Abstract
Solid polymer electrolyte films based on hydroxypropyl methylcellulose (HPMC) complexed with sodium iodide (NaI) were prepared using solution cast method. The dissolution of the salt into the polymer host and the structural properties of pure and complexed HPMC polymer electrolyte films were confirmed by X-ray diffraction (XRD) studies. XRD results revealed that the amorphous domains of HPMC polymer matrix were increased with increase in NaI salt concentration. The degree of crystallinity was found to be high in pure HPMC samples. The thermal properties were studied using differential scanning calorimetry (DSC). DSC results revealed that the presence of NaI in the polymer matrix increases the melting temperature; however, it is observed that fusion heat is high for pure HPMC films. The variation of film morphology was examined by scanning electron microscopy. Fourier transform infrared spectral studies revealed vibrational changes that occurred due to the effect of dopant salt in the polymer. Direct current conductivity was measured in the temperature range of 313–383 K. The magnitude of electrical conductivity was found to increase with the increase in salt and temperature concentration. The data on the activation energy regions (regions I and II) indicated the dominance of ion-type charge transport in these polymer electrolyte films. The composition HPMC:NaI (5:4) is found to exhibit the least crystallinity and the highest conductivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of polymeric systems with high ionic conductivity is one of the main objectives in polymer science. Ion-conducting polymer electrolytes have many advantages such as flexibility, ease of processing into thin films of large surface area, and their potential applications in solid-state electrochemical devices [1–4]. Most of the studies in this field is reported on poly(ethylene oxide) (PEO)-based polymer electrolytes using alkali salts [5–8]. PEO is known to solvate a large number of ionic salts and form stable complexes to give high ionic conductivity. Though the widely studied polymer continues to be PEO and its modifications, there will be a liquid–solid coexistence regime in the phase diagram of many PEO–metal salt complex systems above ambient temperature [9]. To overcome this drawback, several novel polymers like poly(propylene oxide) (PPO), poly(methylmethacrylate), and poly(vinyl alcohol) were utilized for the purpose of designing polymer electrolyte systems [10]. Solid-state lithium polymer batteries are also most widely studied and preferred over others due to their high energy densities good recyclability, reliability, and safety [11, 12]; but recent studies have explored lithium-ion conducting systems for their potential use in solid-state batteries with high cell voltages and energy densities.
Many research articles, which incorporates sodium ion in its polymeric system for its application as electrolyte materials in sodium batteries, are available [13–16]. There also exists some sodium ion-conducting polymer electrolytes based on PEO and PPO complexed with NaSCN, sodium iodide (NaI), NaClO3, NaPF4, NaYF4, NaCF3SO4, NaNO3, and NaClO4 [15, 17] which has been reported for its application as electrolyte materials in sodium batteries [18]. In the present study, hydroxypropyl methylcellulose (HPMC) polymer electrolytes were prepared by incorporating NaI as the doping salt. Relatively small number of studies has been reported on the development of HPMC-based polymer electrolyte systems. HPMC polymer has been chosen because of its appealing properties. HPMC is a biopolymer, ecofriendly, and water soluble [19–22]. Its ability to dissolve high concentrations of a wide variety of metal salts and its charge storage capacity, which can be influenced by the addition of inorganic salt like NaI, provides good amorphous and compatible nature. It has a semicrystalline nature which occurred in two phases (amorphous and crystalline). The amorphous phase enhances higher ionic conduction in the system; meanwhile, the crystalline phase provides strong mechanical support to the polymer electrolytes. Sodium is much more abundant and has a cheaper cost than lithium. The softness of sodium metal promotes the stability of electrode–electrolyte interface in solid-state ionic devices [21]. Keeping these aspects in view, this study has been undertaken and efforts have been made to form stable polyelectrolyte complexes. Several experimental techniques like X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), and direct current (DC) electrical conductivity were carried out to characterize these polymer electrolyte films.
Experimental
Materials and preparation of polymer electrolyte films
Pure HPMC and various compositions of complexed films of HPMC with NaI salt were prepared by solution cast method using double-distilled water as solvent. Hydroxypropyl methylcellulose was purchased from Loba Chemicals, India and sodium iodide was purchased from S. D. Fine Chemicals, India. To prepare the polymer electrolyte films, HPMC polymer was blended with inorganic salt of NaI. Here, 5 g (weight ratio) of HPMC is dissolved in 100 ml double-distilled water and desired amounts of NaI (1–4 %) in the weight ratio was added and stirred continuously for 8 h. The completely soluble homogeneous solution was filtered through Whatmann no. 41 filter paper and degassed. Then, the clear HPMC:NaI solution was poured on to a clean and leveled glass plates (30 × 40 cm) to evaporate at room temperature for 5 days and peeled off from the glass plate. The final films were vacuum dried thoroughly and stored in desiccators to avoid the absorption of moisture.
NaI is hygroscopic in nature and the higher concentration of NaI has the tendency to absorb more moisturizer. However, HPMC:NaI (5:5) polymer films were also prepared and was found that with increasing concentration of NaI, the film is shifting towards an amorphous structure and the films obtained are not stable and are not with uniform thickness. Hence, the optimal doping with NaI is carried out only up to 4 % and polymer electrolyte films HPMC:NaI (5:1, 5:2, 5:3, and 5:4) were prepared.
Instrumentation
The XRD studies of the films were made with an X-ray source with CuKα radiation of wavelength 1.5406 Å. The surface morphology of these polymer films was observed using JEOL 840, resolution at 20 kV, and 10 nm SEM. The samples were gold coated using the sputter coater at 10 mA current under 10−2 Torr vacuum for 3 min before imaging. FTIR spectra of these films were recorded using Thermo-Nicolet 6700. The measurements were taken over a wave number range of 400–4,000 cm−1. The DSC measurements have been taken in the temperature range of 30–200 °C with the help of Differential Scanning Calorimeter Model METTLER-TOLEDO DSC1 thermal analysis system at a heating rate of 10 °C per minute. The temperature-dependent DC conductivity of the reported polymer electrolyte samples were measured in the temperature range of 313–383 K using Keithley Electrometer (Model 617). The disk sample (13 mm in diameter) was sandwiched between the finely polished stainless steel electrodes. Silver paste is deposited on both sides of the sample for good electrical contact. The bulk resistance is determined from the intersection of the high frequency semicircle with the real axis in the complex impedance plots. The electrical conductivity is calculated using the formula
where ‘t’ is the thickness of the sample in millimeter, ‘R b ’ is the resistance in megaohm, and ‘A’ is the area of the sample in square millimeter.
Results and discussion
X-ray diffraction studies
X-ray diffraction is most useful for the determination of the nature of the sample, phase identification, and quantitative identification of the mixture of phases, particle size analysis, etc. The ionic conductivity in a polymer electrolyte is determined by the amorphous nature of the polymer; hence, it is necessary to determine the crystalline and amorphous nature of the polymer electrolyte films.
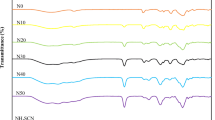
In Fig. 1, pure HPMC shows a broad peak at 2θ = 21.26° (d spacing = 4.175 Å) indicating its semicrystalline polymer nature. This diffraction peak is shifted to 21.42° (d spacing = 4.14 Å) in 5:1 system and almost at 22.5° in other complexes. There is a nucleation of crystalline order which gives an additional Bragg-like reflection almost at 2θ = 27.83° (d spacing = 3.20 Å) for pure HPMC and is shifted to around 29° in all other complexed systems with broad peaks. The mentioned peaks are less intense and are almost found to be disappearing in the polymer electrolyte systems indicating that the addition of NaI salt causes a decrease in the degree of crystallinity and a simultaneous increase in the amorphicity of the HPMC complex. Absence of peaks corresponding to the NaI salt in these complexes indicates that the inorganic salt is thoroughly mixed with the host polymer matrices. No sharp peaks were observed for the higher concentration of NaI salt in the polymer suggesting the dominant presence of the amorphous phase [23]; therefore, it may be confirmed that complexation has taken place in the amorphous phase. From Table 1, it is clear that the percentage of crystallinity decreases with addition of NaI salt and values are in good agreement with the interpreted results from XRD patterns. The percentage of the degree of crystallinity (X c ) was determined from the ratios of the area under the crystalline peak and the respective halos using the method [24, 25]:
where A c and A a are the area of crystalline and amorphous (halo) regions, respectively.
Morphological studies
Scanning electron microscopy is often used to study the compatibility between the various components of the polymer electrolytes through the detection of phase separation and interfaces [26, 27]. The compatibility of the polymer matrix and the inorganic dopants has great influence on properties like mechanical, thermal, and ionic conductivity of the polymer electrolyte systems. The SEM of the pure HPMC and HPMC:NaI (5:1) and (5:4) polymer electrolytes shown in Fig. 2 is of uniform type but has different degrees of roughness. The SEM of pure HPMC films exhibit no features attributable to any crystalline morphology, so the semicrystallinity of HPMC is likely to be submicroscopic in nature. The increase in the degree of roughness and dense structure with the increase of NaI salt concentration indicates the segregation of the dopant in that host polymer matrix. The two-phase microstructure in the SEM image reflects the phase separation at different concentrations (5:1, 5:4) of NaI salt. Therefore, morphological studies clearly indicate the phase segregation phenomenon in these complexed polymer electrolyte systems.
FTIR spectral studies
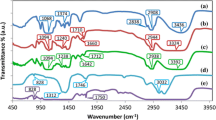
The FTIR spectra of pure HPMC and NaI complexed HPMC of different compositions (5:1, 5:4) are shown in Fig. 3. The following changes in the spectral features have been observed after comparing the spectrum of complexed HPMC with that of pure HPMC and NaI. The absorption band in the 3,498–3,996 cm−1 regions is due to the intermolecular hydrogen-bonded O–H stretching frequency of HPMC which is shifted to 3,496–3,585 and 3,762 cm−1 in the 5:1 and 5:4 NaI salt-complexed HPMC films, respectively. In addition to this, the stretching of methyl and hydroxypropyl showed an absorption band at 2,900 cm−1 in pure HPMC and is shifted to 2,961 and 3,110 cm−1, respectively. The C–H, OCH, and CCH asymmetric bending vibration of the methyl group in CH3O in pure HPMC exhibited absorption at 1,500–1,450 cm−1 in the complexed films, respectively. The IR peak assignments are listed in Table 2. Notably, the deformation is found to be six-membered cyclic groups and asymmetric bending vibration of methyl group in CH3O gives rise to a peak at 1,650 and 1,459 cm−1 in pure HPMC, and is shifted to 1,669 and 1,471 cm−1 in the complexed films due to salt complexation. The ethereal C–O–C group stretching vibration occurring at 1,091 cm−1 in pure HPMC is shifted to 1,026–1,076 and 1,306–1,087 cm−1 in 5:1 and 5:4 salt-complexed polymer electrolyte films, respectively. The peak at 850 cm−1 is associated with the CH2 rocking mode of HPMC. All these changes in the FTIR spectra are clear indications for the complexation of HPMC with NaI salt.
DSC studies
The DSC thermograms for pure HPMC and HPMC:NaI (5:1) and (5:4) are shown in Fig. 4. Pure HPMC shows the endothermic transition starts from 50 to 91 °C with a broad peak value at 72.16 °C [25]. This shifts to 81.33 and 83.33 °C in the HPMC:NaI (5:1, 5:4) systems indicating that the presence of NaI in the polymer matrix increases the melting temperature. Both pure and HPMC:NaI systems show only one endothermic broad peak. This peak shifts towards higher temperature as the NaI salt concentration increases. The enthalpy was calculated from the heat flow integral. Table 3 shows the enthalpy of fusion (ΔH f ) which is given by the area under the melting endotherm and decreases for all HPMC:NaI complexes. Here, the enthalpy of fusion is normalized to the weight of HPMC in the complexes. The relative percentage of crystallinity reduces in all HPMC:NaI polymer films. From Table 1, it is clear that the percentage of crystallinity reduces in all HPMC:NaI complexes when compared with the pure HPMC polymer films. However, crystallinity reduces to a minimum of 28 % for the HPMC:NaI complex of a 5:4 ratio compared with the 65 % crystallinity for the polymer host.
Electrical conductivity studies
The variation of conductivity as a function of temperature for pure HPMC and HPMC:NaI polymer electrolyte films in the temperature range of 313–383 K are as shown in Fig. 5. The temperature dependence of electrical conductivity (σ) follows the Arrhenius equation:
Where σ 0 is the pre-exponential factor, E α is the activation energy, K is the Boltzmann constant, and T is absolute temperature.
From the plots, it is clear that conductivity is found to increase with increase in temperature for pure HPMC as well as in all the compositions of HPMC:NaI polymer electrolyte films. The conductivity versus temperature plots follow the Arrhenius behavior; similar behavior was observed in the number of other polymer electrolyte systems [28–33]. From Fig. 5, pure HPMC shows the conductivity of about 6.530 × 10−9 S cm−1 at a temperature of 313 K, which shows that the polymer is almost nonconducting; it may be because, at sub-ambient temperature, HPMC shows a higher crystalline ratio which decreases the ionic conductivity. However, for pure HPMC and HPMC:NaI complexed films, increase in temperature leads to increase in ionic conductivity because as the temperature increases, the polymer chain flex at increased rate to produce more free volume resulting in enhanced polymer segmental mobility. The increase in conductivity with increase in temperature may be attributed to the transitions from the crystalline to the semicrystalline phase and then to the amorphous phase and is interpreted as hopping mechanism between local coordinate sites, local structural relaxation, and segmental motion of the polymer [34]. This decrease in the degree of crystallinity and increase in the amorphicity was confirmed with XRD studies. As the amorphous region increases progressively, the polymer chain acquires faster internal modes in which bond rotations produce segmental motion; this in turn favors the hopping interchain and intrachain ion movements, hence the conductivity of the polymer electrolytes become high. The E α is a combination of defect formation and defect migration; this can be calculated from the slope of log σ versus 1,000/T plots from Fig. 5 using the expression
where E α is the activation energy (in electron volts) and K is the Boltzmann constant.
Values of temperature-dependent ionic conductivity and activation energy for pure HPMC as well as in NaI-complexed polymer electrolyte films are listed in Table 4. The activation energies in the two regions (regions I and II) shows a decreasing trend as the dopant concentration increases which may be explained in terms of formation of charge transfer complexes. This is in agreement with the earlier reported results [31] and indicates the low activation energy for sodium ion transport is due to dominant presence of amorphous nature of polymer electrolyte that facilitates the fast Na+ ion motion in polymer network. The amorphous nature also provides a bigger free volume in polymer electrolyte system with the increase in temperature [35]. It is observed that HPMC:NaI (5:4) system has the highest conductivity and low activation energy region when compared with pure and other complexes of HPMC.
Conclusions
The complexation of the salt with the host polymer is confirmed by XRD and FTIR studies. XRD studies show the decrease in the degree of crystallinity and the simultaneous increase in amorphicity of the polymer electrolyte films with increasing NaI salt concentration. SEM analysis of pure and complexed polymer electrolytes shows a uniform type but with different degrees of roughness and exhibits no features attributable to any crystalline morphology. The DSC results revealed that the presence of NaI in the polymer matrix increases the melting temperature; however, it is observed that the ∆H f is high for pure HPMC films. The increase in conductivity with the increasing temperature and NaI concentration is attributed to the decrease in the degree of crystallinity and the simultaneous increase in amorphicity also the hopping mechanism of ion movement as ions primarily transport in amorphous phase. The activation energy values obtained from conductivity data for both the regions (regions I and II) decreases as the ionic conductivity increases which confirms the conduction in these polymer electrolytes is predominantly ionic. Therefore this material established a new polymer electrolyte system. The HPMC:NaI (5:4) system increases the amorphous phase and enhances the conductivity. Thus, the polymer electrolyte systems HPMC:NaI with an enhanced amorphous phase and conductivity requires further investigation for electrochemical cells device application.
References
Armand MB (1993) Solid State Ion 9/10:745
Papke BL, Ratner, Shriver DF (1992) J Electrochem Soc 129:1694
Berthier C, Gorecki W, Miner M, Amand MB, Chabagno JM, Riguad P (1983) J Solid State Ion 11:91
Armand MB (1986) Annu Rev Mater Sci 16:245–261
Jaipal Reddy M, Chu PP (2002) Electrochim Acta 47:1189–1196
Reitman EA, Kaplan ML, Kava RJ (1985) J Solid State Ionics 17:67
Mclin M, Angell CA (1992) J Solid State Ionics 56:1027
Lee YL, Crist BJ (1986) J Appl Phys 60:2683
Lascaud S, Perrier M, Vallee A, Besner S, Prud home J, Armand M (1994) Macromolecules 27:7469
Balaki Bhargav P, Madhu Mohan V, Sharma AK, Rao VVRN (2007) J Ionics 13:173–178
Mohammad Saleem Khan, Abdul Shakur (2010) J Ionics 16:539–542
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Subba Reddy CV, Jin AP, Zhu QY, Mai LQ, Chen W (2006) Eur Phys 19:471
Anantha PS, Hariharn K (2003) J Phys Chem Solids 64:1131
Sreekanth Reddy T, Jaipal Reddy M, Ramalingaiah S, Subbarao UV (1999) J Power Sources 79:105
Greenbaum SG, Pak YS, Wintersgill MC, Fontanella JJ, Schultz JW (1988) J Electrochem Soc 135:235
Sreepathi Rao S, Jaipal Reddy M, Laxmi Narsaiah E, Subba Rao UV (1995) Mater Sci Eng B 33:173
Tripathi SK (2012) Bull Mater Sci Indian Acad Sci 35(6):969–975
S. Honary, P. Ebrahimi, N. Emrani (2010) Int J Pharma Bio Sci l(2)
Hardy IJ, Cook WG, Melia CDS (2006) Compression and compaction properties of plasticized high molecular weight hydroxypropyl methylcellulose (HPMC) as a hydrophilic matrix carrier. Inter J Pharm 311(1–2):26–32
Hunter CC, Ingram MD (1984) Solid State Ionics 14:31
Bruce PG, Vincent CA (1993) J Chem Soc Faraday Trans 89:3187–3203
Madhu Mohan V, Raja V, Sharma AK, Narasimha Rao VVRN (2004) J Mater Chem Phys 94:177
Hermans PH, Weidinger A (1961) Makromol Chem 24:44
Sangappa, Demappa et al (2008) Nucl Instr Meth Phys Res B 266:3975–3980
Zhang S, Lee JY, Hong L (2004) J Power Sources 115:288
Chu PP, Reddy MJ (2003) J Power Sources 115:288
Subba Reddy CHV, Sharma AK, Narasimha Rao VVR (2006) J Polymer 47:1318
Bhide A, Hariharan K (2006) J Power Sources 159(2):1450
HiranKumar G, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2005) J Power Sources 144:262
Balaji Bhargav P, Mahy Mohan V, Sharma AK, Rao VVRN (2007) J Ionics 13:173–178
Chakraborty G et al (2011) J Solid State Commun 151:754–758
Janaki Rami Reddy T, Achari VBS, Sharma AK, Rao VVRN (2007) Ionics 13:435–439
Devendrappa H, Subba Rao UV, Ambika Prasad MVN (2006) J Power Sources 155(2):368
Michael MS, Jacob MME, Prabhaharan SRS, Radhakrishnan S (1997) Solid State Ion 98:167
Acknowledgments
The authors acknowledge all the support and the useful discussion by Professor Srikantaiah, Retired Scientist, BARC, Mumbai. We are thankful to the technical staff at SID and Material Science Department, IISc, Bangalore for DSC, FTIR, and SEM analysis. We thank Grian Technologies Pvt. Limited, Bangalore for their support in electrical conductivity studies. Special thanks to Dr Shibu M Eappen, Scientist in Charge, SAIF Cochin University of Science and Technology, Cochin, for XRD measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rani, N.S., Sannappa, J., Demappa, T. et al. Structural, thermal, and electrical studies of sodium iodide (NaI)-doped hydroxypropyl methylcellulose (HPMC) polymer electrolyte films. Ionics 20, 201–207 (2014). https://doi.org/10.1007/s11581-013-0952-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0952-8