Abstract
This paper introduces a carbon paste electrode modified with ferrocene and carbon nanotubes as a voltammetric sensor for determination of sulfite at pH 7.0. The results showed that under the optimum condition (pH 7.0) in cyclic voltammetry, the oxidation of sulfite occurred at a potential about 280 mV less positive than the unmodified carbon paste electrode. Kinetic parameters such as electron transfer coefficient (α) and heterogeneous rate constant (k) for sulfite were also determined using electrochemical approaches. Under the optimized conditions, the electrocatalytic oxidation peak current of sulfite showed two linear dynamic ranges with a detection limit of 0.1 μM for sulfite. The proposed method was examined as a selective, simple, and precise method for voltammetric determination of sulfite in some real samples such as weak liquor from wood and paper industry, boiler water, river water, industrial water, and tap water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sulfites are commonly used in the food and pharmaceutical industries as preservatives and antioxidants, and in the brewing industry as an antibacterial agent. In large quantities, sulfite and its oxidation products are pollutants. Sulfite is a typical example of sulfur oxoanions. In particular, sulfiting agents have received widespread attention as a result of their allergenic effect on those individuals who are hypersensitive. Prior methods for determining sulfite have included titration [1] HPLC [2], chemiluminescence [3], spectrophotometry [4], capillary electrophoresis [5], and electrochemical methods [6–9].
Carbon paste electrode (CPE) is a special kind of heterogeneous carbon electrode consisting of mixture prepared from carbon powder and a suitable water-immiscible or non-conducting binder [10–12]. The use of carbon paste as an electrode was initially reported in 1958 by Adams [13]. In afterward researches, a wide variety of modifiers [14–16] have been used with these versatile electrodes. CPEs are widely applicable in both electrochemical studies and electroanalysis, thanks to their advantages such as very low background current (compared to solid graphite or noble metal electrodes), facility to prepare, low cost, large potential window, simple surface renewal process, and easiness of miniaturization [17]. Besides the advantageous properties and characteristics listed before, the feasibility of incorporating different substances during the paste preparation (which results in the so-called modified carbon paste electrode) allows the fabrication of electrodes with desired composition and, hence, with pre-determined properties [17].
Since the discovery of carbon nanotubes (CNTs) in 1991 [18], numerous investigations were focused on the studies of their properties and applications [19]. Because of the special tube structure, CNTs possess several unique properties such as good electrical conductivity, high chemical stability, and extremely high mechanical strength [20]. In addition, the subtle electronic behavior of CNTs reveals that they have the ability to promote electron-transfer reaction and have a high electrocatalytic effect when used as electrode materials [21]. All these fascinating properties make CNTs as a suitable candidate for the modification of electrodes [22].
In the continuation of our recent studies concerning the preparation of modified electrodes [23–26], in the present work we describe the preparation of a new electrode composed of multiwall carbon nanotubes paste electrode (CNTPE) modified with ferrocene (FC). Then, the performance of the modified electrode for the electrocatalytic determination of sulfite in aqueous solutions was investigated. Moreover, we used the modified electrode as a new and sensitive sensor for determination of sulfite in different real samples such as weak liquor from the wood and paper industry, boiler water, river water, industrial water, and tap water.
Experimental part
Apparatus and reagents
Cyclic voltammetry (CV), chronoamperometry, and differential pulse voltammetry (DPV) were performed in an analytical system, BHP 2063 potentiostat/galvanostat electrochemical analysis system, Behpajooh, Iran. A conventional three-electrode cell assembly consisting of a platinum wire as an auxiliary electrode and an Ag/AgCl(KClsat) electrode as a reference electrode was used. The working electrodes were an unmodified CNTPE or FC-modified CNTPE (FCCNTPE). A Metrohm 710 pH/ion meter was used for pH measurements.
All chemicals used were of analytical reagent grade purchased from Merck (Darmstadt, Germany) unless otherwise stated. Doubly distilled water was used throughout.
Phosphate buffer solutions (sodium dihydrogen phosphate and disodium monohydrogen phosphate plus sodium hydroxide, 0.1 mol L−1), PBS, with different pH values were used.
High viscosity paraffin (d = 0.88 kg L−1) from Merck was used as the pasting liquid for the preparation of the carbon paste electrodes.
Synthesis of carbon nanotubes
Multiwall carbon nanotubes (MWCNTs) were grown by chemical vapor deposition. Several transition metal catalysts have been shown to be active for generation of carbon nanotubes [27].
In this paper, MWCNTs were synthesized from acetylene on a Fe/Co/CaCO3 catalyst at 720 °C, which was previously reported, and the reaction conditions were kept as described before [28]. For the production of MWCNTs, approximately 100 mg of the catalyst containing 5% (w/w) total metals Fe–Co and with a mole ratio 1:1 was weighed and spread into a thin layer onto a graphite subsector centered inside a quartz tube positioned horizontally inside of a resistive tube furnace under nitrogen flow. The furnace temperature was then set at the reaction temperature while it was accurately controlled. When the temperature reached 720 °C, acetylene was introduced at 3.0 mL/min, while the flow of nitrogen was maintained at 200 mL/min. After rinsing the system with nitrogen, the reaction product was collected from the quartz tube. For purification, raw MWCNTs were sonicated (40 kHz) in diluted nitric acid (30% HNO3) for 30 min, and then filtered and washed with distilled water and finally dried at 120 °C overnight. The residue of as-prepared MWCNTs is placed inside a Pyrex tube and oxidized in a furnace at 350 °C in air for different time periods. The diameter, length, purity, and other characteristics of synthesized MWCNTs are summarized in Table 1.
Preparation of the modified electrode
FCNTPEs were prepared by dissolving 0.010 g of ferrocene in diethyl ether and hand mixing, with 89 times its weight of graphite powder and 10 times its weight of MWCNTs, with a mortar and pestle. Stirring evaporated the solvent. A 70:30 (w/w) mixture of ferrocene spiked carbon nanotubes powder and paraffin oil was blended by hand mixing for 30 min until a uniformly wetted paste was obtained. The paste was then packed into the end of a glass tube. Inserting a copper wire into the glass tube at the back of the mixture made electrical contact. When necessary, a new surface was obtained by pushing an excess of paste out of the tube and polishing it on a weighing paper.
For comparison, FC-modified carbon paste electrode (FCCPE) (without MWCNTs), MWCNTs paste electrode (CNTPE) without FC, and unmodified CPE (in the absence of both FC and MWCNTs) were also prepared in the same way.
Recommended procedure
FCCNTPE was polished with a white and clean paper. To prepare a blank solution, 10.0 mL of PBS (pH 7.0) was transferred into an electrochemical cell. The initial and final potentials were adjusted to 0.00 and +0.70 V vs. Ag/AgCl/KCl (3.0 M), respectively. The DPV was recorded to give the blank signal and labeled as I pb. Then, different amounts of sulfite solution were added to the cell, using a micropipette, and the DPVs were recorded to get the analytical signals (I ps). Calibration curve was constructed by plotting the catalytic peaks current vs. the sulfite concentration.
Results and discussion
Electrochemistry of the mediator
Cyclic voltammetry was employed for the investigation the electrochemical properties of the modified electrode in PBS (pH 7.0). The cyclic voltammogram exhibits an anodic and corresponding cathodic peaks with E pa = 0.360 V and E pc = 0.255 V vs. Ag/AgCl/KCl (3.0 M) (Fig. 1, a). The experimental results show well-defined and reproducible anodic and cathodic peaks related to ferrocene/ferricenium redox couple with quasi-reversible behavior because of the peak separation potential, ΔE p = (E pa − E pc), is greater than that of 59/n mV expected for a reversible system. Also, the obtained result from cyclic voltammetry of this modified electrode in various buffered solutions does not show any shift in the anodic and cathodic peak potentials. Therefore, the electrochemical behavior of the redox process of ferrocene/ferricenium couple in the modified electrode is independent on the pH of the solution.
pH optimization
It is well known that the electrochemical behavior of sulfite is dependent on pH value of the solution, whereas the electrochemical properties of Fc/Fc+ redox couple are pH independent. Therefore, optimization of the solution pH seems to be necessary. Thus, we studied the electrochemical behavior of sulfite in 0.1 M PBS in different pH values (3.0 < pH < 9.0) at the surface of FCCNTPE using cyclic voltammetry. As can be seen from Fig. 2, the anodic peak current for electrooxidation of sulfite reached to a maximum value at pH 7.0. The electrochemical behavior of sulfite (K 1 = 1.7 × 10−2 and K 2 = 5.0 × 10−6) is dependent on the pH value of the aqueous solution:
The dependence of the reaction rate on pH is due to variations in the composition of the species that are subjected to the oxidation of sulfite. When sulfite (SO 2−3 ) is dissolved in an aqueous solution, it is in equilibrium with both HSO −3 and SO2, and their relative concentrations depend on the pH of the solution [29] The decreasing of the electrocatalytic activity of the modified electrode in acidic media is due to the increasing of proton concentration hence affect the reactions (1) and (2), and thus, decreasing the oxidation peak current. On the other hand, in higher pH values (pH > 7.0), hydroxide ions affect the electrocatalytic role of the mediator.
Stability and reproducibility
The repeatability and stability of FCCNTPE was investigated by cyclic voltammetric measurements of 50 μM sulfite. The relative standard deviation (RSD) for ten successive assays was 1.2%. When five different FCCNTPEs were used, the RSD for five measurements was 1.3%. When the electrode is stored in the laboratory, the modified electrode retains 97% of its initial response after a week and 95% after 45 days. These results indicate that FCCNTPE has good stability and reproducibility, and could be used for sulfite measurements.
Catalytic effect
Figure 3 depicts the cyclic voltammetric responses from the electrochemical oxidation of 400 μM sulfite at FCCNTPE (curve c), ferrocene modified carbon paste electrode (FCCPE) (curve b), carbon nanotubes paste electrode (CNTPE) (curve d), and bare CPE (curve e). As can be seen, the anodic peak potential for the oxidation of sulfite at FCCNTPE (curve c) and at FCCPE (curve b) is about 340 mV, whereas at CNTPE (curve d) the peak potential is about 610 mV, and at the bare CPE (curve e), the peak potential is about 620 mV. From these results, it is concluded that the best electrocatalytic effect for sulfite oxidation is observed at FCCNTPE (curve c). For example, the results show that the peak potential of sulfite oxidation at FCCNTPE (curve c) shifted by about 270 and 280 mV toward the negative values compared with that at CNTPE (curve d) and at bare CPE (curve e), respectively. Similarly, when we compared the oxidation of sulfite at FCCNTPE (curve c) and FCCPE (curve b), there is an enhancement of the anodic peak current at FCCNTPE relative to that value obtained at FCCPE. In other words, the data obtained clearly shows that the combination of carbon nanotubes and mediator definitely improved the characteristics of sulfite oxidation. FCCNTPE in 0.1 M phosphate buffer (pH 7.0), without sulfite, exhibits a well-behaved redox reaction (curve a), and upon the addition of 400 μM sulfite, the anodic peak current of mediator was greatly increased. In addition, the corresponding cathodic peak disappeared on the reverse scan of the potential (curve c). This behavior is typical of that expected for electrocatalysis at chemically modified electrodes (Diagram 1) [30].
Chronoamperograms obtained at the FCCNTPE in the absence (A) and in the presence (B) of 100 and (C) 200 μM sulfite in a buffer solution (pH 7.0). Inset A charge–time curves: a’ for curve a and b’ for curve b. Inset B Cottrell’s plot for the data from the chronoamperograms. Inset C Dependence of Ic/I L on the t 1/2 derived from the chronoamperogram data
Differential pulse voltammograms of FCCNTPE in the buffer solution (pH 7.0) containing different concentrations of sulfite. 1–10 corresponds to 0.0, 0.4, 1.5, 2.5, 4.0, 10.0, 30.0, 80.0, 100.0, and 120.0 μM sulfite. Insets A and B show the plots of electrocatalytic peak current as a function of sulfite concentration
To obtain further information on the rate-determining step, a Tafel plot was developed for FCCNTPE using the data derived from the raising part of the current–voltage curve (Fig. 4). The Tafel slope was found to be 14.41 V (Fig. 2, inset c), which indicates that transfer coefficient (α) is about 0.57.
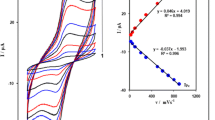
Figure 5 shows the relation of anodic peak current (I pa) versus square root of the scan rate (ν 1/2) for 600 μM of sulfite. The results confirm that anodic peak currents increase linearly with the square root of the scan rate, suggesting that at sufficient over-potential, the reaction is mass transfer controlled.
It can also be noted from Fig. 5 (inset) that with increasing scan rate, the peak potential for the electrooxidation of sulfite shifts to more positive potentials, suggesting a kinetic limitation in the reaction between the oxidized sites of FCCNTPE with sulfite.
Chronoamperometric study
Double step potential chronoamperometry was also employed to investigate the electrochemical behavior of various concentrations of sulfite at FCCNTPE in buffer solution (pH 7.0) by setting the working electrode potential at 0.25 V (at the first potential step) and 0.45 V (at the second potential step) vs. Ag/AgCl/KCl (3.0 M) (Fig. 6). As can be seen, there is no net cathodic current corresponding to the reduction of the mediator in the presence of sulfite when the potential is stepped from 0.25 V to 0.45 V. However, in the presence of sulfite, the charge value associated with forward chronoamperometry is significantly greater than that observed for backward chronoamperometry [Fig. 6, inset A (b’)].
The linearity of electrocatalytic current vs. ν 1/2 shows this current is controlled by diffusion of sulfite from bulk solution toward surface of the electrode that caused to near-Cottrellian behavior. Therefore, the slope of the linear region of Cottrell’s plot can be used to estimate the diffusion coefficient of sulfite (Fig. 6, inset B). A plot of I versus t −1/2 for FCCNTPE in the presence of sulfite gives a straight line, of which the slope of such lines can be used to estimate the diffusion coefficient (D) of sulfite. The mean value of D was found to be 1.75 × 10−5 cm2 s−1.
The rate constant for the chemical reaction between sulfite and redox sites in FCCNTPE, k, can be evaluated by chronoamperometry according to the method of Galus [31]:
where I C is the catalytic current of sulfite at FCCNTPE, I L the limited current in the absence of sulfite, and t is the time elapsed (s). Based on the slope of the I C/I L versus t 1/2 plots, k can be obtained for a given sulfite concentration. Using the values of the slopes, the average value of k was found to be k = 1.3 × 104 mol−1 L s–1 (Fig. 6, inset C).
Calibration plot and limit of detection
Differential pulse voltammetry was used to determine the concentration of sulfite (Fig. 7). The results show two linear segments with different slope for sulfite concentration; namely, for 0.4–4.0 μM of sulfite (Fig. 7, inset A), the regression equation was I p (μA) = (3.348 ± 0.121)C sulfite + (147.640 ± 0.234) (R 2 = 0.9919, n = 5) and for 4.0–120.0 μM of sulfite (Fig. 7, inset B), the regression equation was I p (μA) = (0.189 ± 0.052)C sulfite + (163.150 ± 0.2) (R 2 = 0.9996, n = 5) where C sulfite is concentration of sulfite (μM). The decrease in the sensitivity (slope) of the second linear segment is likely due to kinetic limitation.
The detection limit (3σ) of sulfite was found to be 0.1 μM. This value of detection limit, the linear dynamic range, and the sensitivity for sulfite observed for the FCCNTPE are comparable and even better than those obtained for several other modified electrodes (Table 2).
Interference study
To evaluate the selectivity of the modified electrode for the determination of sulfite in real samples, a group of wood extractive materials (which have phenolic compounds and seem to be electroactive, plus heavy metals) were checked as potential interfering compounds [32]. So, the interference effect of some phenolic compounds such as gallic acid, ellagic acid, and chrysin in the determination of sulfite in a weak liquor solution has been investigated. Results have shown that these kinds of material have no interference in the determination of sulfite in real samples. Also, the result of interfering studied showed that substances such as Ni2+, CN−, Ca2+, Br−, Zn+2, SO 2−4 , Pb+2, and Mn+2 did not show any interferences (at 500-fold) for electroctalytic determination of sulfite using FCCNTPE. Although sulfide ions act as interference for determination of sulfite, it can be minimized, if necessary, using 1.0 mmol L−1 Zn(II).
Real sample analysis
To evaluate the applicability of the proposed method to real sample analysis, it was applied to the determination of sulfite in weak liquor from the wood and paper industry, boiler water, river water, industrial water, and tap water. The determination of sulfite in samples was carried out by the standard addition method. Accuracy was examined by comparison of data obtained from this method with a recognized common method [33] for determination of sulfite (oxidation–reduction titration in acid solution of KIO3/KI in the presence of starch as an indicator). The results are given in Tables 3 and 4.
Conclusion
A ferrocene-modified MWCNTs electrode has been fabricated and used for the electrocatalytic determination of sulfite. The modified electrode showed to be promising for sulfite detection with many desirable properties including good reproducibility, high sensitivity, excellent catalytic activity, low detection limit, and especially its antifouling properties towards sulfite and its oxidation products. Finally, this method was used for the determination of sulfite in different real samples.
References
Monnier GW (1927) Analyst 52:415
Pizzoferrato L, Di Lullo V, Quattrucci E (1998) Food Chem 63:275
Bonifácio RL, Coiche N (2004) Anal Chim Acta 517:125
Hassan SSM, Hamza MSA, Mohamed AHK (2006) Anal Chim Acta 570:232
Jankovskiene G, Daunoravicius Z, Padarauskas A (2001) J Chromatogr A 934:67
Ensafi AA, Karimi-Maleh H, Keyvanfard M (2011) Intern J Environ Anal Chem doi:10.1080/03067319.2011.637198
Mazloum Ardakani M, Habibollahi F, Zare HR, Naeimi H (2008) Int J Electrochem Sci 3:1236
Dadamos TRL, Teixeira MFS (2009) Electrochim Acta 54:4552
Tu W, Lei J, Jian G, Hu Z, Ju H (2010) Chem Eur J 16:4120
Ensafi AA, Karimi-Maleh H (2010) Int J Electrochem Sci 5:392
Raoof JB, Ojani R, Karimi-Maleh H (2008) Asian J Chem 20:483
Ensafi AA, Karimi-Maleh H (2010) Electroanalysis 22:2558
Adams RN (1958) Anal Chem 30:1576
Beitollahi H, Sheikhshoaie I (2011) Anal Methods 3:1810
Mashhadizadeh MH, Khani H (2010) Anal Methods 2:24
Beitollahi H, Raoof JB, Hosseinzadeh R (2011) Electroanalysis 23:1934
Sun W, Gao R, Jiao K (2007) J Phys Chem B 111:4560
Iijima S (1991) Nature 354:56
Chen L, Liu C, Liu K, Meng C, Hu C, Wang J, Fan S (2011) ACS Nano 5:1588
Jacobs CB, Peairs MJ, Venton BJ (2010) Anal Chim Acta 662:105
Beitollahi H, Mazloum Ardakani M, Ganjipour B, Naeimi H (2008) Biosen Bioelec 24:362
Ensafi AA, Rezaei B, Krimi-Maleh H (2011) Ionics doi:10.1007/s11581-011-0562-2
Beitollahi H, Karimi-Maleh H, Khabazzadeh H (2008) Anal Chem 80:9848
Khalilzadeh MA, Karimi-Maleh H (2010) Anal Lett 43:186
Ensafi AA, Karimi-Maleh H (2010) J Electroanal Chem 640:75
Yaghoubian H, Karimi-Maleh H, Khalilzadeh MA, Karimi F (2009) Int J Electrochem Sci 4:993
Sugai T, Yoshida H, Shimada T, Okazaki T, Shinohara H, Bandow S (2003) Nano Lett 3:769
Couteau E, Hernadi K, Seo JW, Thien-Nga L, Miko C, Gaal R, Forro L (2003) Chem Phys Lett 378:9
Dadamos TRL, Teixeira MFS (2009) Electrochim Acta 54:4552
Karimi-Maleh H, Ensafi AA, Allafchian AR (2010) J Solid State Electrochem 14:9
Galus Z (1976) Fundamentals of electrochemical analysis. Ellis Horwood, New York
Sjöström E (1981) Wood chemistry, fundamentals and applications. Academic, New York
Berglund J, Werndrup P, Eiding LI (1994) J Chem Soc Dalton Trans 9:1435
Matsumoto K, Matsubara H, Ukeda H, Osajima Y (1989) Agric Biol Chem 53:2347
Raoof JB, Ojani R, Karimi-Maleh H (2007) Int J Electrochem Sci 2:257
Zhou H, Yang W, Sun C (2008) Talanta 77:366
Scampicchio M, Lawrence NS, Arecchi A, Mannino S (2008) Electroanalysis 20:444
Pournaghi-Azar MH, Hydarpour M, Dastangoo H (2003) Anal Chim Acta 497:133
Acknowledgments
The authors wish to thank Islamic Azad University, Qaemshahr and Majlesi Branch, and the Iranian Nanotechnology Initiative Council for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karimi-Maleh, H., Ensafi, A.A., Beitollahi, H. et al. Electrocatalytic determination of sulfite using a modified carbon nanotubes paste electrode: application for determination of sulfite in real samples. Ionics 18, 687–694 (2012). https://doi.org/10.1007/s11581-011-0654-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0654-z