Abstract
A novel group of polymer blend electrolytes based on the mixture of poly(vinyl acetate) (PVAc), poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP), and the lithium salt (LiClO4) are prepared by solvent casting technique. Ionic conductivity of the polymer blend electrolytes has been investigated by varying the PVAc and PVdF-HFP content in the polymer matrix. The maximum ionic conductivity has been obtained as 0.527 × 10−4 Scm−1 at 303 K for PVAc/PVdF-HFP ((25/75) wt.%)/LiClO4 (8 wt.%). The complex formations ascertained from XRD and FTIR spectroscopic techniques and the thermal behavior of the prepared samples has been performed by DSC analysis. The surface morphology and the surface roughness are studied using SEM and AFM scanning techniques respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ever since the discovery of ionic conductivity in alkali metal salt complexes of solid polymers such as poly(ethylene oxide) (PEO) [1] and the recognition of their technological importance for the construction of solid state rechargeable batteries, electrochromic devices, and sensors [2], the first generation polymer electrolytes were investigated by Armand et al. based on the combination of high molecular weight poly(ethylene oxide) and lithium salts. The second generation consists of modified PEO and new polymers based electrolytes. In the development of third generation polymer electrolytes, attention was focused on increasing the room temperature ionic conductivity as well as the dimensional stability of the polymer electrolyte [3]. PEO and lithium salt-based polymer electrolytes invented by Armand et al. have very low ambient temperature conductivity typically of the order of 10−8 Scm−1 [4]. Due to the poor performance of this electrolyte, many attempts have been made on various polymers such as PVC [5], PAN [6], PMMA [7], PVdF [8], etc. Among the various polymers, Poly(vinyl acetate) (PVAc) offers good mechanical stability, low glass transition temperature, and easy film formation properties. Poly(vinyl acetate) films have high tensile strength (29.4 ± 49.0 MPa) [9] and abrasion resistance; hence, it is used as binder, adhesive applications in packaging, wood gluing, and chewing gum bases. Apart from that PVAc have some special properties such as tasteless, odorless, and non-toxic. Baskaran et al. [10] reported that PVAc-based lithium electrolytes have conductivity in the range 10−5 to 10−3 S cm−1. The conductivity of PVAc polymer complexes also show high values by blending PVAc with other suitable polymer. Ionic conductivity of PVAc with poly(methyl methacrylate) (PMMA) and poly(vinyledinefluoride) (PVdF) based blend electrolytes was first reported by Baskaran et al. [11, 16], and it has good compatible nature. In the present study, a new polymer electrolyte composed of PVAc–poly(vinylidene fluoride-co-hexaflouropropylene) (PVdF-HFP) blend as the host polymer and lithium perchlorate as the doping salt has been prepared. The salt LiClO4 was chosen since it has higher anionic radius [12].
In connection with this, copolymerization or blending technique for matrix polymers of polymer electrolytes has been considered for enhancing the properties of polymer electrolytes [13–15]. In comparison, blending has been found to be more useful because of ease of preparation and control of the properties of polymer electrolytes by changing the composition of blended polymer matrix.
In the present study, an attempt has been made on PVAc/PVdF-HFP based blend electrolyte with constant proportion of lithium salt (LiClO4). The polymer blend electrolytes were prepared using solvent casting technique. Tetrahydrofuran was used as a solvent. The prepared films were characterized under various spectroscopic techniques such as Impedance analysis, X-ray diffraction (XRD), Fourier Transform Infra Red analysis (FTIR), differential scanning calorimetry (DSC), Scanning electron microscope (SEM), and atomic force microscope (AFM).
Experiment
PVAc (Mw = 140,000) and PVdF-HFP of high molecular weight (Mw = 400,000) and LiClO4 were purchased from Aldrich chemicals limited USA. The polymer electrolytes of various blend ratios were prepared by solution casting technique with Tetrahydrofuran as solvent. The complex was stirred well around 24 h with the help of magnetic stirrer and degassed to remove air bubbles. Finally, the solution thus obtained was cast on a glass plate and the solvent was allowed to evaporate slowly in air at room temperature for 48 h. This procedure yielded mechanically stable, free-standing, and flexible films. The films were further dried for 5 h in vacuum at 60°C to remove any trace of solvent. The polymer blends of various (PVAc/PVdF-co-HFP) blend ratios such as 100:00, 75:25, 50:50, 25:75, and 00:100 with constant salt concentration were prepared with a view to optimize the blend ratio on the basis of ionic conductivity and mechanical stability of the film. The ionic conductivity studies were carried out with the help of stainless steel blocking electrodes by using a computer controlled micro auto lab type III Potentiostat/Galvanostat of frequency range 1 Hz–300 KHz in the temperature range 303–373 K. The XRD equipment used in this study was X'pert PRO PANlytical X-ray diffractometer. FTIR spectroscopy studies were carried out using SPECTRA RXI, Perkin Elmer spectro-photometer in the range 400–4,000 cm −1. DSC thermal analyses of blend electrolytes were studied using METLER, model 822R. Roughness parameter and the surface morphology of the maximum ionic conductivity sample were obtained by AFM and SEM, respectively.
Results and discussion
XRD Analysis
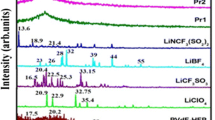
Figure 1(a–h) shows the XRD pattern of pure PVAc, PVdF-HFP, LiClO4, and PVAc/PVdF-HFP/LiClO4-based polymer blend electrolytes respectively. Figure 1(a) of LiClO4 shows intense peaks at angles 2θ = 20.9°, 22.92°, 26.56°, 32.75°, and 35.4°, which reveal the crystalline nature of the ionic salt. Three peaks are found at 2θ = 17.3°, 18.59°, 38.78° for P(VdF-HFP; Fig. 1(b)), and they confirm the partial crystallization of PVDF units in the copolymer, to give an overall semi-crystalline morphology for P(VdF-HFP) [16]. Two broad peaks appear at 2θ = 14.5° and 19° for PVAc (Fig. 1(c)), which reveal its complete amorphous nature. It has been observed that crystallinity of the blended complex gets decreased with the addition of PVAc. Also the intensity of all diffraction peaks are significantly lower in the blended electrolyte system. This implies that the two polymers PVAc and PVdF-HFP have good compatibility with each other. Absence of the peaks corresponding to LiClO4 indicates the complete dissolution of salt in the polymer matrix. This shows that LiClO4 does not contain any separate phase in the complex system.
FTIR studies
Infrared spectral analysis is a powerful tool for identifying the nature of bonding and different functional groups present in a sample by monitoring the vibrational energy levels of the molecules, which are essentially the fingerprint of different molecules [17, 18].
Figure 2 depicts the FTIR transmittance spectra in the range 400–4,000 cm−1 for pure polymers and their blends with different blend ratios. The vibrational bands observed at 2,933 cm−1 and 2,465 cm−1 are ascribed to –CH3 asymmetric and symmetric stretching vibrations respectively for Pure PVAc. The strong absorbance at 1,734 cm−1 represents the C = O stretching vibration mode. The existence of C–O band has been confirmed by the strong absorbance band at around 1,033 cm−1. The strong bands at 1,373 cm−1 is ascribed to –CH3 symmetric bending vibration of pure PVAc. The band at 1,243 cm−1 is assigned to C–O–C symmetric stretching mode of vibration. The peak at 947 cm−1 is ascribed to CH bending vibration, and the peak at 609 cm−1 is assumed to be linked with CH3 (C–O) group. The C–H rocking mode of vibration has been confirmed by the presence of a band at 799 cm−1 [19, 20].
The vibrational peaks at 502 and 416 cm−1 are assigned to bending and wagging vibrations of –CF2, respectively. Crystalline phase of the PVdF-HFP polymer is identified by the vibrational bands at 985, 763, and 608 cm−1 [21]. The strong absorption peak appeared at 1,173 cm−1 is assigned to the symmetrical stretching of –CF2 group. The peak appeared at 1,390 cm−1 is assigned to the CH2 groups [22–24].
Table 1 shows the comparison of band spectra of pure polymers and their blends with different blend ratios. From the table, it is clear that the band assignments of FTIR spectra of blend samples are shifted from their pure spectra. For all the blends, some peaks are found above 3,000 cm−1, which correspond to the C–H stretching vibration modes of blend electrolytes. In addition, some new peaks are present and some of them disappeared in the blended electrolytes. Thus the spectral analysis confirms the complexation of these two polymers and lithium salt.
Conductivity analysis
Impedance spectroscopic analysis
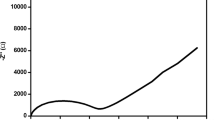
Temperature dependent complex impedance plot of PVAc/PVdF-HFP ((25/75) wt.%)/LiClO4 (8 wt.%) blended electrolytes are shown in the Fig. 3. The complex plot shows semicircular portion which corresponds to the bulk resistance (R b) with the parallel combination of the frequency-dependent capacitance C g and the low-frequency spike is due to the interfacial impedance of the cell setup. The bulk resistance value R b is determined from the low frequency intercepts on the x-axis of the complex impedance plots. The ionic conductivity of solid polymer blended electrolytes is calculated using the equation σ=L/R b A, where L is the thickness of the polymer electrolyte film and A is the surface area of the film. From Fig. 3, it was observed that the bulk resistance (R b) values decrease with the increase of temperature from 303 to 363 K and the ionic conductivity values are found to increase from 0.527 × 10−4 to 4.802 × 10−4 Scm−1. Even though the PVAc-salt and PVdF-HFP-salt complexes contain maximum ionic conductivity, the 2.5/75 wt.% ratio of polymer blend has free-standing film nature and hence they are very much suitable for device fabrication than the other samples (Table 2).
Temperature-dependent conductivity
Figure 4 shows the temperature-dependent ionic conductivity of various blends and polymer salt complexes. From the plot, it is evident that, as the temperature increases the ionic conductivity also increases for all the complex systems. The increase in conductivity with temperature may be due to the decrease in viscosity and hence increased chain flexibility [25]. The conductivity can be expressed as \( \sigma = {\sigma_0}\exp \left( {{{{E_{\rm{a}}}} \mathord{\left/{\vphantom {{{E_{\rm{a}}}} {\hbox{KT}}}} \right.} {\hbox{KT}}}} \right) \), where σ is the ionic conductivity of polymer electrolyte, σo is the pre-exponential factor, E a is the activation energy, and T the absolute temperature in Kelvin scale. In polymer electrolyte, the change in conductivity with the temperature has been explained in terms of segmental motion that results in an increasing free volume of the sample and the motion of ionic charge. The complete amorphous nature of the PVAc polymer provides a greater free volume in the blend electrolyte system upon increasing the temperature [26]. Thus, the segmental motion either permits the ions from one site to another or provides the pathway for ions to move. This inter-chain or intra-chain ion movements are responsible for the high ionic conductivity. Among the various blended samples PVAc/PVdF-HFP ((25/75) wt.%)/LiClO4 (8 wt.%) composition shows high ionic conductivity of the order of 0.527 × 10−4 Scm−1 at 303 K, and they also have better mechanical property than the other samples. Choi et al. reported the ionic conductivity value 2.3 × 10−3 Scm−1 at 298 K for the system PVAc/PVdF-HFP with LiClO4 employing double plasticizer (Ec, PC) [27]. In this study, we have optimized the blend ratio of the polymers and the conductivity value is of the order of 10−4 Scm−1 without plasticizers.
Differential scanning calorimeter
Thermal behavior of polymer-salt complex and the polymer blend electrolytes are measured by using a differential scanning calorimeter. All the samples are scanned at a heating range of −100 °C to 350 °C under nitrogen atmosphere.
The thermograms of blend electrolytes are shown in Fig. 5. Presence of small endothermic peaks in all blends may due to the absorption of the heat energy by the samples. This absorption leads to the changes of blend electrolytes from one state to another state. The glass transition temperature of pure PVAc polymer is 30 °C but in the present study the glass transition temperature shifted to 52 °C. This shift in transition temperature is due to the addition of lithium salt. In fact, it is difficult to determine the glass transition temperature of PVdF-HFP polymer because of its semicrystalline nature. However, the position and the intensity change of the melting peak of samples can be observed. The endothermic peak observed at 137,142 and143 °C for the samples A3, A4, and A5 is attributed to PVdF-HFP melting [28]. It has been found that the endothermic peak varies with the various blend compositions of these two polymers. Appearance of the broad exothermic peaks at 280, 283, 278, 265, and 263 °C in all samples indicates the decomposition of the blended complexes A1, A2, A3, A4, and A5 respectively. The maximum ionic conductivity sample (A4) is thermally stable up to 265 °C. Hence, it can be operated up to this temperature in Li-ion batteries.
SEM analysis
The SEM is one of the most versatile instrument available for the examination and analysis of the microstructure morphology and chemical composition characterizations. Scanning electron microscope (SEM) image of PVAc-salt, PVdF-HFP-salt, and PVAc (25 wt.%)/PVdF-HFP (75 wt.%)/LiClO4 (8 wt.%) polymer blend electrolyte films are shown in Fig. 6. Figure 6a clearly shows smooth and uniform surface morphology of the PVAc-salt complex. This smooth morphology confirms the complete amorphous nature of PVAc polymer. Figure 6b shows the photograph of PVdF-HFP and LiClO4 salt complex with maximum number of pores giving rise to high ionic conductivity of this sample. The appearance of number of uniform tracks of few micrometer size is responsible for the high ionic conductivity of PVAc/PVdF-HFP ((25/75) wt.%)/LiClO4 (8 wt.%) blend electrolyte (Fig. 6c). The maximum ionic conductivity of the polymer blend electrolyte also depends on the segmental motion of the PVAc and PVdF-HFP. The better miscibility of these two polymers can be depicted from the microstructural photograph. The miscibility of these two polymers has also been confirmed from FTIR and DSC analysis. Finally, the SEM photograph of blend polymer electrolyte indicates the good compatibility of these two polymers.
Atomic force microscopic studies
An AFM is a mechanical imaging instrument, which is used to obtain the three dimensional topography images of the samples. In the present study, the scanning spectroscopic method was used to measure the pore size of the prepared sample. The two dimensional, three dimensional topography images are shown in the Fig. 7a, b. The topography images of PVAc/PVdF-HFP ((25/75) wt.%): LiClO4 (8 wt.%) sample clearly shows the presence of pores within the scanned area of 3 µm × 3 µm and the measured pore size of the sample is approximately 600 nm. The presence of the small pores is responsible for the high ionic conductivity of the prepared samples. The size of the chain segment is obtained in the order of 650 nm. The root mean square roughness of the topography image over the scanned area is 107 nm [29].
Conclusion
PVAc/PVdF-HFP/LiClO4 polymer blend electrolytes with different blend ratios have been prepared by solution casting technique. The complex formation has been confirmed by XRD and FTIR spectroscopic studies. DSC thermal analysis has been performed for all the samples. The maximum ionic conductivity of 0.527 × 10−4 Scm−1 at 303 K has been observed for PVAc/PVdF-HFP ((25/75) wt.%) blend electrolyte sample. The surface morphology and the topographic images have been obtained from SEM and AFM scanning probe techniques, respectively.
References
Wright PV (1975) Brit Poly Journal 7:319–327
Armand MB, Chabango JM, Duclot M, (1978) second International Meeting on Solid Electrolytes, Standee's, Scotland, Extented Absts
Munishi MZA (1995) Handbook of Solid State Batteries and Capacitors. World Scientific Publishing Co. Pte. Ltd, Singapore, pp 384–385
Shodai T, Owens BB, Ohtsuka H, Yamaki JC (1994) J Electrochem Soc 141:2978–2981
Rajendran S, Uma T (2000) J Power Sources 87:218–222
Akashi H, Tanaka K-I, Sekai K (1998) J Electrochem Soc 145:881–887
Abraham KM, Alamgir M (1990) J Electrochem Soc 137:1657–1658
Tager A (1972) Physical chemistry of polymers. Mir Publications, Moscow, pp 289–290
Daniels W (1987) In: Mark HF (ed) Encyclopedia of polymer science and technology, vol 17. Wiley, New York, p 402
Selvasekarapandian S, Baskaran R, Kamishima O, Kawamura J, Hattori T (2006) Spectrochim Acta Part A 65:1234–1240
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Solid State Ionics 177:2679–2682
Rajendran S, Sivakumar M, Subadevi R (2004) Solid State Ionics 167:335–339
Kim C-H, Kim H-T, Park J-K, Moon S-I, Yoon M-S (1996) J Polymer Sci B Polymer Phys 34:2709–2714
Sung H-Y, Wang Y-Y, Wan C-C (1998) J Electrochem Soc 145:1207
Rhoo H-J, Kim HT, Park J-K, Hwang T-S (1997) Electrochim Acta 42:1571–1579
Saikia D, Kumar A (2004) Electrochim Acta 49:2581–2589
Nagatomo T, Ichikawa C, Omoto O (1987) J Electrochem Soc 134:305
Nagamoto T, Kakehata H, Ichikawa C, Omoto O (1985) Jpn J Appl Phys 24:305
Baskaran R, Selvasekarapandian S, Hirankumar G, Bhuvaneswari MS (2004) J Power Sources 134:235–240
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Mater Chem Phys 98:55–61
Rajendran S, Mahendran O, Mahalingam T (2002) Eur Poly J 38:49–55
Rajendran S, Mahendran O, Kannan R (2002) Mater Chem Phys 74:52–57
Missan HPS, Chu PP, Sekhon SS (2006) J Power Sources 158:1472–1479
Li Z, Su G, Wang X, Gao D (2005) Solid State Ionics 176:1903–1908
Michael MS, Jacob MME, Prabaharan SRS, Radhakrishna S (1997) Solid State Ionics 98:167–174
Tsunemi K, Ohno H, Tsuchida E (1983) Electrochim Acta 28:833–837
Choi NS, Lee YG, Park JK, Ko JM (2001) Electrochim Acta 46:1581
Jiang Y-X, Chen Z-F, Zhuang Q-C, Xu J-M, Dong Q-F, Huang L-S, Sun S-G (2006) J Power Sources 160:1320–1328
Horcas I, Fernandez R, Rodriques J-M, Colchero J, Herrero J-G, Mbaro A (2007) Rev Sci Instrum 78:13705
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulaganathan, M., Rajendran, S. Preparation and characterizations of PVAc/P(VdF-HFP)-based polymer blend electrolytes. Ionics 16, 515–521 (2010). https://doi.org/10.1007/s11581-009-0415-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0415-4