Abstract
Experimental investigation of a passive direct ethanol fuel cell (DEFC) is presented in this work. A passive DEFC of active area 25 cm2 with Nafion 115 membrane is developed and tested. Effect of several parameters such as ethanol feed concentration, ambient temperature, bolt torque, and operating orientation on the cell performance is investigated. It is found that all these parameters significantly affect the cell performance. The cell performance improves with initial increase in the ethanol feed concentration, reaches to a maximum, and then decreases on further increasing the ethanol feed concentration. A similar trend in the cell performance is observed on increasing the ambient temperature and on increasing the bolt torque too. In this study, the maximum cell performance is observed with 4 M ethanol feed concentration, 60 °C of ambient temperature, and 7 Nm of bolt torque. Cell orientation too affects the cell performance. Horizontal cell orientation is proved to deliver better cell performance compared to vertical orientation. This study depicts that an improved DEFC performance can be obtained by carefully selecting working parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fuel cell is an electrochemical device which directly converts the chemical energy of fuel into electrical energy. Fuel cell has advantages of high efficiency, zero pollution, and noiseless and therefore considered as potential device for powering variety of applications viz. portable application, automotive application, and stationary application [1,2,3,4,5]. Fuel cell uses hydrogen as a preferred fuel due to weak hydrogen-hydrogen bond provides high electrochemical activity. But, the hydrogen is not freely available and it has to be produced by some hydrocarbon fuel through external mechanism. It makes the entire fuel cell system bulky, complex, and costlier. Moreover, hydrogen has very low volumetric energy density and it is highly flammable. Therefore, handling, storage, and transportation of hydrogen are very complicated [6, 7].
Direct liquid fuel cell (DLFC) uses liquid fuel directly into the fuel cell without any extra reforming step. DLFC overcomes the drawbacks associated with the hydrogen. DLFC has the advantages of high energy density, compact size, easy handling of liquid fuel, and easy membrane hydration and therefore it is considered as highly potential power source for portable devices viz. laptop, mobile phones, toys, and drones [8, 9]. However, it has some major issues viz. slow reaction kinetics of liquid fuel, higher anodic over potentials, and fuel crossover through membrane which lowers the fuel cell performance [9]. There are two types of DLFC: active DLFC and passive DLFC. Active DLFC uses pumps and blowers for supply of reactants. On the other hand, passive DLFC does not have pumps and blowers but relies on passive means viz. diffusion, natural convection, and capillary action for the transport of reactants and therefore is simpler in construction [8, 10, 11].
Among the liquid fuels, methanol has caught the most attention and significant research has been reported on the direct methanol fuel cell (DMFC) in the recent past [12,13,14,15,16,17]. Methanol is the simplest alcohol and it is easy to oxidize therefore provides high electrochemical activity. DMFC too suffers from many technological issues viz. high methanol crossover, poisoning of anode catalyst by intermediate compounds generated during the methanol electro-oxidation, cost, and durability. Moreover, methanol is non-renewable fuel, toxic in nature, highly volatile, and highly inflammable [18,19,20].
Another alcohol, ethanol, has also been tested for the fuel cell. Ethanol is less toxic than methanol, it has higher gravimetric energy density (8 kWh kg−1) compared to methanol (6.1 kWh kg−1) and can be produced from agriculture wastes and biomass [21]. Therefore, ethanol is an attractive fuel choice for DLFC. A significant research work on direct ethanol fuel cell (DEFC) has been reported in the recent past. The DEFC can be divided into two types based on the electrolyte used: acid DEFC and alkaline DEFC. Acid DEFC uses polymer electrolyte membrane, typically Nafion to conduct proton from anode to cathode. Whereas alkaline DEFC uses anion exchange membrane, typically A201 by Tokuyama to conduct hydroxyl ions from cathode to anode [22]. The present work mainly focuses on the acidic DEFC. DEFC suffers from many technological challenges viz. sluggish ethanol oxidation kinetics at anode, ethanol crossover, low efficiency, water management, degradation, and durability [21, 23,24,25,26]. Song et al. [27] studied the effect of membrane electrode assembly (MEA) preparation method on the DEFC performance and found that the MEA prepared by the decal transfer method exhibits better cell performance and lower internal resistance compared to conventional hot-pressed MEA. Ma et al. [28] reported a DEFC that uses nitrate reduction reaction instead of conventional oxygen reduction reaction on the cathode. The cell showed higher open-circuit voltage and two times maximum power density. Heysiattalab [29] studied the effect of operating parameters such as ethanol concentration, temperature, reactant’s flow rate, and cathode pressure on the DEFC performance. Alzate et al. [30] also studied the effect of operating conditions on the DEFC performance. It was found that the cell performance was improved with increase in cell temperature and cathode back pressure but was independent of reactant’s flow rate.
Passive DEFC exhibits different mass and heat transport pattern than active DEFC. Passive DEFC does not use any energy consuming auxiliary device for supply of reactants. Therefore, it can deliver higher power density and could be suitable to power portable electronic devices. Literature review shows that significant research has been reported on the active DEFC, but the research on the passive DEFC is rare [31, 32]. To the author’s best knowledge, the only available experimental work on the passive DEFC is by Pereira et al. [31], where the effect of ethanol concentration, membrane thickness, anode diffusion layer material, and anode catalyst loading on cell performance have been reported.
In this research, the effect of working parameters such as ethanol feed concentration, ambient temperature, bolt torque, and operating orientation on a passive DEFC have been studied. A passive DEFC of 25-cm2 active area having Nafion 115 membrane as electrolyte has been developed and used for the experimentation. This study would be useful for the better understanding about the passive DEFC and for its performance improvement.
Experimental
Membrane electrode assembly
Membrane electrode assembly (MEA) of 25-cm2 active area is used for fuel cell. A Nafion® 115 membrane is sandwiched and hot pressed between two diffusion electrodes to obtain MEA. Hot pressing is performed at 135 °C, 8 MPa for 3 min. The Nafion membrane is pre-treated before hot pressing to remove contaminants. For the pre-treatment, membrane is boiled in 3 wt% H2O2 solution for 1 h, boiled in DI water for 1 h, boiled in 0.5 M H2SO4 solution for 1 h, and finally boiled in DI water for 1 h. The pre-treated membrane is kept in DI water until MEA fabrication. Polytetrafluoroethylene (PTFE)-treated carbon cloth (Avcarb 1071) with micro-porous layer is used as diffusion layer. Diffusion electrode is obtained by coating the catalyst ink onto the diffusion layer. Catalyst ink is prepared by dispersing catalyst in a solution of deionized (DI) water, isopropyl alcohol, and Nafion solution. The catalyst ink is applied onto the diffusion layer using paintbrush technique followed by drying in an oven at 50 °C for 2–3 min. Several coatings are applied to obtain the desired catalyst loading. Once the desired catalyst loading is achieved, the diffusion electrode is sintered at 70 °C for 1 h. The Pt-Ru/C (80 wt%) and Pt/C (55 wt%) are used as anode and cathode catalyst respectively. A catalyst loading of 4 mg/cm2 and 2 mg/cm2 respectively are used on anode and cathode respectively.
Single-cell fixture
A schematic diagram of passive DEFC is shown in Fig. 1a, b and a photograph is shown in Fig. 1c. The cell consists of anode end plate, ethanol reservoir, anode current collector, membrane electrode assembly, cathode current collector, and cathode end plate. Teflon-coated glass fiber gaskets are used between cell components to prevent leakage. Anode end plate, ethanol reservoir, and cathode end plate are made of transparent acrylic plate of 10 mm thickness, which enable observing various phenomena occurring inside the cell, mainly, carbon dioxide bubble transport. The cathode end plate has window-frame structure to allow oxygen and water transport. The ethanol reservoir has the dimension of 50 mm × 50 mm × 10 mm and a volume of 25 mL. An opening was provided on the fuel reservoir for fuel injection and carbon dioxide exhaust. The function of current collector is to collect current generated in the MEA, to support the MEA, and to provide passage for reactants and products. Current collectors are made of 3-mm-thick stainless steel (SS 316 L) plates. To provide passage for the transport of reactants, 121 holes of 3.6-mm diameter each are drilled in the current collectors, resulting in an open ratio of 49.2%. Eight nut bolts with appropriate toque are arranged circumferentially on the cell fixture to keep the fuel cell assembly intact, to reduce the electrical contact resistance of the cell, and to avoid leakage. A torque wrench (Tristar, TVT 100A, accuracy ± 3%) was used to tighten the nut-bolts. Electrical insulation was provided on the bolt surface to avoid short-circuiting.
Experimental setup and test conditions
A DC electronic load (N3300A Mainframe with N3302A Module) is used to perform polarization test and open-circuit voltage (OCV) test. Before experiments, passive DEFC is activated by filling the reservoir with 2 M ethanol solution and let it stand for 12 h. Then, the cell is run at constant voltage of 0.35 V for 12 h and then a polarization test (I-V) is conducted. The process is repeated until stable polarization curve is obtained. For experiments, ethanol of appropriate concentration is fed in the ethanol reservoir. Then the cell is kept at OCV for 60 min so that it can reach stable condition. Then, the polarization test is performed. During the test, for each discharging current point, a waiting time of 60 s is used to obtain a stable voltage. Experiments are conducted at a room temperature of 22–25 °C and relative humidity of 60–70%.
Results and discussion
Effect of ethanol feed concentration
Effect of ethanol feed concentration on the cell performance is shown in Fig. 2. Ethanol solution of seven different concentrations 0.5 M, 1 M, 2 M, 3 M, 4 M, 5 M, and 6 M are used in the study. Figure 2a shows variation in OCV with ethanol feed concentration. After filling the ethanol solution in the anode reservoir, the OCV is recorded until it becomes stable, usually it takes 60 min. It can be seen from the figure that the OCV decreases on increasing the ethanol concentration. The ethanol concentration of 0.5 M gives the highest (0.568 V) and 6 M gives the lowest OCV (0.470 V). The OCV is closely related to the ethanol crossover. Ethanol is stored in the reservoir; it diffuses through current collector and diffusion layer and reaches at anode catalyst layer where the electrochemical reaction takes place. Some of the unused ethanol at anode catalyst layer permeates through the membrane and reaches at cathode catalyst layer. It is termed as ethanol crossover. At cathode catalyst layer, the ethanol directly reacts with oxygen and produces water and carbon dioxide. This creates mixed potential and results in reduction in OCV. The ethanol oxidation on the cathode generates water and therefore increases the cathode water flooding. This restricts the supply of oxygen on the cathode catalyst layer. Oxygen is essential for the electrochemical reaction. This also makes adverse effect on to the OCV. Ethanol crossover rate is proportional to the ethanol concentration gradient across the membrane. On increasing the ethanol feed concentration, the ethanol crossover increases and therefore the OCV decreases.
Figure 2b, c shows the effect of ethanol concentration on the current density—voltage curve (I-V) and current density—power curve (I-P) respectively. It is seen that the cell performance improves continuously on increasing the ethanol concentration from 0.5 to 4 M. The 4 M gives the maximum cell performance. The cell performance deteriorates on further increasing the ethanol concentration to 5 M and 6 M. The results indicate that the ethanol concentration is an important parameter for the DEFC performance. On increasing the ethanol concentration: (i) ethanol mass transport rate from reservoir towards the anode catalyst layer increases which decreases the concentration loss and makes positive effect on the cell performance. Relatively higher concentration loss for 0.5 M and 1 M is clearly visible in the I-V curves at the higher current densities. (ii) Ethanol crossover increases on increasing the ethanol concentration, which increases the mixed potential and cathode water flooding and make adverse effect on the cell performance. (iii) Oxidation of ethanol is an exothermic reaction. Ethanol permeates through membrane and is oxidized at cathode releases heat and increases the cell temperature. Higher cell temperature increases the rate of electrochemical reaction and improves the cell performance. On increasing the ethanol concentration, ethanol crossover increases; therefore, the cell temperature also increases. Summarily, effects (i) and (iii) improves the cell performance while the effect (ii) deteriorates the cell performance on increasing the ethanol feed concentration.
The overall effect of the ethanol feed concentration on the cell performance is the combined effect of the three effects mentioned above. When the ethanol concentration is varied from 0.5 to 4 M the effect (i) and (iii) have dominating effect on the overall cell performance and therefore the cell performance improves. On the other hand, when the ethanol concentration is increased to 5 M and 6 M, the effect (ii) has dominating effect over the cell performance; therefore, the cell performance decreases. The 4 M ethanol concentration gives the best cell performance. The maximum power density with 0.5 M is 0.771 mW cm−2, while that with 4 M is 1.124 mW cm−2, which is 45.7% higher than the former. It signifies the use of appropriate ethanol concentration in the fuel cell.
Effect of ambient temperature
The cathode of a passive DEFC is open to ambient air; therefore, the change in ambient temperature has an impact over the inside phenomena and over the cell performance. Figure 3 shows the effect of ambient temperature on the performance of passive DEFC. The cell performance is measured at five different ambient temperatures: 30 °C, 40 °C, 50 °C, 60 °C, and 70 °C. To measure the effect of ambient temperature, the passive DEFC is kept in a convection oven at specified temperature. Ethanol feed concentration of 4 M is used in the study. Figure 3a shows the OCV variation with temperature. It is seen that when the ambient temperature is increased from 30 to 60 °C, the OCV gradually increases and then decreases slightly on increasing the temperature to 70 °C. Similar trend is found for I-V curve and I-P curve, which is shown in Fig. 3b, c respectively. The cell performance improves on increasing the cell temperature from 30 to 60 °C. The cell gives maximum performance at 60 °C. On increasing the temperature further to 70 °C, the cell performance deteriorates. The maximum power density at 30 °C is 0.438 mW cm−2 and that at 60 °C is 1.02 mW cm−2, which is 132.8% higher than the former. This shows the importance of the ambient temperature for the betterment of cell performance. Increase in the ambient temperature results in the following effects: (i) higher ambient temperature reduces the rate of heat dissipation out of the cell and therefore increases the cell temperature. An increased cell temperature increases the electrochemical reaction rate and improves the cell performance (ii) internal resistance of the cell decreases with increase in the cell temperature, which reduces the ohmic loss and improves the cell performance (iii) water evaporation rate at cathode increases at higher temperature, which removes the cathode water faster and provides better access to oxygen. It makes positive effect on the cell performance (iv) ethanol crossover rate and its adverse effect on the cell performance increases with increase in the cell temperature. In summary, on increasing the ambient temperature effects (i), (ii), and (iii) provide improvement while the effect (iv) provides decrement in the cell performance. Combine effect of these four effects governs the overall cell performance. On increasing the ambient temperature from 30 °C to 60 °C, the effects (i), (ii), and (iii) have dominating effect over the cell performance; therefore, the cell performance is improving. Thereafter, the effect (iv) is dominating the overall cell performance; therefore, the cell performance is decreasing on increasing the cell temperature to 70 °C.
Effect of bolt torque
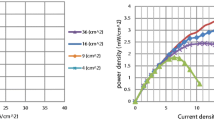
In the fuel cell assembly, all components are held together by applying suitable toque on to the bolts. It prevents any leakage of reactants. A sufficient bolt torque is required to ensure proper contact between fuel cell components and therefore it is an important parameter to study. The effect of bolt torque on the DEFC performance is shown in Fig. 4. Experiments are done for seven different bolt torques between 4 and 11 Nm. Initially, the cell is tightened by applying a bolt torque of 3 Nm, but the cell is found dripping. Next, the torque is increased to 4 Nm and the dripping is stopped. Therefore, 4 Nm is used as starting torque in experiments. Ethanol solution of 4 M is used during experiments. It is seen in Fig. 4 that the cell performance is improving continuously when bolt torque is increased from 4 Nm to 5 Nm to 6 Nm and then to 7 Nm. The best cell performance is obtained with 7 Nm. Thereafter, on increasing the bolt toque further to 8 Nm, 9 Nm, and then 11 Nm, the cell performance is decreasing continuously. The maximum power density for 4-Nm torque is 0.382 mW cm−2 while that for 7 Nm is 0.586 mW cm−2, which is 53.4% higher than the former. This indicates importance of the bolt torque in the fuel cell assembly. The fuel cell should be run with optimum bolt torque to obtain the maximum cell performance. An increase in the bolt torque has following effects: (i) contact resistance between diffusion layer and current collector decreases as a result ohmic loss decreases and the cell performance improves (ii) in-plane and through-plane electron conductivity of the diffusion layer increases as a result ohmic loss decreases. (iii) Carbon cloth is used as diffusion layer in this study which is highly porous. Pore volume and porosity of the diffusion layer decrease with increase in the bolt torque. It makes transport of the reactants difficult, increases the mass transport loss, and deteriorates the cell performance.
The effect of bolt toque depicted in Fig. 4 is the combined effect of above three effects. On initially increasing the bolt torque from 4 to 7 Nm, effects (i) and (ii) have dominating effect over the cell performance; therefore, the cell performance is improving. But, at relatively higher bolt torque of 8, 9, and 11 Nm, the diffusion layer is excessively compressed and mass transport losses have dominating effect; therefore, the cell performance is decreasing.
Effect of cell orientation
The effect of cell orientation on passive DEFC performance is shown in Fig. 5. DEFC is tested at two different orientations: horizontal (anode facing up) and vertical orientation. Tests are conducted for three different ethanol feed concentrations: 2 M, 4 M, and 6 M and the cell performance curves (I-V and I-P) are shown in Fig. 5a–c respectively. It is seen that the cell kept in horizontal orientation performs better than the vertical orientation for all ethanol feed concentrations. The maximum power obtained at vertical orientation are 0.8818, 0.9342, and 0.8323 mW cm−2 and that at horizontal orientation are 0.9952, 1.019, and 0.9043 mW cm−2 for 2 M, 4 M, and 6 M ethanol feed concentration respectively. Horizontal orientation shows 8.5% to 13% better performance compared to vertical one. Carbon dioxide is generated on the anode side due to electrochemical reaction. Effective carbon dioxide removal is essential to facilitate better cell performance. Horizontal orientation facilitates more effective removal of carbon dioxide. In the vertical orientation of the cell, carbon dioxide bubbles move towards the top of the anode chamber and accumulate there. If carbon dioxide removal is not effective, it gets accumulated at the anode catalyst layer. The catalyst layer provides three phase boundaries between reactant, catalyst, and Nafion ionomer, where the electrochemical reaction takes place. Catalysts promote electrochemical reaction as well as provide path for the electron transport. Nafion ionomer facilitates proton transport. If the carbon dioxide is accumulated at the catalyst layer, it prevents reactants to reach catalyst sites, to form three phase boundaries and to proceed with electrochemical reaction. Therefore, the cell performance decreases. With the horizontal orientation, carbon dioxide bubbles are uniformly distributed over the entire active area, facilitating better carbon dioxide removal.
Similar to ethanol crossover, some of the unused water at anode permeates through membrane and reaches at cathode. This is termed as water crossover. Water is generated also at the cathode due to electrochemical reaction and due to oxidation of permeated ethanol. Generated and crossovered water starts accumulating at cathode. Effective removal of water is essential to facilitate uninterrupted supply of oxygen. In the vertical orientation, due to gravity, liquid water accumulates at the bottom portion of the cathode. On the other hand, horizontal cell orientation facilitates better liquid water removal due to the gravity effect. Therefore, the cell performs better in the horizontal orientation which is seen in Fig. 5.
It is also seen in Fig. 5c that the difference in the cell performance gets wider at higher current densities. At higher current, more water is produced on the cathode side. Also, at higher ethanol feed concentration, the ethanol crossover is higher which upon oxidation produces more water. As a result, cathode flooding is more severe at higher ethanol concentration and higher current. Due to inability of vertical cell orientation to remove water, the cell performance gets worse.
Conclusions
Experimental investigation of a passive DEFC is reported in this work. A passive DEFC of active area 25 cm2 is used for the study. Effect of ethanol feed concentration, ambient temperature, bolt torque, and cell orientation on the cell performance is studied. An ethanol feed concentration from 0.5 to 6 M, cell temperature from 30 to 70 °C, bolt torque from 4 to 11 Nm, and horizontal and vertical cell orientations are used for the investigation. It is found that all these parameters significantly affect the cell performance. The cell gives 8.5% to 13% better performance when operated in the horizontal orientation compared to vertical orientation. Horizontal orientation provides better carbon dioxide removal as well as better cathode water removal. On increasing, the ethanol feed concentration, ambient temperature, and bolt torque, the cell performance improves first, reaches to a maximum, and then decreases on further increasing these parameters. In this study, the maximum cell performance is obtained at 4 M ethanol feed concentration, 60 °C ambient temperature, and 7 Nm of bolt torque. Based on the results, it is recommended to choose the working parameters of the cell carefully in order to achieve the maximum cell performance.
References
Mekhilef S, Saidur R, Safari A (2012) Comparative study of different fuel cell technologies. Renew Sust Energ Rev 16:981–989
Lucia U (2014) Overview on fuel cells. Renew Sust Energ Rev 30:164–169
Jayakumar A, Sethu S, Ramos M, Robertson J, Al-Jumaily A (2015) A technical review on gas diffusion, mechanism and medium of PEM fuel cell. Ionics 21:1–18
Pollet BG, Staffell I, Shang JL (2012) Current status of hybrid, battery and fuel cell electric vehicles: from electrochemistry to market prospects. Electrochim Acta 84:235–249
Hu Z, Xu L, Li J, Ouyang M, Song Z, Huang H (2018) A reconstructed fuel cell life-prediction model for a fuel cell hybrid city bus. Energy Convers Manag 156:723–732
Sharaf OZ, Orhan MF (2014) An overview of fuel cell technology: fundamentals and applications. Renew Sust Energ Rev 32:810–853
Acres GJK (2001) Recent advances in fuel cell technology and its applications. J Power Sources 100:60–66
Qian W, Wilkinson DP, Shen J, Wang H, Zhang J (2006) Architecture for portable direct liquid fuel cells. J Power Sources 154:202–213
Lamy C, Lima A, LeRhun V, Delime F, Coutanceau C, Leger JM (2002) Recent advances in the development of direct alcohol fuel cells (DAFC). J Power Sources 105:283–296
Kamarudin SK, Achmad F, Daud WRW (2009) Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int J Hydrog Energy 34:6902–6916
Cao J, Wang L, Song L, Xu J, Wang H, Chen Z, Huang Q, Yang H (2014) Novel cathodal diffusion layer with mesoporous carbon for the passive direct methanol fuel cell. Electrochim Acta 118:163–168
Yousefi S, Zohoor M (2013) Investigating the effect of operating parameters on the open circuit voltage of a passive DMFC. Ionics 19:1195–1201
Hashemi R, Yousefi S, Faraji M (2015) Experimental studying of the effect of active area on the performance of passive direct methanol fuel cell. Ionics 21:2851–2862
Chen R, Zhao TS (2007) Porous current collectors for passive direct methanol fuel cells. Electrochim Acta 52:4317–4324
Yousefi S, Ganji DD (2012) Experimental investigation of a passive direct methanol fuel cell with 100 cm2 active areas. Electrochim Acta 85:693–699
Munjewar S, Thombre S, Mallick R (2017) A comprehensive review on recent material development of passive direct methanol fuel cell. Ionics 23:1–18
Shrivastava N, Thombre S, Chadge R (2016) Liquid feed passive direct methanol fuel cell: challenges and recent advances. Ionics 22:1–23
Faghri A, Li X, Bahrami H (2012) Recent advances in passive and semi passive direct methanol fuel cells. Int J Therm Sci 62:12–18
Zhao TS, Chen R, Yang WW, Xu C (2009) Small direct methanol fuel cells with passive supply of reactants. J Power Sources 191:185–202
Zhao TS, Yang WW, Chen R, Wu QX (2010) Towards operating direct methanol fuel cells with highly concentrated fuel. J Power Sources 195:3451–3462
Akhairi M, Kamarudin S (2016) Catalysts in direct ethanol fuel cell (DEFC): an overview. Int J Hydrog Energy 41:4214–4228
An L, Zhao T, Li Y (2015) Carbon-neutral sustainable energy technology: direct ethanol fuel cells. Renew Sust Energ Rev 50:1462–1468
Abdullah S, Kamarudin S, Hasran U, Masdar M, Daud W (2015) Development of a conceptual design model of a direct ethanol fuel cell (DEFC). Int J Hydrog Energy 40:11943–11948
Song S, Zhou W, Tian J, Cai R, Sun G, Xin Q, Kontou S, Tsiakaras P (2005) Ethanol crossover phenomena and its influence on the performance of DEFC. J Power Sources 145:266–271
Badwal S, Giddey S, Kulkarni A, Goel J, Basu S (2015) Direct ethanol fuel cells for transport and stationary applications – a comprehensive review. Appl Energy 145:80–103
Kamarudin M, Kamarudin S, Masdar M, Daud W (2013) Review: direct ethanol fuel cells. Int J Hydrog Energy 38:9438–9453
Song S, Wang G, Zhou W, Zhao X, Sun G, Xin Q, Kontou S, Tsiakaras P (2005) The effect of the MEA preparation procedure on both ethanol crossover and DEFC performance. J Power Sources 140:103–110
Ma K, Han S, Kwon S, Kwak D, Park K (2018) In press) High-performance direct ethanol fuel cell using nitrate reduction reaction. Int J Hydrog Energy 43:17265–17270
Heysiattalab S, Shakeri M, Safari M, Keikha M (2011) Investigation of key parameters influence on performance of direct ethanol fuel cell (DEFC). J Ind Eng Chem 17:727–729
Alzate V, Fatih K, Wang H (2011) Effect of operating parameters and anode diffusion layer on the direct ethanol fuel cell performance. J Power Sources 196:10625–10631
Pereira J, Falcão D, Oliveira V, Pinto A (2014) Performance of a passive direct ethanol fuel cell. J Power Sources 256:14–19
Oliveira V, Pereira J, Pinto A (2017) Modeling of passive direct ethanol fuel cells. Energy 133:652–665
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shrivastava, N.K., Chadge, R.B., Ahire, P. et al. Experimental investigation of a passive direct ethanol fuel cell. Ionics 25, 719–726 (2019). https://doi.org/10.1007/s11581-018-2797-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2797-7