Abstract
The Li-rich layered Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19) cathode materials have been successfully synthesized through a one-pot facile co-precipitation route. The synthesized MCO3 (M = Mn0.6Ni0.2Co0.2) precursor mixing with Li2CO3 was annealed at 500 °C and calcinated at 900 °C. The morphology and structure of the samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The results indicate that the sample Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) exhibits the most outstanding electrochemical performance, which may be ascribed to uniform particle size and high crystallinity. It delivers an initial discharge-specific capacity of approximately 241.7 mAh g−1 with an initial coulombic efficiency of 70.3 % at a constant density of 25 mA g−1. A reversible discharge-specific capacity of approximately 207.2 mAh g−1 is still obtained after 100 cycles. The discharge capacities of nearly 226.8, 194.4, 158.9, 143.7, 116.8, 97.5, and 47.6 mAh g−1 can also be attained under 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C (1 C = 250 mA g−1), respectively. The Li-rich layered Li1.165Mn0.501Ni0.167Co0.167O2 will be a promising cathode material for advanced lithium-ion batteries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the contemporary of attaching great importance to green initiative and energy saving, rechargeable lithium-ion batteries have attracted much attention in the commercial applications of electric vehicles (EVs) and hybrid electric vehicles (HEVs) by virtue of their high energy density, high working voltage, no memory effect, long cycle life, and environmental benignity [1–7]. Notoriously, the cathode with low capacity and high cost makes the development of lithium-ion batteries hit a bottleneck (limits). Thus, searching for advanced cathode materials with excellent performance has been on the top of agenda and the focus of research in the field of lithium-ion batteries in recent years [8–11].
Currently, LiCoO2 is still the dominant cathode materials in the business market. Nonetheless, much effort has been made to explore an alternative cathode material to substitute for LiCoO2 on account of its toxicity, high cost, and insecurity [12, 13]. In this regard, Li-rich layered cathodes written as Li1 + x [M]1 − x O2 (M = Mn, Ni, Co, etc.; 0 < x < 1) have captured worldwide attention and interests, being a promising candidate for the novel cathode materials of lithium-ion batteries. This type of cathode material is also considered to be a nano-dimensional solid solution with rock salt structure between Li2MnO3 (C2/m space group) [14, 15] and LiMO2 (R-3m space group) [16] at different proportions, which is also formulated as xLi2MnO3 · (1 − x)LiMO2 [17, 18]. The Li2MnO3 component is conducive to stabilize the structure and improve the cycling performance. These materials display a unique framework of layered-layered structure, delivering a markedly high specific capacity of over 250 mAh g−1 in a wide voltage window of 2.0 to 4.8 V as compared to common cathodes [19–21]. Wang et al. [22] prepared Li1 + x Ni1/6Co1/6Mn4/6O2.25 + x/2 (0.1 ≦ x ≦0.7) cathode materials using a simple carbonate co-precipitation route, devoting to researching the structure, morphology, and electrochemical properties detailedly. Min et al. [23] adopted an electrospinning technique with different polymers to discuss the morphological effects on the electrochemical performance, leading to the formation of a fiber and flower-like nano-structured morphology and splendid performance. A hierarchical micro/nano-structured Li-rich layered 0.5Li2MnO3 · 0.5LiMn0.4Ni0.3Co0.3O2 materials obtained by Fu et al. [24] via a solvothermal method can still maintain a high-valued discharge capacity of 163.3 mAh g−1 when the rate is increased to 10 C (1 C = 200 mA g−1).
Despite the mentioned merits, these integrated materials suffer some unsolved drawbacks, such as low rate capabilities, large irreversible capacity fading, and poor conductivity. Aiming at solving these issues, some strategies, such as surface modification (coating with the positive materials, like carbon [20], oxides, phosphates [6], fluorides [25], etc.), doping [26] with conductive elements, ion substitution [27], or reduction in particle size, have been employed to optimize the morphology and improve the conductivity and cycle stability [23]. Zhang et al. [28] propose a PO4 3− polyanion doping for stabilizing the energy density of Li-rich layered Li(Li0.17Ni0.20Co0.05Mn0.58)O2 oxides, minimizing the local structure change and presenting excellent energy density retention. Lithium-rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 was prepared by Xue et al. [29] through integrating a fast co-precipitation method with polyaniline modification, presenting a high rate capacity of 198.6 mAh g−1 at 10 C. Hou et al. [30] have synthesized festoon-like Li1.13[Ni0.233Mn0.534Co0.233]0.87O2 cathode materials with higher capacity and superior rate capability by adopting hexadecyl trimethyl ammonium bromide (CTAB) as a surfactant, which effectively alleviate the layered/spinel structure change during cycling. As a matter of fact, lithium content is also a key factor, having great impact on the morphology and electrochemical properties of cathode materials. Lithium content has an effect on the Li+ insertion/extraction during the charging-discharging process, thereby influencing the subsequent high-temperature sintering process. Theoretically, the excess of lithium is required due to the Li volatilization in the reaction process. But in fact, metal ions are inevitably wasted to some extent in the experimental early stage, corresponding to the increase of the proportion of lithium in disguise.
In this work, the Li-rich layered Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19) cathode materials were successfully synthesized via one-pot facile co-precipitation route and a subsequent calcination procedure. Here, the valence states of manganese, nickel, and cobalt are +4, +2, and +3, respectively [31–34]. What is more, it should be pointed out that the co-precipitation method has many distinct advantages, such as simple process, controllable conditions, short synthesizing cycle, high yield, low cost, and so on, which is much popular and widely applied. Herein, the influence of lithium content on the morphology and electrochemical properties of the batteries was briefly but systematically investigated. The carbonate precursor showed an excellent spherical shape with uniform particle size. The Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) cathode material has an integrated structure and thus exhibits outstanding electrochemical performance. A high discharge-specific capacity of 241.7 mAh g−1 was acquired in the initial charge-discharge cycling at a current density of 25 mA g−1. Moreover, the discharge capacity of 213.5 mAh g−1 is still remained after 100 cycles. Such excellent performance is mainly due to the uniform and small particle size, which is in favor of the Li+ extraction/insertion during the charge/discharge process.
Experimental
Synthesis of LMNCO materials
All chemicals (Sigma Aldrich) were of commercially available analytical grade and were used without further purification. Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19) cathode materials were synthesized via one-pot facile co-precipitation method and two-step calcinations subsequently. 1.0 mol L−1 metal acetate salt solutions (Mn(CH3COO)2 · 4H2O, Ni(CH3COO)2 · 4H2O, and Co(CH3COO)2 · 4H2O in a stoichiometric ratio of 3:1:1) and 1.0 mol L−1 Na2CO3 solutions were simultaneously pumped into the NH4HCO3 solutions (0.3 mol L−1) in a continuous stirred reactor with a stirring speed of 800 rpm. After stirring for 6 h, the obtained precipitation was filtered, rinsed thoroughly with deionized water, and then dried at 60 °C overnight in a vacuum oven. Afterwards, the obtained manganese-nickel-cobalt carbonate precursor was thoroughly mixed with Li2CO3 in stoichiometric amounts. After thorough grinding, the mixture was annealed at 500 °C for 5 h and then calcinated at 900 °C for 12 h in air atmosphere. The final materials were gained by quenching them to room temperature and ground for the fabrication of the electrode.
Material characterization
The phase analysis of the as-prepared products were characterized by X-ray diffraction (XRD; PANalytical X’Pert PRO, Cu Kα radiation, λ = 0.15406 nm) with Cu Kα radiation in a 2θ range from 10° to 80° at a scan rate of 5°min−1. The particle morphology and crystalline structure of the as-prepared products were performed by scanning electron microscopy (SEM; ZEISS ULTRA 55) and transmission electron microscopy (TEM; JEM-2100HR), accompanying with the high resolution transmission electron microscopy (HRTEM).
Electrochemical performance test
The electrochemical performance was carried out using coin-type half cells (CR2430) assembled in an argon-filled glove box. The working electrodes were made by casting the slurry with the composition of 80 wt% active materials, 10 wt% Super-P (conductive agent), and 10 wt% PVDF (the binder). The slurry was coated on aluminum foil with thickness of about 10 μm and then dried at 60 °C for 12 h in a vacuum. Subsequently, the electrodes were pressed and punched (φ = 18 mm), while the metallic lithium foil served as the counter and reference electrodes. 1.0 mol L−1 LiPF6 dissolved in ethylene carbonate (EC)—diethyl carbonate (DEC)—ethylene methyl carbonate (EMC) (1:1:1 in volume) was used as the electrolyte and a polypropylene microporous film (Cellgard 2400) as the separator.
The charge-discharge tests were galvanostatically performed on a LAND battery program-control test system between 2.0 and 4.65 V at the charge-discharge rates from 0.1 C to 10 C (1 C = 250 mA g−1) at room temperature. Electrochemical impedance spectroscopy (EIS) of the cells was conducted with a frequency range from 105 to 0.01 Hz at a complete charge state. Cyclic voltammograms (CV) were carried out on an electrochemical workstation (Solartron Analytical 1470E) in the potential window of 2.5–4.65 V. The potentials in this work are in reference to the Li/Li+ couple.
Results and discussion
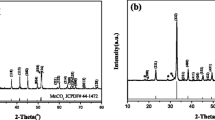
The Li-rich layered cathode xLi2MnO3 · (1 − x)LiMn1/3Ni1/3Co1/3O2 is a nano-dimensional solid solution between monoclinic layered Li2MnO3 with C2/m space group [14, 15] and rhombohedral LiMO2 with R-3m space group [16]. The atomic radius of Mn, Ni, and Co is very close to each other, which has little influence on the crystal structure. The XRD patterns of the precursor and samples are shown in Fig. 1. As can be seen from the XRD patterns of the precursor in Fig. 1a, the peaks are almost in accordance with the PDF card of MnCO3 (PDF#44–1472), but deviate seriously from that of NiCO3 (PDF#12–0771) and CoCO3 (PDF#11–0692). That is because the ratio of the amount of Mn, Ni, and Co is fixed at 3:1:1 in our study. Comparatively speaking, the amount of Mn is relatively higher than that of Ni and Co. Besides, it illustrates the precursor is a kind of carbonate. Figure 1b shows the XRD patterns of the active materials. The diffraction peaks except for the low intensity part within 20° and 25° reflect a hexagonal α-NaFeO2 layered structure with R-3m space group [13, 35, 36]. The small superlattice peaks observed between 20° and 25° represent the existence of monoclinic Li2MnO3 phase with C2/m space group [37], conforming to the LiMn6 cation arrangement in the transition metal layers of the extremely small Li2MnO3 regions [9]. Obviously, the weak peaks become symmetrical and sharp with the decrease of x. The distinct splitting peaks of (006)/(102) and (108)/(110) indicate that the Li-rich cathode materials exhibit a well-formed layered hexagonal structure and high crystallinity. No any impurity peak is detected, suggesting the high purity of the oxides. With respect to the formation of Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19), all samples present small differences in the sharpness and position of the peaks. Besides, as can be seen from Fig. 1b, the peaks shift slightly toward higher 2θ values from x = 0.14 to x = 0.19, which may be ascribed to the bigger ion radius of Li+ (0.68 Å) than Mn4+ (0.60 Å) and Co3+ (0.63 Å) [38].
The morphologies of the precursor and composites are investigated by SEM in Fig. 2. As shown in Fig. 2a, the precursor presents a spherical shape with small particle size (2 μm). Figure 2b–d show the morphologies of the three samples (x = 0.14, 0.165, 0.19), respectively, after high-temperature solid-state reaction. Apparently, the spherical shape is well maintained for the sample Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) after calcination process owning to surface force, distinctly accompanying with the formation of layered structure, while the shapes of the Li1.14Mn0.516Ni0.172Co0.172O2 (x = 0.14) and Li1.19Mn0.486Ni0.162Co0.162O2 (x = 0.19) are irregular but have the trend of agglomerating to spheres. The average diameters of the primary particles of the three samples (x = 0.14, 0.165, 0.19) are determined to be about 200–600, 100–300, and 200–600 nm, respectively. The secondary particles of the Li1.165Mn0.501Ni0.167Co0.167O2 have a bigger size (about 1.8 μm) owing to agglomeration. Comparatively speaking, the Li1.165Mn0.501Ni0.167Co0.167O2 is well shaped with smooth surfaces and sharp edges and has uniform particle size distribution, which is believed to be beneficial to shorten the Li+ diffusion distance and increase specific surface areas and facilitate the Li insertion/extraction; thus, it is advantageous for the depth of the charge and discharge and enhance the electrochemical reaction activity.
The microstructure of the as-prepared Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19) was explored by transmission electron microscopy (TEM). The TEM images (Fig. 3a–c) could indicate the morphology and particle size. As can be seen from Fig. 3b, the TEM image of the Li1.165Mn0.501Ni0.167Co0.167O2 presents regularly agglomerated spherical shapes with a size of 1.8 μm in diameter, which is consistent with the SEM images of Fig. 2c. Figure 3d–f shows the HRTEM images of the selected area in Fig. 3a–c, respectively. The HRTEM image of the Li1.165Mn0.501Ni0.167Co0.167O2 in Fig. 3e exhibits distinct parallel lattice fringes, while the lattice fringes of Li1.14Mn0.516Ni0.172Co0.172O2 and Li1.19Mn0.486Ni0.162Co0.162O2 cannot distinguish clearly in Fig. 3d, f. According to our previous studies [30], the atomic arrangement tends to be in good order due to atomic diffusion during the high-temperature calcination process, forming a complete crystal structure. Relatively speaking, it is considered that Li1.165Mn0.501Ni0.167Co0.167O2 exhibits better crystallization. About 0.47 nm of the interplanar spacing between two lattice fringes can be measured, which corresponds to (003) planes from rhombohedral phase with R-3m space group or (001) planes from monoclinic phase with C2/m space group [22]. It is consistent with the above XRD analysis.
Figure 4a presents the cycling performance of the as-prepared materials at a current density of 25 mA g−1 (0.1 C) between 2.0 and 4.65 V. All of the three samples display excellent cycle performance with high capacity retention. As shown in Fig. 4a, the charge/discharge capacity has a gradually decreased trend for the following cycles. Moreover, the coulombic efficiencies of all samples are mostly over 98.0 % during the following cycles. Herein, the Li1.14Mn0.516Ni0.172Co0.172O2 (x = 0.14) and Li1.19Mn0.486Ni0.162Co0.162O2 (x = 0.19) deliver an initial discharge capacity of 194.7 mAh g−1 and 198.4 mAh g−1, respectively, revealing the corresponding initial coulombic efficiency of 63.6 and 69.6 %. And the discharge capacities of 194.0 and 179.2 mAh g−1 can be obtained after 100 cycles for the Li1.14Mn0.516Ni0.172Co0.172O2 and Li1.19Mn0.486Ni0.162Co0.162O2, respectively. Comparatively speaking, the Li1.165Mn0.501Ni0.167Co0.167O2 delivers the highest initial charge and discharge capacity of 344.0 and 241.7 mAh g−1, respectively, indicating a high initial coulombic efficiency of 70.3 %. Moreover, its discharge capacity of 207.2 mAh g−1 is still maintained after 100 cycles. Such high specific capacity and cycling stability are mainly attributed to well-formed structure and uniform particle size, making for the Li+ extraction and shortening of the Li+ diffusion distance. In addition, it can be obviously seen from the figure that the capacity of the Li1.19Mn0.486Ni0.162Co0.162O2 increases gradually in the first 10 cycles until reaching a stable point, which may be caused by the electrochemical activation process of the battery.
Figure 4b reveals the rate capability of the as-prepared materials from 0.1 C to 10 C between 2.0 and 4.65 V. For the Li1.14Mn0.516Ni0.172Co0.172O2, the discharge capacities of 210.8, 190.2, 161.0, 139.9, 111.1, 87.7, and 52.2 mAh g−1 can be obtained at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C, respectively. The delivered capacities of the Li1.19Mn0.486Ni0.162Co0.162O2 at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C are about 190.8, 172.4, 142.2, 110.2, 80.4, 64.2, and 46.2 mAh g−1, respectively. With respect to the Li1.165Mn0.501Ni0.167Co0.167O2, the discharge capacities of 226.8, 194.4, 158.9, 143.7, 116.8, 97.5, and 47.6 mAh g−1 are attained at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C, respectively. And when the current goes back to 0.1 C, the discharge capacity reaching up to 224.7 mAh g−1 still can be achieved for the Li1.165Mn0.501Ni0.167Co0.167O2. Therefore, the Li1.165Mn0.501Ni0.167Co0.167O2 exhibits better rate capability due to its small primary particle size and large specific surface areas, decreasing ion diffusion distance and contributing to the Li+ extraction during the charge/discharge process. All samples reflect a gradual loss of the capacity with the increasing of the current densities. The decrease is mainly ascribed to the lattice disorders at high rates [9]; thus, the extraction of Li+ is hindered, producing high polarization and capacity attenuation.
The initial charge-discharge curves of three samples at 0.1 C between 2.0 and 4.65 V are displayed in Fig. 4c. Two charge plateaus in the initial charge process can be seen from the graph. The first one at 3.9–4.5 V is relative to the de-lithiation of LiMO2 phase accompanied with the oxidation of Ni2+→Ni4+ and Co3+→Co4+. The second one, which is located above 4.5 V, embodies the extraction of Li2O from the layered Li2MnO3 lattice producing a high irreversible charge capacity simultaneously [22]. As can be clearly observed, the charge and discharge capacities in the first cycle in Fig. 4c are completely consistent with what Fig. 4a depicts, equally demonstrating the outstanding electrochemical performance of the Li1.165Mn0.501Ni0.167Co0.167O2 compared with the Li1.14Mn0.516Ni0.172Co0.172O2 and Li1.19Mn0.486Ni0.162Co0.162O2. Apparently, an abnormal plateau at 2.6 V is newly formed for the Li1.14Mn0.516Ni0.172Co0.172O2 and Li1.165Mn0.501Ni0.167Co0.167O2, which is usually considered to be a sign of spinel formation [39].
Electrochemical impedance spectroscopy (EIS) has been measured to deeply study the electrochemical performance. The Nyquist plots of EIS spectra for these composites at the charge state of 4.65 V after 100 cycles are shown in Fig. 5a. The plots consist of a semicircle in the high frequency, a semicircle in the intermediate frequency, and a tail in the low frequency. Generally, the high-frequency semicircle is assigned to the solid electrolyte interface (SEI) resistance (R f) of Li+ diffusion in the passive SEI layer, which is formed due to the oxidation of electrolyte accompanying the formation of Li2CO3 during the electrochemical activation of Li2MnO3 [17, 40]. The intermediate-frequency semicircle is ascribed to the charge transfer electrochemical resistance (R ct) of the electrode/electrolyte interface. Nevertheless, the lattice rearrangement process during cycling can result in a disordered surface structure, and thus, the charge transfer especially for Li+ suffers obstructions [17]. The low-frequency tail is associated with the Warburg impedance (Z w) of the Li+ diffusion in the solid-phase active material. When the slope of this line is bigger, the impedance is smaller on the contrary, suggesting an easier diffusion. Comparing the three magnified curves in the magnified inset of Fig. 5a, the Li1.14Mn0.516Ni0.172Co0.172O2 and Li1.165Mn0.501Ni0.167Co0.167O2 perform the smaller radius of the high frequency far less than that of the Li1.19Mn0.486Ni0.162Co0.162O2, illustrating a smaller interfacial film impedance and easier Li+ diffusion. Figure 5b shows the equivalent circuit of the Nyquist plots. R e, CPE f, and CPE ct stand for the solution impedance, non-ideal capacitance of the surface layer, and non-ideal capacitance of the double layer, respectively [9, 13]. The fitting results of R e, R f, and R ct are listed in Table 1. Obviously, the Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) owns the lowest impedance, suggesting superior electrochemical performance as compared with the other two samples.
Based on the above-mentioned analysis, the Li1.165Mn0.501Ni0.167Co0.167O2 exhibits the best electrochemical performance; thus, its cyclic voltammetry (CV) test is performed to acquire deep insights into the electrochemical behavior during the charge/discharge process. Figure 6a displays the CV curves of the Li1.165Mn0.501Ni0.167Co0.167O2 in the first three cycles at a scan rate of 0.1 mV s−1 in the voltage range of 2.5–4.65 V. The curves in the initial cycle are sharp, symmetrical, and accordant with what are shown in the initial charge-discharge curves. Two main sharp oxidation peaks in the charging state can be discerned in the initial cycle, located at 4.14 and 4.63 V, respectively. The first oxidation peak located at 4.14 V corresponds to the extraction of Li+ from the LiMO2 structure along with the oxidation of Ni2+ to Ni4+ and Co3+ to Co4+. It is worthy to point out that the excessive Li will begin to extract from the Li2MnO3 phase when charged above 4.4 V [13], so the activation peaks at the high potential 4.63 V, which become weaker with the increase of cycle number, are ascribed to the activation of Li2MnO3 component and the loss of oxygen. What is more, three reduction peaks are discernable at 3.3, 3.8, and 4.4 V. Therein, the reduction peak at 3.3 V can be clearly observed especially in the second and third cycles, which is attributed to the reduction reaction of Mn4+ due to the loss of oxygen in the first charge. The other two reduction peaks at 3.8 and 4.4 V are related to the Li+ reinsertion to LiM1 − x O2 phase accompanying the reduction of Ni4+ to Ni2+ and Co4+ to Co3+ [41]. The reduction peaks at 3.8 and 4.4 V are highly overlapped, demonstrating a fairly well cyclic reversibility. The redox peaks have the trend of shifting to lower potential, which is due to the modification of the bulk material structure after the first cycle or the oxidation of Co3+ to Co4+ and part of the electrolyte [42, 43], and it means a smaller potential difference between oxidation peak and reduction peak, indicating the smaller polarization effect.
CV curves of the Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) between 2.5 and 4.65 V: at the scan rate of 0.1 mV s−1 (a); at different scan rates of 0.1, 0.2, 0.4, and 0.8 mV s−1 (b). The current of peaks (i p) vs the square root of scanning rates (υ 1/2) plots (c) of the Li1.165Mn0.501Ni0.167Co0.167O2
The improved rate capability of the Li1.165Mn0.501Ni0.167Co0.167O2 can also be explained by means of CV analysis. Figure 6b shows the CV curves of the Li1.165Mn0.501Ni0.167Co0.167O2 at different scan rates between 2.5 and 4.65 V. As clearly indicated, the oxidation peaks move to higher potentials, while the reduction peaks move to lower potentials with the increase of scan rate. In addition, the area surrounded by the oxidation peak and reduction peak is significantly increased with the increase of scan rate. Figure 6c shows the current of peaks (i p) vs the square root of scanning rates (υ 1/2) plots of the Li1.165Mn0.501Ni0.167Co0.167O2. The lithium-ion diffusion coefficient of the Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) can be calculated as the following equation:
where n is the electron transfer numbers of redox reactions (n = 1), A is the effective surface area of the electrode (2.54 cm2), D Li+ is the lithium-ion diffusion coefficient, C is the molar concentration of lithium-ion (9.8975 × 10−2 mol cm−3), and υ is the scan rates. It can be calculated for the Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) that the lithium-ion diffusion coefficient of the oxidation and reduction peaks are 8.30946 × 10−9 and 3.27828 × 10−9 cm2 s−1, respectively. Besides, the R-square of the fitting lines and the accuracy rating of the calculated diffusion coefficient of oxidation and reduction peaks are 0.99603 and 0.98914, respectively, indicating a low error and high reliability of the fitting data.
Conclusions
The Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 (x = 0.14, 0.165, 0.19) cathode materials were successfully synthesized by a one-pot facile co-precipitation method. The impact of lithium content on the electrochemical properties was mainly discussed. It comes to a conclusion that the Li1.165Mn0.501Ni0.167Co0.167O2 (x = 0.165) exhibits an outstanding electrochemical performance in terms of cycling stability and rate capability. Its charge- and discharge-specific capacity of 344.0 and 241.7 mAh g−1 can be achieved in the first cycle at 0.1 C (1 C = 250 mA g−1), and the discharge capacity of 207.2 mAh g−1 is still maintained after 100 cycles. Furthermore, the discharge capacities of approximately 226.8, 194.4, 158.9, 143.7, 116.8, 97.5, and 47.6 mAh g−1 can also be attained at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C, respectively. The superior performance can be ascribed to small particle size and good crystallinity, which can shorten the Li+ diffusion distance and increase specific surface areas, facilitating the Li insertion/extraction and enhancing the electrochemical reaction activity. In summary, the Li-rich layered Li1.165Mn0.501Ni0.167Co0.167O2 cathode material with excellent performance will be appealing and alternative for large-scale commercial applications in advanced lithium-ion batteries. On the side, the long-term project concerning lithium content is still urgent to in-depth study to further improve the electrochemical performance of lithium-ion batteries.
References
Ye DL, Wang B, Chen Y, Han G, Zhang Z, Hulicova-Jurcakova D, Zou J, Wang LZ (2014) J Mater Chem A 2:18767–18774
Noh HJ, Youn S, Yoon CS, Sun YK (2013) J Power Sources 233:121–130
Harry KJ, Hallinan DT, Parkinson DY, MacDowell AA, Balsara NP (2014) Nat Mater 13:69–73
Yao LM, Hou XH, Hu SJ, Ru Q, Tang XQ, Zhao LZ, Sun DW (2013) J Solid State Electrochem 17:2055–2060
Yu DYW, Prikhodchenko PV, Mason CW, Batabyal SK, Gun J, Sladkevich S, Medvedev AG, Lev O (2013) Nat Commun 4:2922–2929
Yao YL, Liu HC, Li GC, Peng HR, Chen KZ (2013) Electrochim Acta 113:340–345
Li M, Hou XH, Sha YJ, Wang J, Hu SJ, Liu X, Shao ZP (2014) J Power Sources 248:721–728
Zheng JM, Wu XB, Yang Y (2011) Electrochim Acta 56:3071–3078
Shi SJ, Tu JP, Tang YY, Yu YX, Zhang YQ, Wang XL (2013) J Power Sources 221:300–307
Yu HJ, Zhou HS (2013) J Phys Chem Lett 4:1268–1280
Ebin B, Lindbergh G, Gürmen S (2015) J Alloys Compd 620:399–406
Li J, Klöpsch R, Stan MC, Nowak S, Kunze M, Winter M, Passerini S (2011) J Power Sources 196:4821–4825
Shi SJ, Tu JP, Tang YY, Zhang YQ, Wang XL, Gu CD (2013) J Power Sources 240:140–148
Riou A, Lecerf A, Gerault Y, Cudennec Y (1992) Mater Res Bullet 27:269–275
Strobel P, Lambert-Andron B (1988) J Solid State Chem 75:90–98
Akimoto J, Gotoh Y, Oosawa Y (1998) J Solid State Chem 141:298–302
Zhang QG, Peng TY, Zhan D, Hu XH (2014) J Power Sources 250:40–49
Gu M, llias Belharouak, Zheng JM, Wu HM, Xiao J, Genc A, Amine K, Thevuthasan S, Baer DR, Zhang JG, Browning ND, Liu J, Wang CM (2013) ACS Nano 7:760–767
Yang XK, Wang XY, Zou GS, Hu L, Shu HB, Yang SY, Liu L, Hu H, Yuan H, Hu BN, Wei QL, Yi LH (2013) J Power Sources 232:338–347
Deng YH, Liu SQ, Liang XX (2013) J Solid State Electrochem 17:1067–1075
Miao XW, Yan Y, Wang CG, Cui LL, Fang JH, Yang G (2014) J Power Sources 247:219–227
Wang J, Yuan GX, Zhang MH, Qiu B, Xia YG, Liu ZP (2012) Electrochim Acta 66:61–66
Min JW, Kalathil AK, Yim CJ, Im WB (2014) Mater Charact 92:118–126
Fu F, Deng YP, Shen CH, Xu GL, Peng XX, Wang Q, Xu YF, Fang JC, Huang L, Sun SG (2014) Electrochem Commun 44:54–58
Liu H, Du CY, Yin GP, Song B, Zuo PJ, Cheng XQ, Ma YL, Gao YZ (2014) J Mater Chem A 2:15640–15646
Wang J, Lin WQ, Wu BH, Zhao JB (2014) Electrochim Acta 145:245–253
Li HX, Fan LZ (2013) Electrochim Acta 113:407–411
Zhang HZ, Qiao QQ, Li GR, Gao XP (2014) J Mater Chem A 2:7454–7460
Xue QR, Li JL, Xu GF, Zhou HW, Wang XD, Kang FY (2014) J Mater Chem A 2:18613–18623
Hou XH, Zou XL, Huang YL, Hu SJ, Ru Q, Gao YM (2014) RSC Adv 4:29534–29541
Li Q, Li GS, Fu CC, Luo D, Fan JM, Li LP (2014) ACS Appl Mater Interfaces 6:10330–10341
Zhang J, Lu QW, Fang JH, Wang JL, Yang J, NuLi YN (2014) ACS Appl Mater Interfaces 6:17965–17973
Zhang XH, Luo D, Li GS, Zheng J, Yu C, Guan XF, Fu CC, Huang XD, Li LP (2013) J Mater Chem A 1:9721–9729
Song BH, Lai MO, Liu ZW, Liu HW, Lu L (2013) J Mater Chem A 1:9954–9965
Toprakci O, Toprakci HAK, Li Y, Ji LW, Xue LG, Lee H, Zhang S, Zhang XW (2013) J Power Sources 241:522–528
Gao M, Lian F, Liu HQ, Tian CJ, Ma LL, Yang WY (2013) Electrochim Acta 95:87–94
Zhao TL, Chen S, Li L, Zhang XF, Chen RJ, Belharouak I, Wu F, Amine K (2013) J Power Sources 228:206–213
Tang ZH, Wang ZX, Li XH, Peng WJ (2012) J Power Sources 208:237–241
Song BH, Liu HW, Liu ZW, Xiao PF, Lai MO, Lu L (2013) Sci Rep 3:3094–3106
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) J Am Chem Soc 133:4404–4419
Manikandan P, Periasamy P, Jagannathan R (2014) RSC Adv 4:40359–40367
Wei X, Zhang SC, Du ZJ, Yang PH, Wang J, Ren YB (2013) Electrochim Acta 107:549–554
Li HF, Pang J, Yin YP, Zhuang WD, Wang H, Zhai CX, Lu SG (2013) RSC Adv 3:13907–13914
Acknowledgments
This work was financially supported by the National Science Foundation of China (NSFC, Nos. 51201066 and 51171065), the Natural Science Foundation of Guangdong Province (No. S2012020010937), Science and Technology Project Foundation of Zhongshan City of Guangdong Province of China (No. 20123A326), and College Students’ Innovative Entrepreneurial Training Program of South China Normal University (No. 2014058).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, S., Hou, X., Lin, Z. et al. One-pot facile co-precipitation synthesis of the layered Li1 + x (Mn0.6Ni0.2Co0.2)1 − x O2 as cathode materials with outstanding performance for lithium-ion batteries. J Solid State Electrochem 20, 95–103 (2016). https://doi.org/10.1007/s10008-015-3006-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3006-5