys Lett 31:834
Bathe SR, Patil PS (2008) Solid State Ionics 179:314
Rougier A, Blyr A, Garcia J, Zhang Q, Impey SA (2002) Sol Energy Mater Sol Cells 71:343
Penin N, Rougier A, Laffont L, Poizot P, Tarascon JM (2006) Sol Energy Mater Sol Cells 90:422
Kondrachova L, Benjamin PH, Vijayaraghavan G, Williams RD, Stevenson KJ (2006) Langmuir 22:10490
Gesheva K, Cziraki A, Ivanova T, Szekeres A (2005) Thin Solid Films 492:322
Rueda de Leon JMO, Acosta DR, Pal U, Castaneda L (2011) Electrochim Acta 56:2599
Kalidindi NR, Manciu FS, Ramana CV (2011) ACS Appl Mater Interfaces 3:863
Kalidindi NR, Bharathi KK, Ramana CV (2011) Appl Phys Lett 97:142107
Lethy KJ, Beena D, Mahadevan Pillai VP, Ganesan V (2008) J Appl Phys 104:033515
Lethy KJ, Beena D, Mahadevan Pillai VP, Ganesan V (2009) J Phys D Appl Phys 42:85407
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addition-Wesley, Reading, MA
Kovendhan M, Joseph DP, Kumar ES, Sendilkumar A, Manimuthu P, Sambasivam S, Venkateswaran C, Mohan R (2011) Appl Surf Sci 257:8127
Papadimitropoulos G, Vourdas N, Giannakopoulos K, Vasilopoulou M, Davazoglou D (2011) J Appl Phys 109:103527
Gullapalli SK, Vemuri RS, Ramana CV (2010) Appl Phys Lett 96:171903
Gaury J, Kelder EM, Bychkov E, Biskos G (2013) Thin Solid Films 534:32
Swanepoel R (1983) J Phys E Sci Instrum 16:1214
Patil CE, Jadhav PR, Tarwal NL, Deshmukh HP, Karanjar MM, Patil PS (2011) Mater Chem Phys 126:711
Mahajan SS, Mujawar SH, Shinde PS, Inamder AI, Patil PS (2009) Sol Energy Mater Sol Cells 93:183
Nicholson RS, Shain I (1964) Anal Chem 36:706
Chen HS, Jan DJ, Chen CH, Huang KT (2013) Electrochem Acta 93:307
Acknowledgments
One of the authors, V. Madhavi, is thankful to the University Grant Commission, India for the award of UGC - RFSMS Junior Research Fellowship to carry out the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhavi, V., Kumar, P.J., Kondaiah, P. et al. Effect of molybdenum doping on the electrochromic properties of tungsten oxide thin films by RF magnetron sputtering. Ionics 20, 1737–1745 (2014). https://doi.org/10.1007/s11581-014-1073-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1073-8