Abstract
LiZnVO4 particles ware synthesized via solid-state reaction route. It was characterized by X-ray diffraction and scanning electron microscopy. As anode material for rechargeable lithium-ion battery, the electrochemical performance of the LiZnVO4 samples was measured. It was found that a large capacity of 330 mAh g−1 can be retained after 70 cycles. The electrochemical measurements indicate that the anode material made of LiZnVO4 exhibits excellent cycling stability even at a high current density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries (LIBs) are being intensively pursued for hybrid electric vehicle and plug-in hybrid electric vehicle applications. Nowadays, most of the commercial LIBs use graphite as anode material for its excellent cycling behavior. However, low theoretical capacity and poor cycling behavior for graphite anode at a high current density limit its large-scale application in high energy-density battery systems. Thus, much attention has been paid to develop new anode materials [1–3]. Recently, the vanadates have generated a new interest shown to be potential candidates for anode material in LIBs [4–9]. For instance, brannerite structural MnV2O6 and inverse spinel structural LiNiVO4 can react with a large number of lithium ions per formula unit at a low voltage [10, 11]. Although these vanadate compounds have large initial capacity, they would become amorphous after the first cycle and exhibited a large capacity loss upon the subsequent cycles [4, 12]. So far, several methods have been developed to enhance their electrochemical properties. MnV2O6 synthesized by a hydrothermal route can deliver a reversible capacity of 630 mAh g−1 after 30 cycles [13]. Amorphous LiNiVO4 thin films obtained by the physical vapor deposition technique exhibited discharge capacity of 800–1,100 mAh g−1 after 20 cycles [14]. According to a report by Denis et al. [12, 15], during the electrochemical reduction of these vanadates, oxygen acts as a redox center of Li–O bonds thereby leading to an enhancement in capacity. Accordingly, it can be stated that the 2p orbital of oxygen may play an important role for the compensation of charge equivalence besides transition metals during the insertion and extraction of Li ion.
In the present work, we synthesized LiZnVO4 via a solid-state reaction route and used it as anode material in a rechargeable lithium-ion battery. The electrochemical measurements indicated that the cells made of LiZnVO4 exhibited a large capacity and excellent cycling stability.
Experimental
Synthesis of LiZnVO4
LiZnVO4 was synthesized by a solid-state reaction. In a typical process, lithium carbonate (Li2CO3), zinc oxide (ZnO), and vanadium pentoxide (V2O5) were ground in an agate mortar at a Li/Zn/V = 1:1:1. The mixture was sintered at 750 °C for 3 h. X-ray diffraction (XRD) patterns were recorded on a PANalytical X′Pert spectrometer using CoKα radiation (λ = 1.78897 Å), and the data were converted to CuKα data. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were taken on a Philips-XL30 instrument and a JEOL 2010 instrument, respectively.
Electrochemical measurements
The electrochemical behaviors of the materials were examined via CR2025 coin-type cells with lithium metal as the counter electrode. The anode materials were made by mixing active materials, acetylene black and PVDF, in a weight ratio of 60:30:10, respectively. The electrolyte was 1 M LiPF6 in a 1:1:1 (volume ratio) mixture of ethylene carbonate, ethylene methyl carbonate, and dimethyl carbonate. The separator was a UP 3093 (Japan) microporous polypropylene membrane. Cell assembly was carried out in a glove box under an argon atmosphere. The discharge and charge tests for aqueous lithium-ion battery were carried out by a program-controlled Battery Test System (Land CT 2001A, Wuhan, China) at different constant current density with a cutoff voltage of 0.02–2.5 V. All the electrochemical measurements were performed at room temperature.
Results and discussion
Figure 1 shows the crystal structure of LiZnVO4. It contains three types of tetrahedra, [VO4] and two disordered mixed-site [Li/ZnO4] tetrahedral. The ratio of lithium and zinc in the mixed-site [Li/ZnO4] tetrahedra is 1:1, and the hexagonal channels are along the [0001] direction [16].
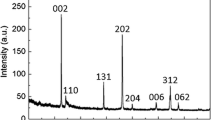
The XRD pattern of the product synthesized at 750 °C for 3 h is shown in Fig. 2a. All the diffraction reflections can be indexed to a rhombohedra structure LiZnVO4 (JCPDS 38-1332). According to JCPDS 38-1332, LiZnVO4 belongs to the rhombohedra crystal system with space group R-3h. The unit cell volume is 1,653.49 Å3, and the lattice parameters are a = b = 14.1857 Å, c = 9.4877 Å, α = β = 90°, γ = 120°, Z = 18 [17]. Figure 2b shows the SEM image of LiZnVO4 synthesized at 750 °C for 3 h. It clearly shows that the product is composed of irregular particles, and their size is found to be several micrometers.
The electrochemical properties of LiZnVO4 have been investigated, and the results are shown in Fig. 3. Figure 3a shows cyclic voltammetry (CV) curves of the cells made of LiZnVO4 at a scan rate of 0.5 mV s−1. As can be seen from Fig. 3a, the CV curves between the first cycle and the following cycles are different. This result might be attributed to the fact that the irreversible structured transformation has been taking place in the initial cycle. After the first cycle, the CV curves changed slightly, indicating that the lithium ions' intercalation/deintercalation into/out of the LiZnVO4 electrode is well reversible. Figure 3b shows the charge–discharge profiles of anodes made of LiZnVO4 particles at the 1st, 2nd, 20th, 50th, and 70th cycles recorded at a current density of 1 A g−1, and a larger irreversible discharge capacity of 824 mAh g−1 was observed. This might be attributed to the structure transformation to amorphous at the initial cycle [13, 18]. It can be found from Fig. 3c that the capacity tends to decrease in the initial cycling period, then stopped decreasing, and eventually reached a plateau, indicating that the electrochemical properties of LiZnVO4 anode are very stable, and the Li-ion intercalation/deintercalation into/out of the electrode is well reversible even at a current density of 1 A g−1. A reversible capacity of ca. 330 mAh g−1 was obtained after 70 cycles corresponding to x = 2.3 for Li x ZnVO4. Even after the current density was increased to 8 A g−1, a reversible capacity of 110 mAh g−1 was also achieved (see Fig. 3d). On the other hand, the capacity can be recovered, and the cycling stability can be retained after the current density was increased significantly. This might be attributed to the intrinsic characteristics of anode. As mentioned above, the hexagonal channels along the [0001] direction in the crystal structure could provide a diffusion space for Li-ion intercalation/deintercalation into/out of the anode, resulting in very good cycling stability. Meanwhile, the amorphous structure of LiZnVO4 might improve the capacity for creating a smoother pathway during the Li-ion insertion and extraction process. A similar result was also observed for the NaV6O15 electrode [19].
To further research the electrochemical reaction mechanism of LiZnVO4, XRD and TEM were measured on the anode recovered from the cells at selected voltages. Figure 4a shows the XRD patterns of the anode after discharge and charge. The peaks indicated by solid triangle symbols are attributed to Li2CO3, which most likely results from the reaction of Li2O and CO2 in air, and Li2O might come from the electrochemical process of discharge and charge. The similar result was also observed by Orsini and coworkers [4]. As shown in the TEM image, only the Li2CO3 phase is observed, while the crystalline LiZnVO4 cannot be detected, indicating that it was transferred into amorphous phase at the first cycle, which is in agreement with CV measurement.
Conclusions
In summary, LiZnVO4 has been synthesized via a solid-state reaction route and then was used as anode material in a rechargeable Li-ion battery. A large capacity of 330 mAh g−1 was retained after 70 cycles even at a current density of 1 A g−1. The electrochemical measurements indicate that the anode material made of LiZnVO4 particles displayed excellent cycling stability. This might be attributed to the intrinsic characteristics of anode material. These results might renew interest in the design of anode material in the future.
References
Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ (2008) Adv Funct Mater 18:3941–3946
Kim MG, Cho J (2009) Adv Funct Mater 19:1497–1514
Yang R, Huang J, Zhao W, Lai WZ, Zhang XZ, Zheng J, Li XG (2010) J Power Sources 195:6811–6816
Orsini F, Baudrin E, Denis S, Dupont L, Touboul M, Guyomard D, Piffard Y, Tarascon J-M (1998) Solid State Ionics 107:123–133
Denis S, Baudrin E, Orsini F, Ouvrard G, Touboul M, Tarascon J-M (1999) J Power Sources 81–82:79–84
Guyomard D, Sigala C, Le Gal La Salle A, Piffard Y (1997) J Power Sources 68:692–697
Ram M (2011) Int J Mod Phys B 25(12):1619–1628
Prakash D, Masuda Y, Sanjeeviraja C (2012) Ionics 18(1–2):31–37
Clemens O, Bauer M, Haberkorn R, Beck HP (2011) Z Anorg Allg Chem 637(7–8):1036–1044
Kim SS, Ikuta H, Wakihara M (2001) Solid State Ionics 139:57–65
Han XY, Tang WC, Yi ZH, Sun JT (2008) J Appl Electrochem 38:1671–1676
Hara D, Shirakawa J, Ikuta H, Uchimoto Y, Wakihara M, Miyanagab T, Watanabe I (2002) J Mater Chem 12:3717–3722
Huang WD, Gao SK, Ding XK, Jiang LL, Wei MD (2010) J Alloys Compd 495:185–188
Reddy MV, Pecquenard B, Vinatier P, Levasseur A (2007) Surf Interface Anal 39:653–659
Denis S, Baudrin E, Touboul M, Tarascon J-M (1997) J Electrochem Soc 144:4009–4109
Azrour M, Elouadi B, Ammari LE (2010) Acta Cryst E 66:i39
Capsoni D, Bini M, Massarotti V, Mustarelli P, Belotti F, Galinetto P (2006) J Phys Chem B 110(11):5409–5415
Rossignol C, Ouvrard G, Baudrin E (2001) J Electrochem Soc 148:869–877
Liu HM, Wang YG, Li L, Wang KX, Hosonoa E, Zhou HS (2009) J Mater Chem 19:7885–7891
Acknowledgments
This work was financially supported by grants from the Natural Science Foundation of Fujian Province (2011J033).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Y., Xiao, F. & Gao, S. A new anode material LiZnVO4: synthesis and electrochemical measurements. Ionics 19, 391–394 (2013). https://doi.org/10.1007/s11581-012-0766-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0766-0