Abstract
The authors have performed a comparative study of the performance of various carbonaceous material-based electrochemical sensors in order to identify the most appropriate sensor for determination of sulfonamides. The electro-oxidative power of carbon paste electrodes prepared using carbon black, graphite, carbon nanopowder, acetylene black, multiwalled carbon nanotubes and glassy carbon powder was investigated by square-wave voltammetry at pH 6.0 using sulfamethoxazole (SMX) as the model analyte. It is found that carbon paste electrodes prepared with graphite or carbon nanopowder and operated at a voltage of 0.93 (vs. Ag/AgCl) display the highest sensitivity and lowest detection limit. Next, the sulfonamides sulfadiazine, sulfacetamide, sulfadimethoxine, sulfathiazole, sulfamethiazole and sulfamerazine were also tested. The voltammetric response is linear in the 1 to 75 μM concentrations range, with detection limits range from 0.4 to 1.2 μM, and sensitivities were between 10 and 38 nA⋅μM−1. The carbon nanopowder paste electrode (CNPE) showed the lowest detection limit (0.12 μM) for SMX and was successfully applied to its determination in (spiked) water samples and in pharmaceutical formulation.

Schematic illustration of the preparation of paste electrodes based on nanoparticles for use in voltammetric determination of sulfonamides
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A wide range of pharmaceuticals has been detected as micropollutants in fresh and marine waters, which makes the research of their fate and transport in natural aqueous systems and their environmental impact needed [1, 2]. Among these emerging pollutants, the antibiotics have gained more attention due to their biological activity and the possibility to cause the development of resistance genes in bacteria of the environment, reducing, in the long-term, the effectiveness of these compounds [3, 4]. Sulfonamides, commonly known as sulfa drugs, are the most antimicrobial agents used to prevent and to treat the bacterial infective disease, because of their inexpensiveness and wide-spectrum antimicrobial activity. They are very active against a broad spectrum of Gram-positive and many Gram-negative bacteria [5, 6]. However, sulfonamides residues have been detected at a significant concentration levels in many animal products and environmental samples which present a potential health risk due to their allergic properties [7]. They also exhibit a potential toxicity to aquatic organisms and are responsible for the emergence of antibiotic resistant strains. The excessive use of sulfonamides in animal husbandry leads to their presence as a potential hazard to human health [8]. Several methods for sulfonamides detection were reported in literature including high performance liquid chromatography (HPLC) [9, 10], capillary electrophoresis coupled mass spectrometry [11], liquid chromatography (LC) [12] gas chromatography [13], spectrophotometric methods [14, 15]. These techniques are precise and robust but they have many inconvenient such as are time-consuming, expensive, require complicated sample preparation. However the electroanalytical methods offer many advantages for the detection of sulfonamides such as they are simple to use, not need sample preparation, require low cost instrumentation, exhibit low detection limits, high dynamic range high sensitivity and good selectivity in comparison with the other analytical methods [16, 17]. Sulfonamides compounds can be electrochemically oxidized at the –NH 2 group and reduced at the –SO 2 NH– group [18]. Various reports on electroanalytical determination of sulfonamides have been reported in literature using several types of electrodes [6, 18–21]. The electrochemical sensors based on carbon materials are one of the categories of sensors which have unique characteristics as analytical tools comparable to the other kinds of electrodes. The CPEs offer many advantages such as their facility to prepare, chemical inertness, economic, renewability, robustness, low ohmic resistance, stable response, suitability for a variety of sensing, and large detection applications [22, 23]. In our recent work [24] we have successfully reported a general study on the analytical performances of solid-like carbon paste electrodes (SCPEs) using different carbon materials namely carbon black, acetylene black, carbon nanopowder and carbon mesoporous. In this study various carbon nanomaterials namely Carbon Black (CB), Acetylene Black (AB), Carbon Nanopowder (CN), Graphite, Multiwalled Carbon Nanotubes (MWCNTs) and Glassy Carbon (GC) were employed for preparation of paste electrodes and investigated for their responses toward sulfonamides determination. The Carbon Nanopowder Paste Electrode (CNPE) and conventional carbon paste electrode (CPE) showed a high sensitivities and low detection limit for Sulfamethoxazole. The analysis of real samples shows good recoveries for SMX using the developed method.
Experimental
Chemicals
Sulfamethoxazole (SMX), Sulfadiazine (SDZ) ≥ 99.0 %, Sulfamerazine (SMZ) ≥ 99.0 %, Sulfadimethoxine (SDM) ≥ 98.5 %, Sulfathiazole (STZ) ≥ 98.0 %, Sulfamethiazole (SMT) ≥ 99.0 %, Sulfacetamide (SCT) ≥ 98.0 %,, Bisphenol A (BPA) ≥ 99.0 %, Liquid and Solid paraffin, and solvents were of analytical grade and purchased from Sigma-Aldrich. Different grades of manufactured CB (N110, N220, N375 and N772) used in our study were obtained from Cabot Corporation (Ravenna, Italy). GC powder was purchased from HTW (Germany). AB was purchased from Strem Chemicals. Graphite <0.1 mm, CN <50 nm and MWCNTs ≥98 % carbon basis, O.D × L 10 nm ± 1 nm × 4.5 nm × 3- ~6 μm, TEM were purchased from Sigma-Aldrich. The characteristics of different carbon materials are indicated in Table. S1. K2HPO4 and KH2PO4, CH3COOH and H3PO4 were purchased from ProLab. All the other reagents were of analytical grade. The distilled water was used throughout the experiments.
Apparatus
Electrochemical measurements were performed with an electroanalytical instrument PalmSens (Palmsens BV Houten, The Netherlands) in connection with a PC controlled by software PSTrace 3.0. A traditional three-electrode system was used. Paste electrodes were prepared using several carbon materials as the working electrode, Ag/AgCl (saturated with KCl) as the reference electrode, and a bare of stainless steel as the counter electrode were employed. All the electrochemical experiments were performed at room temperature. The pH values of the solutions were measured with HANNA instrument (HI 8521) pH meter.
Preparation of carbon paste electrodes (CPEs)
The CPEs were prepared by hand mixing of a proportion of carbon materials with the paraffin. The adequate amount of carbon material (75 % of graphite powder, 70 % of CN, 80 % GC and 50 % of CB, AB and MWCNTs) was hand mixed with liquid paraffin using a pestle and mortar to form a homogeneous paste. However, in the case of solid CPEs, solid paraffin was first liquefied by heating at around 45 °C before mixing with carbon material. The resulting pastes were packed into the well of the working electrode to a depth of 2 mm with 3 mm of diameter. The surface exposed to the solution was polished on a print paper. The body of the working electrode was a Teflon tube.
Experimental methods
Linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), square-wave voltammetry (SWV) and amperometry were carried out with three electrodes in phosphate buffer.
LSV measurements were recorded by applying a sweep potential from +0.3 V to +1.3 V with a potential step of 10 mV at a scan rate of 0.05 V.s−1. DPV measurements w were performed by applying a sweep potential from +0.7 V to +1.3 V at pulse amplitude of 30 mV and pulse width 0.1 s with a scan rate of 10 mV.s−1 SWV measurements were performed by scanning in the potential range from (+0.7 V) to (+1.3 V) vs. Ag/AgCl reference electrode at the pulse amplitude, 10 mV with the frequency 10 Hz. Amperometric measurements of SMX were carried out under stirring solution at the applied potentials +890 mV and +920 mV using CPE and CNPE respectively.
Analysis in real samples
Drinking water samples were collected from a local market. The analysis of their content on sulfonamides before and after their fortification with a known amount of SMX was performed using the developed method.
The developed method was used for the determination of SMX in tablets (CO-TRIM®) purchased from a drugstore in Casablanca city (Morocco). The sample solution was prepared as follows: 5 tablets were weighed and powdered, then an accurate weight of the powder equivalent to one tablet was mixed with methanol in a 100 ml calibrated flask, stirred for about 10 min, then sonicated for 15 min, and filtered to separate any insoluble matter. The filtrate was collected in a clean flask. After dilution with water, an aliquot of sample solution was measured in phosphate buffer pH 6.0. The amount of SMX per tablet was calculated from standard calibration curve.
Results and discussion
Electrochemical behavior of SMX at different paste electrodes
The Fig. 1 shows the LSV responses of paste electrodes based on various carbon nanoparticles prepared using liquid or solid paraffin in phosphate buffer solution pH 6.0, containing 100 μM of SMX. The results obtained by LSV showed that the oxidation peak of SMX at different tested electrodes varies between +890 mV and +980 mV and the intensity of the oxidation peak current values varies between 0.31 μA and 2.52 μA (Table 1). The results obtained using paste electrodes based on MWCNTs in comparison with the conventional CPEs, showed a slightly shifting in terms of oxidation peak potential due to the oxidation of amino group-NH2 as reported in literature for dopamine and epinephrine [25]. The oxidation peak potential values obtained by CPEs prepared using solid paraffin was generally higher than that obtained by the electrodes prepared by the liquid paraffin. The choice of the optimal ratio between carbon powder and the binder was based on the best ratio that gives a high electrochemical response and leads to a good mechanical aspects of the pastes. The amount of the binder which covers all particles of carbon surface area is highly dependent on the size of the nanoparticles. The optimal ratio has been adopted taking into account the results published in literature [26–29]. Indeed, under the experimental conditions, 50 % was chosen for CB, AB and MWCNTs nanoparticles. However, 70 %, 75 % and 80 % were chosen for CN, Graphite and GC respectively, leads to a compact paste which is mechanically stable under stirred solutions. Both solid CPEs [28] and conventional CPEs [29] exhibit an easy surface renewing. On the other hand, the CPEs prepared with liquid paraffin were more sensitive to gradual surface erosion phenomena in stirred solution and they have an unstable baseline which limits the analytical performances under hydrodynamic conditions as reported in literature [30].
Optimization of the method
The following parameters were optimized:
Choice of voltammetric technique
The voltammetric study of conventional CPE (paste electrodes based on graphite) was carried out by using DPV and SWV waveforms in 0.05 M of phosphate buffer solution containing 10 μM of SMX. As shown in Figure. S1 (Electronic Supplementary Material), the peak current intensity obtained by mean of SWV was higher than the one obtained by DPV. The SWV technique provided the good peak current and well resolved narrow peak. Therefore, it was chosen for further experiment work.
Effect of pH
The electrooxidation of SMX was studied over B-R buffer at a pH range of 3.0 to 9.0. SWV of 10 μM of SMX at different pH in this range was recorded at the surface of a conventional CPE. The results confirm that the oxidation peak is pH dependent. As shown in Figure S2, the peak current has a maximum value at pH 6.0 decreasing for both high and low pH values. Between pH 3.0 and 9.0, the oxidation peak potential is shifted to less positive values with increasing pH. This is a consequence of the deprotonation in the oxidation process that is facilitated at higher pH. It can be concluded that the catalytic oxidation of SMX is more favored at pH 6.0, thus it was fixed as the optimal pH. As shown in Figure S3 the B-R buffer and phosphate buffer pH 6.0 were compared, and it was observed that the peak current was highest, the peak shape was well defined, and the intensity current signal was increased by 19 % at phosphate buffer pH 6.0. Hence, phosphate buffer was chosen for the further experiments.
Effect of scan rate
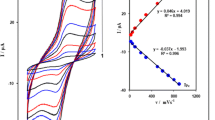
The effect of scan rate on the oxidation peak current (Ipox) was studied by CV. As illustrated in Figure S4, the Ipox of 100 μM SMX shows a linear relationship with scan rate within the range of 10–250 mV/s. The Ipox varies linearly with square root of scan rate indicating that the oxidation of SMX at various paste electrodes based on nanoparticles is controlled by diffusion process.
Analytical performances
Determination of sulfonamides using square-wave voltammetry
To apply the optimized method for the analysis of seven sulfonamide derivatives, the influence of sulfonamides concentration on the peak current, over the potential range of +0.7 to +1.3 V, was studied at conventional CPE.
The detection limits in the range of 0.4–1.2 μM and the sensitivities between 10 and 38 nA.μM−1 were obtained (Table 2). Indeed, a low detection limit of 0.4 μM for SMT, SDZ and SCT sulfonamides derivatives, and a high sensitivity of 38 nA.μM−1 for SMX were achieved.
Sulfamethoxazole, indicated in Figure S5, is one of the most used sulfonamides in human and veterinary medicine [6]. Hence it was selected during the comparative study of the analytical performances of paste electrodes. The oxidation peak current (Ipox) at about 936 mV increased linearly as a function of SMX concentration (CSMX) over the range of 1–75 μM at a CNPE (Fig. 2a). After plotting the corresponding voltammetric Ipox versus CSMX, the calibration curve (Fig. 2b) showed two linear regions. The first region demonstrated a linearity over a concentration range of 1.0–10.0 μM with a regression equation of I (nA) = 44.6 CSMX (μM) + 64.1 and correlation coefficient of 0.991 (inset of Fig. 2b). The second linear region with a correlation coefficient of 0.987 in the concentration range of 10 to 75 μM showed a smaller slope 17.3 nA.μM−1.than the first region. Since the aim of this study is the detection of sulfonamides at low concentrations level, the sensitivity was tested in the range of 1–10 μM for all the paste electrodes. From this plot, the calculated LOD and limit of quantification (LOQ) at CNPE were 0.12 μM and 0.4 μM according to the 3Sb/m and 10Sb/m criteria, respectively, where Sb is the standard deviation of the blank and m is the slope of the calibration curve. The results obtained for the different kinds of electrodes are summarized in Table 3 and showed the highest sensitivity (44.6 nA.μM−1) for CNPE.
SWV of varying concentrations of SMX obtained at the Carbon Nanopowder Paste Electrode in phosphate buffer pH 6.0. (the letters a–j correspond to blank, 1;2;3;4;6;8;10;20;50;75 μM SMX). Inset shows the corresponding calibration curve of SMX in the concentration range of 1.0–10.0 μM, Eampl = 10 mV, Estep = 8 mV, tcond = 10 s, Frequency = 10 Hz
The results of sensitivity obtained for the electrodes based on various kinds of carbon materials were comprised between 7.6 and 44.6 nA.μM−1. In agreement with the work reported [31] the electrodes based on carbon nanotubes paste electrodes showed a high sensitivity due to the high surface area of the nanoparticles. Furthermore the paste electrodes based on AB, CN showed a high sensitivity towards SMX. Taking into account the high sensitivity and the low detection limit values, CNPE and conventional CPE were selected as the best electrodes.
Comparison of the applied method with others reported in literature
CNPE and CPE showed the lowest LOD compared to the other reported in literature (Table 4). Furthermore they are economic, easy to prepare and do not require any surface modification.
Amperometric detection of SMX
The amperometric measurement of SMX using CPE and CNPE was tested in the presence of consecutive increasing concentration. The Fig. 3 illustrates the amperograms responses towards SMX at low concentration with the surface of CPE and CNPE at a constant applied potential 890 mV and 920 mV respectively (Table 1). It can be seen that the CNPE has a better analytical performance for the detection of SMX in term of signal/noise ratio and LOD (0.5 μM) compared to CPE. Amperometric measurements were in correlation with the results obtained by SWV. However, SWV technique is very fast and sensitive for the detection of SMX which makes it the method of choice.
Interference study
The probably interferents species in the environmental samples were phenols. Indeed the electrochemical method showed a good separation between the oxidation peaks of Bisphenol (BPA) and SMX at the surface of CNPE. Furthermore the presence of 10 μM of BPA has no matrix effect toward SMX oxidation as shown in figure S6. Since Trimethoprime (TMP) is often used as a part of synergistic combination with SMX in tablets, its influence on the Ipox of SMX was studied. As indicated in figure S7 the TMP has no matrix effect on SMX oxidation.
Repeatability and reproducibility of measurements
The precision estimated in terms of the relative standard deviation (RSD) for three consecutive calibration curves was equal to 2.2 % which revealed an acceptable repeatability of the electrode. Reproducibility of the method was evaluated by successive determinations (n = 5) of SMX with five different electrodes. The RSD value of less than 3.2 % was obtained for 4 μM of SMX indicating a good reproducibility.
Analytical applications
A commercial pharmaceutical product containing SMX (in the presence of trimethoprime) was analyzed by SWV using the CNPE following the above described electroanalytical determination. The values obtained for SMX 810 ± 33 mg was almost equal to the label value (800 mg). The relative difference between the labeled and obtained value is 1 %. The RSD value of five determinations was lower than 4 %.
The analysis of four drinking water samples before and after their fortification with a known amount of SMX was performed in order to determine the recovery as the ratio of the amounts found/added. The calculated recoveries were between 95.5–100.2 % (Table 5). The results showed that CNPE is effective and sensitive for real water sample analysis. Ongoing work involves the use of solid phase extraction columns for preconcentration of sulfonamides trace in environmental matrix before their quantification using developed sensors.
Conclusions
In this study, paste electrodes based on various carbon nanomaterials were tested for the detection of sulfonamides. A submicromolar concentration of sulfonamides was detected successfully using SWV at unmodified paste electrodes. The carbon nanopowder paste electrode (CNPE) showed the highest sensitivity and the lowest detection limit 0.12 μM towards SMX. The CNPE showed good recoveries for the determination of sulfonamides in water samples and in pharmaceutical formulations. Taking into account the good electroanalytical performances of this electrode, it can be considered as good choice for the electrochemical detection of sulfonamides.
References
Barana W, Adameka E, Ziemianskab J, Sobczaka A (2011) Effects of the presence of sulfonamides in the environment and their influence on human health. J Hazard Mater 196:1–15. doi:10.1016/j.jhazmat.2011.08.082
Dias-Cruz MS, García-Galán MJ, Barceló D (2008) Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography quadrupole linear ion Trap-mass spectrometry. J Chromatogr A 1193:50–59. doi:10.1016/j.chroma.2008.03.029
Cesarino I, Cesarino V, Lanza MRV (2013) Carbon nanotubes modified with antimony nanoparticles in a paraffin composite electrode: simultaneous determination of sulfamethoxazole and trimethoprim. Sensors Actuators B J188:1293–1299. doi:10.1016/j.snb.2013.08.0
Trenholm RA, Vanderford BJ, Snyder SA (2009) On-line solid phase extraction LC-MS/MS analysis of pharmaceutical indicators in Water: a green alternative to conventional methods. Talanta 79:1425–1432. doi:10.1016/j.talanta.200906006
Wutz K, Niessner R, Seidel M (2011) Simultaneous determination of four different antibiotic residues in honey by chemiluminescence multianalyte chip immunoassays. Microchim Acta 173:1–9. doi:10.1007/s00604-011-0548-9
Souza CD, Braga OC, Vieira IC, Spinelli A (2008) Electroanalytical determination of sulfadiazine and sulfamethoxazole in pharmaceuticals using a boron-doped diamond electrode. Sensors Actuators B 135:66–73. doi:10.1016/j.snb.2008.07.020
Munir M, Wong K, Xagoraraki I (2011) Release of antibiotic resistant bacteria and genes in the effluent and biosolids of five wastewater utilities in Michigan. Water Res 45:681. doi:10.1016/j.watres.2010.08.033
Sun N, Wu S, Chen H, Zheng D, Xu J, Ye Y (2012) Determination of sulfamethoxazole in milk using molecularly imprinted polymer monolith microextraction coupled to HPLC. Microchim Acta 179:33–40. doi:10.1016/j.watres.2010.08.033
Yudthavorasit S, Chiaochan C, Leepipatpiboon N (2011) Simultaneous determination of multi-class antibiotic residues in water using carrier-mediated hollow-fiber liquid-phase microextraction coupled with ultra-high performance liquid chromatography tandem mass spectrometry. Microchim Acta 172:39–49. doi:10.1007/s00604-010-0454-6
Font G, Juan-Garcia A, Pico Y (2007) Pressurized liquid extraction combined with capillary electrophoresis-mass spectrometry as an improved methodology for the determination of sulfonamide residues in meat. J Chromatogr A 1159:233–241. doi:10.1016/j.chroma.2007.03.062
Akay C, Ozkan SA (2002) Simultaneous LC determination of trimethoprim and sulphamethoxazole in pharmaceutical formulations. J Pharm Biomed Anal 30:1207–1213. doi:10.1016/S0731-7085(02)00460-0
Chiavrino B, Crestoni ME, Marzio AD, Fornarini S (1998) Determination of Sulphonamide antibiotics by gaz chromatography coupled with atomic emission detection. J Chromatogr B 706(2):269–277. doi:10.1016/S0378-4347(97)00568-9
Nagaraja P, Sunitha KR, Vasantha RA, Yathirajan HS (2002) Iminodibenzyl as a novel coupling agent for the spectrophotometric determination of sulfonamide derivatives. Eur J Pharm Biopharm 53:187–192. doi:10.1016/S0939-6411(01)00235-1
Amin AS, Zareh MM (1996) Acetylacetone–formaldehyde reagent for the spectrophotometric determination of some sulfa drugs in pure and dosage forms. Microchim Acta 124:227–233. doi:10.1007/BF01242820
Msagati TAM, Ngila JC (2002) Voltammetric detection of sulfonamides at a poly (3-methylthiophene) electrode. Talanta 58:605–610. doi:10.1016/S0039-9140(02)00327-2
Sabry SM (2007) Polarographic and voltammetric assays of sulfonamides as α-Oxo-γ-Butyrolactone Arylhydrazones. Anal Lett 40:233–256. doi:10.1080/00032710600867564
Fotouhia L, Hashkavayia AB, Heravia MM (2013) Electrochemicalbehaviour and voltammetric determination of sulphadiazine using a multi-walled carbon nanotube composite film glassy carbon electrode. J Exp Nanosci 8:947–956. doi:10.1080/17458080.2011.624554
Sadeghi S, Motaharian A (2013) Voltammetric sensor based on carbon paste electrode modified with molecular imprinted polymer for determination of sulfadiazine in milk and human serum. Mater Sci Eng C33(8):4884–4891. doi:10.1016/j.msec.2013.08.001
Braga OC, Campestrini I, Vieira IC, Spinelli A (2010) Sulfadiazine determination in pharmaceuticals by electrochemical reaction on a glassy carbon electrode. J Braz Chem Soc 21:813–820. doi:10.1590/S0103-50532010000500008
Dejmkova H, Mikes M, Barek J, Zima J (2013) Determination of Sulfamethizole using voltammetry and amperometry on carbon paste electrode. Electronalysis 25:189–194. doi:10.1002/elan.201200354
Tadi KK, Motghare RV, Ganesh V (2014) Electrochemical detection of sulfanilamide using pencil graphite electrode based on molecular imprinting technology. Electroanalysis 26:1–10. doi:10.1002/elan.201400251
Svancara I, Schachl K (1999) Testing of unmodified carbon paste electrodes. Chem List 93:490–499
Kalcher K, Kauffmann JM, Wang J, Svancara I, Vytras K, Neuhold C, Yang Z (1995) Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990–1993. Electroanalysis 7:5–22. doi:10.1002/elan.1140070103
Malha SIR, Ait Lahcen A, Arduini F, Ourari A, Amine A (2015) Electrochemical characterization of carbon solid-like paste electrode assembled using different carbon nanoparticles. Electroanalysis 27:1–9. doi:10.1002/elan.201500637
Carrazon JMP, Corona PC, Diez LMP (1987) Electroanalytical study of sulphadiazine at solid electrodes, determination in pharmaceutical preparations. Electrochim Acta 32:1573–1575. doi:10.1016/0013-4686(87)90006-5
Campestrini I, De Braga OC, Vieira IC, Spinelli A (2010) Application of bismuth-film electrode for cathodic electroanalytical determination of sulfadiazine. Electrochim Acta 55:4970–4975. doi:10.1016/j.electacta.2010.03.105
Valentini F, Amine A, Orlanducci S, Terranova ML, Palleschi G (2003) Carbon nanotube purification: preparation and characterization of carbon nanotube paste electrodes. Anal Chem 75:5413–5421. doi:10.1021/ac0300237
Petit C, Kauffmann JM (1995) New carbon paste electrode for the development of biosensors. Anal Proc 32:11–12. doi:10.1039/AI9953200011
Rice ME, Galus Z, Adams RN (1983) Graphite paste electrodes: effects of paste composition and surface states on electron-transfer rates. J Electroanal Chem 143:89–102. doi:10.1016/S0022-0728(83)80256-3
Ricci F, Goncalves C, Amine A, Gorton L, Palleschi G, Moscone D (2003) Electroanalytical study of Prussian blue modified glassy carbon paste electrodes. Electroanalysis 15:1204–1211. doi:10.1002/elan.200390148
Amine A, Kauffmann JM, Patriarche GJ, Kaifer AF (1991) Long-term operational stability of a mixed glucose oxidase redox mediator-carbon paste electrode. Anal Lett 24(8):1293–1315. doi:10.1080/00032719108052973
Malha SIR, Mandli J, Ourari A, Amine A (2013) Carbon black-modified electrodes as sensitive tools for the electrochemical detection of nitrite and nitrate. Electroanalysis 25:2289–2297. doi:10.1002/elan.201300257
Acknowledgments
The research presented in this manuscript was supported by the European project ‘Sensing toxicants in Marine waters makes Sense using biosensors’, (SMS) GRANT AGREEMENT N° 613844.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
This work was first presented at the 7th Intl. Workshop on «Biosensors for Food Safety and Environmental Monitoring» (held in Erfoud; Morocco; 19 – 21 Nov. 2015; www.biocap.ma/).
Electronic supplementary material
ESM 1
(DOCX 353 kb)
Rights and permissions
About this article
Cite this article
Ait Lahcen, A., Ait Errayess, S. & Amine, A. Voltammetric determination of sulfonamides using paste electrodes based on various carbon nanomaterials. Microchim Acta 183, 2169–2176 (2016). https://doi.org/10.1007/s00604-016-1850-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1850-3