Abstract
The precursor of (Ni0.15Co0.15Mn0.7)CO3 has been synthesized by a carbonate precipitation method, which was used to prepare high-capacity cathode material Li1.2Ni0.12Co0.12Mn0.56O2 for lithium-ion batteries. Carbonate precipitation was conducted using NH4HCO3 solution as the precipitation reagent. Two different feeding ways were adopted during the precipitation process. The physical properties of the precursor and the resulting Li1.2Ni0.12Co0.12Mn0.56O2 were characterized in detail, and the electrochemical properties of the prepared Li1.2Ni0.12Co0.12Mn0.56O2 powers were evaluated. It was found that the structural and morphological properties of the precursor and the final material were effectively improved by the ordered addition method. Electrochemical studies confirmed that the Li1.2Ni0.12Co0.12Mn0.56O2 synthesized by ordered addition method exhibited a higher capacity of 287.3 mAh g−1, and the capacity retention after 30 cycles was 90.5 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Layered lithium-rich transition metal oxides containing manganese, nickel, and cobalt are currently receiving worldwide attention as a possible replacement for the LiCoO2 electrode of conventional lithium-ion cells [1–3]. These materials can be represented using either (1) structurally integrated two-component solid solution notations such as xLi2MnO3·(1 − x)LiMO2 (M = Ni, Co, and Mn) (layered layered in which the Li2MnO3 component is electrochemically activated above 4.4 V vs. Li/Li+) or (2) standard notation as Li1 + y M1 − y O2 (M = Ni, Co, and Mn) [4–7]. Several methods have been reported to prepare Li1 + y M1 − y O2 (M = Ni, Co, and Mn) cathode materials, such as coprecipitation method [8–11], sol–gel method [12, 13], spray drying method [14], molten salt method [15], solid-state method [16], fast coprecipitation [17], etc. Among these methods, the coprecipitation method is the most commonly adopted to get phase pure oxide precursor with uniform and smaller particle size. However, as precipitation agent and complexing agent are mainly used to prepare the precursor with this method, the balance is difficult to control, and the precipitation efficiency is low. On the other hand, the performance of the materials is strongly correlated not only to the synthesis methods but also to the synthesis conditions. Therefore, it is necessary to explore an appropriate preparation method and optimize the process so that lithium-rich transition metal oxides with good performance can be prepared.
In this study, a carbonate precipitation method was attempted to prepare Li1.2Ni0.12Co0.12Mn0.56O2 (which could be rewritten as 0.5Li2MnO3·0.5LiNi0.3Co0.3Mn0.4O2) cathode materials. The precursor, (Ni0.15Co0.15Mn0.7)CO3, was prepared using NH4HCO3 solution as the precipitation reagent. Two different feeding ways were adopted during the precipitation process. The physical properties of the precursor and the resulting Li1.2Ni0.12Co0.12Mn0.56O2 were characterized in detail, and the electrochemical properties of the prepared Li1.2Ni0.12Co0.12Mn0.56O2 powers were evaluated.
Experimental
Synthesis of Li1.2Ni0.12Co0.12Mn0.56O2
Li1.2Ni0.12Co0.12Mn0.56O2 was synthesized by calcination–crystallization process using carbonate precursor and lithium carbonate as raw materials. The carbonate precursor (Ni0.15Co0.15Mn0.7)CO3 was synthesized in a continuously stirred tank reactor via a carbonate precipitation process. The precursor, (Ni0.15Co0.15Mn0.7)CO3, was prepared according to the following methods: (a) stoichiometric amount of NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O (Ni/Co/Mn = 0.15:0.15:0.7, molar ratio) was dissolved in distilled water with a concentration of 2.0 mol L−1, respectively. The aqueous solution of NiSO4, MnSO4, and CoSO4 was pumped into a continuously stirred tank reactor in sequence. At the same time, NH4HCO3 solution (2.0 mol L−1) which was used as the precipitation reagent was continuously fed into the reactor. This method was named as ordered addition method. (b) Stoichiometric amount of NiSO4·6H2O, CoSO4·7H2O, and MnSO4·H2O (Ni/Co/Mn = 0.15:0.15:0.7, molar ratio) was dissolved together in distilled water with a concentration of 2.0 mol L−1. The mixed solution and a 2.0 mol L−1 NH4HCO3 solution were pumped into the reactor, respectively. This method was named as mixed addition method. Except for the feeding ways, the other experimental conditions were the same. The temperature of the reactor was maintained at 50 °C with continuous stirring for 12 h. The pH (7.5) value of the precipitation solution was maintained via carefully controlling the addition speed of the solutions. The precipitated powders were filtered and washed and then dried at 105 °C for 5 h. The obtained carbonate precursors were thoroughly mixed with a 3 at.% excess of a stoichiometric amount of Li2CO3 to compensate for the calcining loss and then calcined at 950 °C for 10 h in air. The precursors (Ni0.15Co0.15Mn0.7)CO3 made from the ordered and mixed addition methods were marked as a' and b', respectively. The Li1.2Ni0.12Co0.12Mn0.56O2 material made from the ordered and mixed addition methods were marked as a and b.
Measurements
X-ray diffraction measurements of materials were carried out on the Rigaku 2500 X-ray diffractometer using Cu-Kα radiation. The diffraction data were collected over the range 10° < 2θ < 80°. The chemical composition of the synthesized materials was determined by an atom absorption spectrophotometry (AAS) (TAS990; Beijing Purkinje General Instrument, China). The morphological characteristics of the samples were observed by a JEOL JSM-5600LV scanning electron microscopy (SEM). The active surface areas of the materials were measured by the Brunauer, Emmer, and Teller (BET) method with the Beishide Instrument, Beijing, China. Tap densities were determined using the ZA-201 tap density meter (Liaoning Instrument Research Institute Co., Ltd., Liaoning, China).
The cathode electrodes were prepared by mixing 80 wt% active material, 10 wt% carbon black as conductive material, and 10 wt% polyvinylidene difluoride binder in N-methyl-2-pyrrolidone solvent. Then, the slurry was cast onto an aluminum foil current collector, punched in the form of disks typically with a diameter of 14 mm and then dried. The charge–discharge tests were carried out using laboratory half-cell which consisted of a cathode and a lithium metal anode separated by the Celgard 2400 polyethylene/polypropylene film. Cells were assembled and sealed in an argon-filled glove box with the electrolyte of 1 mol L−1 LiPF6 dissolved in EC/DMC/DEC (1:1:1 by volume). The charge–discharge tests were operated in the voltage range of 2.0–4.8 V with a constant current density of 15 mA g−1 (0.06 C) at room temperature on the LAND CT2001A battery test system (Jinnuo Wuhan Co., Ltd., People’s Republic of China).
Results and discussion
Crystal structure properties
Figure 1 shows the X-ray diffraction (XRD) patterns of the (Ni0.15Co0.15Mn0.7)CO3 precursors prepared by different feeding ways. All main characteristic peaks in each pattern were coincided with the diffraction peaks of hexagonal structure with a space group of \( R\overline{3}c \) corresponding to MnCO3 (JCPDS no. 44-1472), and no impurity-phase peaks exist. The peaks of sample a' were relatively broad which is likely due to the small grain size of the precursor particles [18], in agreement with the SEM image.
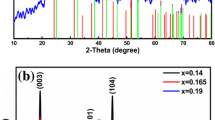
Figure 2 shows the XRD patterns of the Li1.2Ni0.12Co0.12Mn0.56O2 samples synthesized by calcining the mixture of the precursors and lithium carbonate. Most of the XRD peaks in Fig. 2 were indexed with hexagonal α-NaFeO2 structure (space group, \( R\overline{3}m \)) except for the weak peaks around 21° corresponding to integrated monoclinic Li2MnO3 phase (C2/m), originating from the ordering of Li+ ion in the transition metal layer [3]. Another observation in the XRD patterns is the clear splitting of the (006)/(102) and (108)/(110) peaks, indicating that the material has a well-organized layered structure [19]. No peak of any impurity phase is detected in the XRD patterns, illustrating the high purity of the as-synthesized Li1.2Ni0.12Co0.12Mn0.56O2 material prepared by these methods where the carbonate precipitation was conducted using NH4HCO3 solution as the precipitation reagent.
The ratio (R) of I(003)/I(104) is sensitive to the cation distribution in lattice and the degree of cation mixing of materials [20–22]. When the R value is higher, the degree of cation mixing is lower. It was reported that the undesirable cation mixing would appear when R is smaller than 1.2. In our experiment, the R values of samples a and b are 1.701 and 1.432, respectively. The R value of Li1.2Ni0.12Co0.12Mn0.56O2 sample prepared by ordered addition method is higher than that of the sample prepared by mixed addition method. In addition, the diffraction peaks in Fig. 2(a) are more sharp and narrow than those in Fig. 2(b). It reveals that the former has a better layered structure than the latter, which implies that the former might have better electrochemical performance. The possible reason for the structure difference could be attributed to the difference of the solubility product constants (K sp) of NiCO3, CoCO3, and MnCO3, i.e., K sp(NiCO3) = 6.6 × 10−9, K sp(CoCO3) = 1.4 × 10−13, and K sp(MnCO3) = 1.8 × 10−11. Ni2+ is relatively difficult to precipitate completely compared to Mn2+ and Co2+ because of the relatively larger value of K sp(NiCO3). In the ordered addition method, the precipitation reaction started with the formation of NiCO3 seed particles and then dissolved reprecipitation according to the solubility equilibrium of precipitation between NiCO3, CoCO3, and MnCO3 when CoSO4 and MnSO4 were pumped into the reactor in sequence and ended with the production of homogeneous particles of carbonate precursor with a chemical composition close to the nominal one. While in the mixed addition method, the carbonate precipitation reaction with Mn and Co dominated the whole reaction because of the relatively smaller value of K sp(MnCO3) and K sp(CoCO3), and then, the residual concentration of NH3 was high in the solution, which would result in producing more nickel–ammonia complexes. This may result in nonuniform sedimentation with the mixed addition method. The assumption was confirmed by AAS results (Table 1) which shows that the measured molar ratios of Ni/Co/Mn are well in agreement to the intended composition for the sample a', while those for sample b' are slightly deviated from the target stoichiometry.
Morphology characteristics
The morphology of the precursors and the final Li1.2Ni0.12Co0.12Mn0.56O2 samples is shown in Fig. 3. The particles of precursor a' (Fig. 3(a')) have uniform structural morphology and smooth crystal surface with narrow size distribution, which is in agreement with the XRD result (Fig. 1(a')), while the crystal size of sample b' (Fig. 3(b')) is bigger and is not as uniform. The possible explanation is that the transition metal ions were inconsistently precipitated easily due to the difference of the solubility product constant (K sp) of NiCO3, CoCO3, and MnCO3. After lithiation, the particles of sample b exist with some agglomeration. The morphology of sample a remains almost the same as that of the precursor powder except for some decrease in the diameter of the particle. This is consistent with the measurement results of the surface area by the BET method, 1.14 m2 g−1 (sample a) and 0.81 m2 g−1 (sample b), and the tap density of the samples a and b was 1.81 g cm−3 and 1.83 g cm−3, respectively.
Electrochemical properties
Figure 4 shows the first charge–discharge profiles of the Li/Li1.2Ni0.12Co0.12Mn0.56O2 samples. As can be seen, all charge plots are composed of a slope region and a long plateau. The slope region (<4.5 V) is attributed to the extraction of Li+ ions from the LiNi0.3Co0.3Mn0.4O2 component with a concomitant oxidation of Ni2+ and Co3+. The long plateau, characterized by a voltage plateau above 4.5 V, is attributed to the removal of Li2O from Li2MnO3 [10, 11]. Other workers also reported that the excess capacity originating at the 4.5 V plateau resulted from the hybridization of O2− 2p orbital and Mn4+ 3d orbital [23]. The existence of the long plateau will ensure a high capacity in the subsequent cycling. All cells had first irreversible capacity. Some portion of the irreversible capacity may come from the oxidation of the electrolyte since the onset potential for the oxidation of electrolyte is at approximately 4.65 V, depending on the electrolyte composition. The large irreversible capacity loss may be related to the extraction of Li2O followed by elimination of oxygen-ion vacancies from the lattice during first charge, resulting in a lower number of sites for insertion and extraction of Li+ in the subsequent cycles [24]. Further, detailed structural and phase transitions associated with such lithiation–delithiation processes at higher voltage (>4.4 V) are not fully understood yet [6].
The sensitivity of the initial capacity to the synthesis conditions of the precursors is proved to be higher than the sensitivity of common powder XRD method, though the most substantial changes are indicated both by XRD and electrochemical capacity measurements. It can be seen from Fig. 4 that the discharge capacity of sample a (287.3 mAh g−1) is greater than the common value (∼250 mAh g−1) of Li-rich transition metal oxide prepared by other methods [11, 25–28], while the value of sample b (242.8 mAh g−1) is similar to the common value. Moreover, the coulombic efficiency of sample a is larger (75.76 %) than that of sample b (73.58 %).
Figure 5 shows the profiles of differential capacity (dQ/dV) versus voltage (V) for the Li/Li1.2Ni0.12Co0.12Mn0.56O2 cells. The cells from both samples a and b have large oxidation peak (starting at 4.5 V) in differential capacity which corresponds to the 4.5 V plateau in Fig. 4. From Fig. 5(b), three cathodic peaks are evident on discharge curve. Although it is impossible to differentiate the reduction processes of the individual Mn, Ni, and Co ions from the data, it is believed that the reduction process at ∼4.25 V may be associated with the occupation of tetrahedral sites by lithium within the extensively delithiated (lithium) layer and the lower voltage processes between 3.0 and 3.6 V to the occupation of octahedral sites [29]. Another cathodic peak below 2.75 V is consistent with the lithiation of a chemically derived MnO2 component in the electrode. Such a voltage profile in Li/Li1.2Ni0.12Co0.12Mn0.56O2 cell suggests that the layered structure transforms into a spinel phase during the charge process. This may attributed to the nonuniform sedimentation with the mixed addition method. Meanwhile, for the sample a, the cathodic peak of the cell below 2.75 V does not emerge. All the results indicate that the ordered addition method can guarantee the impurity of Li1.2Ni0.12Co0.12Mn0.56O2 because of the difference of the solubility product constant (K sp) of NiCO3, CoCO3, and MnCO3.
The rate performance for the Li/Li1.2Ni0.12Co0.12Mn0.56O2 cells is shown in Fig. 6. As can be seen, the discharge capacities of the sample a were higher than those of sample b at all different C rates. Moreover, the difference in capacity between samples a and b becomes greater as the discharge current rate is increased from 0.06 to 1 C. The good rate performance is attributed to the smaller particle size which shortens the migration path of lithium [17, 25]. However, further efforts are needed to improve the rate properties.
Figure 7 presents the cycling performance of the Li/Li1.2Ni0.12Co0.12Mn0.56O2 cells. The cells were charged/discharged at a 0.06 C rate over the first 30 cycles and at a 0.2 C rate over the following 70 cycles. As can be seen, when cycled at 0.06 C, the discharge of sample a goes up and down at a steady high capacity (260 mAh g−1), whereas the discharge of sample b has an unsteady lower capacity (190 mAh g−1). The sample a delivers a capacity retention of 78.9 % after 100 cycles (30 cycles for 0.06 C and 70 cycles for 0.2 C), which is substantially higher than that of the sample b (65.9 %). The improved electrochemical characteristics may be attributed to the enhanced structural stability reflected by the higher R value, and the better layered structure of sample a is shown in Fig. 2(a). As reported by Thackeray [30], high crystallinity is essential to obtain good electrochemical properties and to maintain its structural integrity during cycling. The slight fluctuation in the capacity versus the cycle number plot is attributed to variations in the room temperature of the laboratory in which the tests were conducted.
The differences in the electrochemical performance of the Li1.2Ni0.12Co0.12Mn0.56O2 cathode can be attributed to the differences in their physical and chemical properties such as the crystal structure and morphology. In this study, by using the ordered addition method with NH4HCO3 solution as the precipitation reagent, the physical and chemistry properties of the precursors and the Li1.2Ni0.12Co0.12Mn0.56O2 cathode material were effectively improved, which results in the superior physical and electrochemical properties for Li1.2Ni0.12Co0.12Mn0.56O2.
Conclusions
An improved carbonate precipitation method was employed to prepare the lithium-rich Li1.2Ni0.12Co0.12Mn0.56O2 cathode material. Carbonate precipitation was conducted using ordered addition method with NH4HCO3 solution as the precipitation reagent. The morphological and structural properties of the precursor and the final Li1.2Ni0.12Co0.12Mn0.56O2 cathode material were effectively improved by the ordered addition method. Electrochemical studies confirmed that the Li1.2Ni0.12Co0.12Mn0.56O2 synthesized by ordered addition method exhibited a higher capacity of 287.3 mAh g−1, and the capacity retention after 30 cycles was 90.5 %. The results in this work suggest a simple and effective way to synthesize the lithium-rich cathode for rechargeable lithium batteries.
References
Ellis BL, Lee KT, Nazar LF (2010) Chem Mater 22:691
Fergus JW (2010) J Power Sources 195:939
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Hackney SA (2006) Electrochem Commun 8:1531
Whittingham MS (2004) Chem Rev 104:4271
Croy JR, Kang SH, Balasubramanian M, Thackeray MM (2011) Electrochem Commun 13:1063
Marth SK, Nand J, Veith GM, Dudney NJ (2012) J Power Sources 199:220
Du K, Hu GR (2012) Chin Sci Bull (Chin Ver) 57:794
Zheng JM, Wu XB, Yang Y (2011) Electrochim Acta 56:3071
Lee DK, Park SH, Amine K, Bang HJ, Parakash J, Sun YK (2006) J Power Sources 162:1346
Park SH, Kang SH, Belharouak I, Sun YK, Amine K (2008) J Power Sources 77:177
Lim JH, Bang H, Lee KS, Amine K, Sun YK (2009) J Power Sources 189:571
Shin SS, Sun YK, Amine K (2002) J Power Sources 112:634
Kim JH, Sun YK (2003) J Power Sources 119–121:166
Zhang L, Muta T, Noguchi H, Wang X, Zhou M, Yoshio M (2003) J Power Sources 117:137
Zhao X, Cui Y, Xiao L, Liang H, Liu H (2011) Solid State Ionics 192:321
Yu L, Qiu W, Lian F, Liu W, Kang X, Huang J (2008) Mater Lett 62:3010
Chen Y, Xu G, Li J, Zhang Y, Chen Z, Kang F (2013) Electrochim Acta 87:686
Wang D, Belharouak I, Koenig GM Jr, Zhou G, Amine K (2011) J Mater Chem 21:9290
Wu F, Wang M, Su Y, Bao L, Chen S (2010) J Power Sources 195:2362
Manikandan P, Ananth MV, Kumar TP, Raju M, Periasamy P, Manimaran K (2011) J Power Sources 196:10148
Luo X, Wang X, Liao L, Wang X, Gamboa S, Sebastian PJ (2006) J Power Sources 161:601
Zhang B, Chen G, Xu P, Lv ZS (2007) Solid State Ionics 178:1230
Hong YS, Park YJ, Ryu KS, Chang SH (2005) Solid State Ionics 176:1035
Armstrong AR, Holzapfel M, Novák P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) J Am Chem Soc 128:8694
Wang J, Yuan G, Zhang M, Qiu B, Xia Y, Liu Z (2012) Electrochim Acta 66:61
Guo XJ, Li YX, Zheng M, Zheng JM, Li J, Gong ZL, Yang Y (2008) J Power Sources 184:414
Shojan J, Rao CV, Torres L, Singh G, Katiyar RS (2013) Mater Lett 104:57
Zhang J, Guo X, Yao S, Zhou W, Qiu X (2013) J Power Sources 238:245
Johnson CS, Li N, Lefief C, Vaughey JT, Thackeray MM (2008) Chem Mater 20:6095
Thackeray MM (1995) J Electrochem Soc 142:2558
Acknowledgments
This work was supported by the Hunan Provincial Innovation Foundation for Postgraduate, China, and the Major Science and Technology Project of Hunan Province, China (2011FJ1005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, Y., Yin, Z. & Li, X. An improved carbonate precipitation method for the preparation of Li1.2Ni0.12Co0.12Mn0.56O2 cathode material. Ionics 20, 163–168 (2014). https://doi.org/10.1007/s11581-013-0981-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0981-3