Abstract
During the caudo-rostral progression of Lewy pathology, the amygdala is involved relatively early in Parkinson’s disease (PD). However, lesser is known about the volumetric differences at the amygdala subdivisions, although the evidence mainly implicates the olfactory amygdala. We aimed to investigate the volumetric differences between the amygdala’s nuclear and sectoral subdivisions in the PD cognitive impairment continuum compared to healthy controls (HC). The volumes of nine nuclei of the amygdala were estimated with FreeSurfer (nuclear parcellation-NP) from T1-weighted images of PD patients with normal cognition (PD-CN), PD with mild cognitive impairment (PD-MCI), PD with dementia (PD-D), and HC. The appropriate nuclei were then merged to obtain three sectors of the amygdala (sectoral parcellation-SP). The nuclear and sectoral volumes were compared among the four groups and between the hyposmic and normosmic PD patients. There was a significant difference in the total amygdala volume among the four groups. In terms of nuclei, the bilateral cortico-amygdaloid transition area (CAT) and sectors superficial cortex-like region (sCLR) volumes of PD-MCI and PD-D were less than those of the PD-CN and HC. A linear discriminant analysis revealed that left CAT and left sCLR volumes classified the PD-CN and cognitively impaired PD (PD-CI: PD-MCI plus PD-D) with 90.7% accuracy according to NP and 85.2% accuracy to SP. Similarly, left CAT and sCLR volumes correctly identified the hyposmic and normosmic PD with 64.8% and 61.1% accuracies. Notably, the left olfactory amygdala volume successfully discriminated cognitive impairment in PD and could be used as neuroimaging-based support for PD-CI diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder mainly defined with motor symptomatology caused by the degeneration and loss of the dopaminergic neurons in the substantia nigra pars compacta (SNpc). The causative intraneuronal inclusion is called the Lewy pathology. The primary structural component is a misfolded protein aggregate called alpha-synuclein. Lewy pathologies are twofold; the first is the Lewy neurite (LN) that aggregates in axons and dendrites, and the second is the Lewy body (LB) that aggregates in the cytoplasm (Spillantini et al. 1998). It seems that the former precedes the latter (Del Tredici and Braak 2012). As per the Braak et al.‘s definition of the pathological progression of alpha-synuclein aggregates in an ascending caudo-rostral manner, from the lower brain stem, first to the midbrain and limbic-mesocortical structures and finally, to the neocortical areas, nigral dopaminergic degeneration that causes the motor symptoms of the disease corresponds to the intermediate stages of the disease (Braak et al. 2003). According to this staging system, LB accumulation in PD starts most caudally in the brainstem from the dorsal motor nucleus of the vagal nerve in the medulla and in parallel in the olfactory bulbs (OB) and the anterior olfactory nucleus at Stage I (Braak et al. 2002, 2003, 2004; Del Tredici et al. 2002). This synchronous initiation at two separate sites was later coined as the “dual hit hypothesis,“ which proposed a pathogen entering the central nervous system via nasal and gastric routes, thus initiating the pathologic cascade (Hawkes et al. 2007, 2009). This proposed cascade of LP spread was later challenged several times due to “failure to fit” of a considerable percentage of cases (14–43%) (Jellinger 2008; Kalaitzakis et al. 2008; Parkkinen et al. 2008) and several modifications have been proposed claiming increased sensitivity (Adler et al. 2019; Beach et al. 2009; Coughlin et al. 2019; Leverenz et al. 2008; Muller et al. 2005). Nevertheless, we retain our reference to Braak’s scheme for practical purposes because the amygdala is almost always an obligatory early relay structure regardless of the staging system.
Although the formal clinical diagnostic criteria consist of motor findings (Hughes et al. 1992), it is now known that there are two sets of non-motor symptoms that precede motor symptoms due to the earlier involvement of extranigral structures. The first set is related to the involvement of the enteric nervous system and caudal brainstem structures, leading to dysautonomia, including chronic constipation, mood changes including depression and anxiety, and REM-sleep behavior disorder. The second is related to the olfactory involvement, leading to hyposmia (Goldman and Postuma 2014; Schapira and Tolosa 2010). Hyposmia is one of the best characterized non-motor symptoms of PD, with a prevalence of 50–90% (Boesveldt et al. 2008; Hawkes et al. 1997).
Cognitive impairment is one of the most common non-motor symptoms in the PD continuum. Although subtle cognitive impairment can be observed at the initial stages of the disease, it becomes more evident with the progression of the disease and is frequently seen in people over 70 years of age (Buter et al. 2008; Kempster et al. 2010; Williams-Gray et al. 2007). Cognitive status can be divided into three phases in PD: cognitively normal PD (PD-CN), PD with mild cognitive impairment (PD-MCI), and PD with dementia (PD-D). Studies have shown that newly diagnosed PD patients have PD-MCI in rates ranging from 19 to 33% (Aarsland et al. 2009; Aarsland and Kurz 2010; Litvan et al. 2011; Santangelo et al. 2015) and approximately 40% of patients with normal cognition later develop MCI (Pigott et al. 2015).
The amygdala is a major component of the limbic system and one of its core structures harboring the neuropathologic insignia of various neurodegenerative diseases, such as Alzheimer’s disease, primary age-related tauopathy, argyrophilic grain disease, limbic-predominant age-related TDP-43 encephalopathy, and various LB-related diseases, including PD. Nelson et al. (Nelson et al. 2018) suggested that “the amygdala may represent an incubator for misfolded proteins.“ Amygdala is one of the extranigral regions to contain LBs early (Stages 3 and 4) in the PD course (Braak et al. 2002, 2003, 2004; Harding et al. 2002; Silveira-Moriyama et al. 2009). Amygdala volume loss was shown in some neuropathological studies [20% loss in a general PD cohort by Harding et al. (Harding et al. 2002), and 18% loss in PD-D by de la Monte et al. (de la Monte et al. 1989)]. Some of the non-motor signs of PD such as olfactory dysfunction (Baba et al. 2012), autonomic dysfunction (Li et al. 2017), cognitive impairment (Bouchard et al. 2008), depression, and anxiety (van Mierlo et al. 2015; Vriend et al. 2016) have been attributed to amygdalar involvement.
The human amygdala is a group of heterogeneous nuclei in the medial temporal lobe. These nuclei have distinct cytoarchitecture and connectivity patterns and can be classified roughly into two cortex-like input and one non-cortex-like output sectors (Yilmazer-Hanke 2012). Based on afferent connectivity, Yilmazer-Hanke names the first input sector as the “superficial cortex-like region (sCLR).“ In primates, its rostral and superior regions receive direct input from the olfactory bulbs via the lateral olfactory tract. Accordingly, de Olmos et al. (De Olmos et al. 2004) named this region “olfactory amygdala”. The second deeply-located cortex-like input sector receives non-olfactory subcortical sensory information, as well as multimodal information from higher-order cortical areas, including fronto-insular cortices and entorhinal-hippocampal complex. Yilmazer-Hanke names this sector as the basolateral nuclear group (BLNG). The outputs of both sectors of the amygdala are principally via the central (Ce) and medial (Me) nuclei. Together this third sector is called the centromedial group (CMG). Yilmazer-Hanke adds the structures in the neighboring amygdalostriatal area (e.g., the bed nucleus of stria terminalis), and calls this third sector as the centromedial extended amygdala (CMEA).
Structural magnetic resonance imaging (MRI) techniques are employed to investigate anatomical structures in vivo. It is now possible to segment amygdalar nuclei with automated segmentation techniques. Saygın et al. (Saygin et al. 2017) created an atlas of the amygdala, which was later distributed in the FreeSurfer software. This atlas made it possible to visualize nine amygdalar nuclei boundaries that may be classified under the above described three sectors. This is considerable progress in the amygdala imaging segmentation since previous approaches enabled only two to five nuclear groups (Amunts et al. 2005; Blair et al. 2019; Entis et al. 2012; Solano-Castiella et al. 2011).
The present study analyzes the imaging data of a separate study, which was previously designed to investigate the multimodal neuroimaging signatures of cognitive impairment in PD. Hitherto, two papers were published related to the main study (Arslan et al. 2020; Azamat et al. 2021). This sub-study was conceived later and aims to determine the volumetric quantifications of the total amygdala, its three sectors, and nine individual nuclei in healthy control subjects and compare the findings with PD patients aligned along the three stages of the cognitive impairment continuum. Our objective was to find volumetric loss in amygdala along this continuum, likely more prominent in the “olfactory amygdala,“ that is, the sCLR sector and its component nuclei since it is one synapse away from the OB, which is one of the initiation sites of LB accumulation, followed by the other two sectors and nuclei.
Materials and methods
Participants
All participants provided written informed consent to the main study according to a protocol approved by the Local Ethics Committee of Istanbul University, Turkey, per the Declaration of Helsinki. In total, 18 healthy controls (HC) with no history of neurological or psychiatric illness and 54 right-handed PD patients (18 PD-CN, 18 PD-MCI, 18 PD-D) diagnosed with idiopathic PD according to the UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria at the Istanbul University, Faculty of Medicine, Behavioral Neurology and Movement Disorders Unit between November 2015 and July 2018. The Unified Parkinson’s Disease Rating Scale (UPDRS) was applied to all PD patients in the study. Patients with motor symptoms severe enough to cause motion artifact during imaging, patients with a history of any neuropsychiatric disease other than PD, and patients with Geriatric Depression Scale (GDS) score > 14 were excluded from the study. For the PD-MCI classification, Movement Disorder Society (MDS) Level I diagnostic criteria for PD-MCI were used (Litvan et al. 2012). Accordingly, Addenbrooke’s Cognitive Examination-Revised (ACE-R) cut-off score of 83 was used for PD-CN and PD-MCI distinction (Uysal-Canturk et al. 2018). For the PD-D classification, the diagnostic criteria recommended by Emre et al. (Emre et al. 2007) were used. Since this study was not conceived during the design of the original study, an objective assessment of smell perception is missing. However, questioning the sense of smell is part of the routine history taking in PD patients, and hyposmia information was gathered from the patient files.
MRI Acquisition
All MRI data were acquired using a 32-channel SENSE coil with a 3T clinical MR scanner (Phillips, Achieva, Best, Netherlands) installed at the Istanbul University Hulusi Behçet Life Sciences Research Laboratory Neuroimaging Unit. High-resolution anatomical MR images were recorded in the axial plane aligned with the anterior commissure-posterior commissure using a T1-weighted 3D Turbo Field Echo (TFE) sequence. The scan time was 5 min and 55 s (repetition time (TR) = 8.4 ms, echo time (TE) = 3.9 ms, flip angle = 8°, number of slices = 180, slice thickness = 1 mm (without gap), voxel size = 1 mm3 and field of view (FOV) = 250 mm).
Processing of MRI data
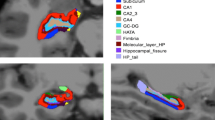
FreeSurfer software package (stable v6.0.0) (Fischl 2012; Fischl and Dale 2000), which provides several algorithms to measure the structural properties of the human brain, was used for the processing of the structural MRI data. Cortical parcellation and subcortical segmentation procedures were performed with FreeSurfer’s standard recon-all analysis flow. Briefly, image processing was performed on T1-weighted MR images, which were corrected for within-subject head motion, removal of non-brain tissue, affine registration to Talairach space, intensity normalization, segmentation of the cortical and subcortical structures, and consequently the estimation of the cortical thickness and volumes (Fischl et al. 2002). Amygdala volumes were calculated using the automated amygdala segmentation algorithm included with the development version of FreeSurfer 6.0 (dev-20,190,118), which uses a probabilistic atlas based on the ultra-high resolution MRI data and a modified version of the Van Leemput algorithm to segment the nuclei of the amygdala (Saygin et al. 2017). After segmentation, the amygdala was divided into nine nuclei, including lateral (La), basal (Ba), central (Ce), medial (Me), cortical (Co), accessory basal (AB), paralaminar (PL), anterior amygdala area (AAA), and cortico-amygdaloid transition area (CAT). The amygdala subfield segmentation results are illustrated in Fig. 1. Additionally, to make our analyses compliant with Amunts et al.’s (Amunts et al. 2005) sectoral segmentation and using Yilmazer-Hanke’s (Yilmazer-Hanke 2012) terminology for the amygdalar sectors, we created three sectors by merging lateral, basal, paralaminar, and accessory basal nuclei as the BLNG; anterior amygdala area, cortico-amygdaloid transition area and cortical nuclei as sCLR; and medial and central nuclei as the CMG (Fig. 2).
Representative images of automated amygdala subfield segmentation in a PD-CN subject (top row). (A), (B) and (C) represent the image view of sagittal, coronal, and axial planes, respectively. 3-Dimensional rendering of manual segmentation based on MRI in same PD-CN case (bottom row). (D) Sagital, (E) anterior, and (F) posterior views. La: Lateral nucleus, Ba: Basal nucleus, Ce: Central nucleus, Me: Medial nucleus, Co: Cortical nucleus, AB: Accessory basal nucleus, CAT: Cortico-amygdaloid transition area, AAA: Anterior amygdala area, PL: Paralaminar nucleus. Ant.: anterior, Post.: posterior, R: right, and L: left
Representative images of Amunts et al.‘s (2005) parcellation in a PD-CN subject (top row). (A), (B) and (C) represent the image view of sagittal, coronal, and axial planes, respectively. 3-Dimensional rendering of manual segmentation based on MRI in same PD-CN case (bottom row). (D) Sagital, (E) coronal, and (F) axial views. BLNG: Basolateral nuclear group, sCLR: Superficial cortex-like region, CMG: Centromedial group. Ant.: anterior, Post.: posterior, R: right, and L: left
Statistical analysis
Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS) software version v25.0. The Pearson chi-square test was used for gender comparison between groups, and one-way ANOVA was used for other demographic and clinical comparisons. Three separate MANCOVA designs were applied to compare the total volumes, the nuclear parcellations and the sectoral parcellations of the left and right amygdalae among the four groups. In MANCOVAs, age, gender, and education were added as covariates to eliminate the demographic variability of the cohort. Additionally, the estimated total intracranial volume of each subject was calculated using the standard FreeSurfer processing pipeline to correct for personal differences in head size by including it also as a covariate in the MANCOVA designs.
In the first MANCOVA design for total amygdala volumes, the Bonferroni corrected statistical significance thresholds for univariate results were set at 0.05/2 = 0.025 for comparison of the left and right total amygdala volumes, 0.05/(2 × 9) = 0.0028 for the comparison of the nine bilateral nuclear amygdala segments, and 0.05/(2 × 3) = 0.008 for the comparison of the three bilateral sectoral segments. For comparing hyposmic and normosmic PD patients regarding any significant differences of left and right sCLR or the left and right CAT volumes, two separate MANCOVA analyses were performed. In each MANCOVA the univariate left, and right hemisphere findings were Bonferroni-corrected with significance value set at 0.05/2 = 0.025. Subsequently, 6 pairwise comparisons between all pairs of 4 groups were Bonferroni-corrected with significance values set at 0.05/(6 × 2) = 0.004 for the left and right whole amygdala volumes, 0.05/(6 × 18) = 0.00046 for the nine nuclear amygdala segments bilaterally, and 0.05/(6 × 6) = 0.0014 for the three bilateral sectoral amygdala segments.
Based on the segments that significantly differed among the subject groups in the MANCOVA, the performance of the related variables to discriminate the cognitive impairment in PD was further tested by using linear discriminant analysis (LDA) between PD-CN cases (n = 18) and PD cases with any severity of cognitive impairment (PD-MCI and PD-D groups were collapsed as PD with cognitive impairment [PD-CI], n = 36), with leave-one-out cross-validation and 1000 times bootstrapping. Finally, the all-PD group (n = 54) was divided into two subgroups according to having a normal sense of smell (normosmic PD = 32) and abnormal sense of smell (hyposmic PD = 22), regardless of being cognitively impaired or not. The LDA was repeated this time with left CAT and left sCLR volumes to predict whether a PD patient was hyposmic PD (n = 22) or normosmic PD (n = 32). Age, gender, education, and intracranial volume were added as covariates in all LDAs.
Results
Demographic and clinical results
The demographic and clinical variables of HC, PD-CN, PD-MCI, and PD-D groups are provided in Table 1. There was no significant difference among the groups in terms of age (F(3) = 2.670, P = 0.054) and gender (χ2 (3, n = 72) = 5.776, P = 0.134) while the education was significantly different between the groups (F(3) = 10.549, P < 0.001). Post-hoc analysis revealed that the education level of the HC group was significantly higher than all the PD groups. Still, there was no significant difference among the PD groups in terms of education. The three PD groups were found to be significantly different with regards to disease duration (F(2) = 4.293, P = 0.019), total UPDRS (F(2) = 25.384, P < 0.001) and UPDRS-III scores (F(2) = 13.395, P < 0.001). According to the post-hoc tests, the disease duration, UPDRS, and UPDRS-III scores of the PD-D group were significantly higher than those of the PD-CN and PD-MCI groups. Yet, there were no significant differences between the PD-CN and PD-MCI groups.
Structural MRI results obtained with MANCOVA
The overall group effect of the MANCOVA design for the total left and right amygdala volumes revealed a significant change of the total amygdala volumes among the four groups (Wilks’ λ = 0.792, F(6,126) = 2.596, P = 0.021). This effect was in the direction of shrinkage of the amygdala volume in cognitively impaired PD groups (Supplementary Table 1). The univariate results for each of the left and right amygdalae displayed that the group effect on the left amygdala volume was significant (F(3) = 4.591, P = 0.0066, η2 = 0.17), while the group effect on the right amygdala volume was non-significant (F(3) = 2.325, P = 0.083, η2 = 0.10).
The differences among the groups were further investigated with two MANCOVA designs covering the nine nuclear or three sectoral amygdala segments in each hemisphere, respectively. The overall group effects were significant both with regard to nuclear (Wilks’ λ = 0.109, F(54,140.8) = 2.878, P = 3.2 × 10− 7) and sectoral (Wilks’ λ = 0.291, F(18,167.4) = 5.088, P = 3.7 × 10− 9) amygdala segments. The univariate MANCOVA results for each nuclear and sectoral segment and the post-hoc pairwise comparisons between the four groups are shown in Tables 2 and 3, respectively. The univariate MANCOVA results revealed a significant difference among the groups in terms of the left CAT (F(3) = 32.583, P = 6.60 × 10− 13, η2 = 0.604), and right CAT volumes (F(3) = 14.929, P = 1.79 × 10− 7, η2 = 0.412) in nuclear (Table 2), and left sCLR (F(3) = 22.847, P = 3.59 × 10− 10, η2 = 0.517) and right sCLR (F(3) = 9.698, P = 2.30 × 10− 5, η2 = 0.313) volumes in sectoral segmentation (Fig. 3 A). The pairwise comparisons showed that the left and right CAT volumes of the PD-MCI and PD-D groups were lower than those of the HC and PD-CN groups. Similarly, the left and right sCLR volumes of PD-MCI and PD-D groups were lower than those of the HC and PD-CN groups (Table 3). Other amygdala segments did not statistically significantly differ between the groups (Supplementary Tables 2 and Table 3).
As the significant group differences were in amygdala segments linked with olfactory processing, the CAT and sCLR volumes were further compared between the hyposmic and normosmic PD patients, which revealed that both CAT (Wilks’ λ = 0.824, F(2,47) = 5.015, P = 0.011) and sCLR volumes (Wilks’ λ = 0.866, F(2,47) = 3.630, P = 0.034) significantly differed between the two groups (Supplementary Table 4). The univariate MANCOVA results refined these findings in terms of the presence of significant group effect, specifically in the left CAT (F = 10.243, P = 0.002, η2 = 0.180) and left sCLR volumes (F = 7.335, P = 0.009, η2 = 0.13) (Fig. 3B).
Cortico-amygdaloid transition area’s (CAT) and superficial cortex-like region’s (sCLR) volumetric changes in (A) HC, PD-CN, PD-MCI, and PD-D groups, (B) Normosmic PD and hyposmic PD groups. HC: Healthy control, PD-CN: Cognitively normal PD, PD-MCI: PD with mild cognitive impairment, PD-D: PD with dementia. *P < 0.025, **P < 0.0014, ***P < 0.00046
Classification performance
Two LDAs conducted with the left-right CAT and left-right sCLR volumes to predict whether a patient belongs to PD-CN or PD-CI groups revealed significant associations between the groups and both sets of predictors, accounting for 60.37% (left and right CAT volume) and 49.56% (left and right sCLR volume) of between-group variability. However, a closer analysis of the structure matrix revealed that mainly the left CAT volume (r = 0.899) or left sCLR volume (r = 0.983) were significantly contributing to the linear discriminant functions. In contrast, the right CAT (r = 0.198) or right sCLR volumes (r = 0.028) did not significantly help in the discrimination. The cross-validated overall classification accuracy was 90.7%, with a sensitivity of 91.7% (correct classification of the PD-CI cases) and a specificity of 88.9% (correct classification of the PD-CN cases) in nuclear parcellation. In comparison, the cross-validated overall classification accuracy was 85.2%, with a sensitivity of 88.9% and a specificity of 77.8% in sectoral parcellation.
Finally, to analyze the relation of the volume changes associated with the cognitive impairment in PD with olfactory function, two LDAs were carried out with either the left CAT volume or the left sCLR volume as predictors for the hyposmia in PD. Significant associations between the left CAT or left sCLR volumes and the presence of hyposmia were found, accounting for 12.04% and 8.12% between-group variability, respectively. The cross-validated overall classification accuracy was 64.8%, with a sensitivity of 54.5% (correct classification of the hyposmic PD cases) and a specificity of 71.9% (correct classification of the normosmic PD cases) for the left CAT volume, while the cross-validated overall classification accuracy was 61.1%, with a low sensitivity of 31.8% but a high specificity of 81.3% for the left sCLR volume.
Discussion
A significant difference among the healthy subjects and three PD groups with varying degrees of cognitive impairment for the total left amygdala volume was identified in this study. In the analyses at sectoral and nuclear levels, the volumes of the bilateral sCLR sectors, which correspond to the olfactory amygdala, and its largest component, the bilateral CAT nuclei of the PD-MCI and PD-D groups, were significantly smaller than those of the PD-CN and HC groups. However, no significant difference was found between PD-MCI and PD-D and the PD-CN and HC groups in any amygdala segments. Additionally, the comparison of hyposmic and normosmic PD groups revealed smaller left sCLR and left CAT volumes in the hyposmic group. In LDA analysis conducted to determine the discriminant functions of these structures on the cognitively normal and impaired PD groups, classification accuracy was very high for CAT according to the nuclear parcellation of Saygin et al. (Saygin et al. 2017), while it was relatively high for sCLR as generally matched to the sectoral parcellation of Amunts et al. (Amunts et al. 2005). Additionally, classification accuracies were obtained for the discrimination of the normosmic and hyposmic PD cases using the left CAT (64.8%) and the left sCLR volumes (61.1%), with a low sensitivity for the latter analysis.
The sCLR, being one of the direct recipients of the OB input, is also considered one of the primary olfactory cortices. In addition to its role in elementary odorant discrimination as a primary sensory cortex, the olfactory amygdala has probably a major function in linking olfactory information with emotional behavior and establishing olfactory-mediated emotional memories (Buchanan et al. 2003; Kadohisa 2013). The sCLR also receives afferents from intra-amygdalar and insular connections (Yilmazer-Hanke, 2012). The second cortex-like region, that is the BLNG is essential for maintaining attention and vigilance/arousal, particularly in ambiguous contexts. The salient stimuli are identified in such situations, and an affective valence is appraised. This appraisal will become the “current value” of the stimulus at stake, transforming it into a conditioned stimulus and determining the decision to approach or avoid during the future exposures (Gallagher and Schoenbaum 1999; Janak and Tye 2015; Pessoa 2010). The third non-cortex-like sector, which is also the output sector of the amygdala, the CMG receives glutamatergic excitatory inputs from both cortex-like regions (The Me mainly from sCLR and Ce from BLNG) and sends GABAergic inhibitory efferents. Hence, it is homologous to the basal ganglia for cortex-like amygdala sectors. Through its connections with the insula, hypothalamus, and brainstem nuclei, it seems that the CMG mediates the somatomotor and autonomic responses to the conditioned stimulus, food intake, reproductive behavior, and hormonal homeostasis (Gallagher and Schoenbaum 1999).
The research on amygdala involvement in PD does not have a deep history. In the neuropathological studies, changes have been shown in accessory cortical and central nuclei (Braak et al. 1994), cortical and basolateral nuclei (Harding et al. 2002), periamygdaloid cortex, and accessory cortical nucleus (Price 1987; Price 1990), accessory cortical nucleus, cortical and central nuclei of the amygdala (Churchyard and Lees 1997). One of the earliest is a neuropathological study from 1989 that found 18% tissue loss in the amygdalae of the four PD-D brains (de la Monte et al. 1989). There was no attempt to delineate amygdala components in this study. Braak et al. reported their neuropathological findings on 16 PD brains (Braak et al. 1994). The patients had Hoehn and Yahr staging ratings between III-V, and it was stated that “some of the patients also had cognitive impairment.“ The researchers found no macroscopic amygdalar atrophy but saw abundant LBs and LNs in the accessory cortical nucleus and central nucleus, followed by the periamygdaloid cortex (PAC), while BLNG nuclei were relatively spared. It was suggested that PAC and accessory cortical nucleus receive projections of the olfactory system, which may contribute to olfaction deficits. According to Yilmazer-Hanke (Yilmazer-Hanke 2012), the accessory cortical nucleus is the sulcal PAC or the parahippocampal-amygdaloid transition area located in the caudal sCLR, which “receives few olfactory inputs.“ The PAC largely overlaps with the CAT. Churchyard and Lees reported their neuropathological findings in 27 PD cases, 17 of whom were demented before death (Churchyard and Lees 1997). They divided the amygdala into four nuclear groups: the accessory cortical nucleus, cortical nuclei, central nucleus, and lateral nuclei. Similar to Braak et al.’s findings, accessory cortical nucleus, cortical nuclei, and central nucleus were involved, and lateral nuclei were spared (Braak et al. 1994). Furthermore, the severity of cognitive impairment correlated with the density of LNs in the hippocampal CA2 field, whereas amygdalar involvement was not different between the cognitively intact and impaired groups. In another neuropathological study, dystrophic neurites have been shown in PAC, and the neuritic change in the PAC was more prominent in PD patients with more severe cognitive impairment (Mattila et al. 1999). Harding et al. reported neuropathologic findings on prospectively studied 18 PD patients, seven of whom had experienced visual hallucinations late in the disease course (Harding et al. 2002). The amygdala was subdivided into the basolateral and the corticomedial complex. Total amygdala volume was reduced by 20%, and corticomedial complex volume by 30%. LBs were concentrated in the cortical and basolateral nuclei. While there was no volume loss in the basolateral nucleus, LBs in this nucleus were doubled in patients with hallucinations. Bertrand et al. (Bertrand et al. 2004) reported findings on 41 PD brains, 31 cases of whom were demented. They found significantly higher densities of LBs and LNs in the transentorhinal cortex, CA2-3 region of the hippocampus, and cortical complex of the amygdala.
In the clinical course of PD, amygdala atrophy has been reported in several studies (Beyer et al. 2007; Junque et al. 2005; Melzer et al. 2012; Weintraub et al. 2011), but it has been suggested that this atrophy is subtle and cannot be detected visually (Braak et al. 1994). Probably the earliest volumetric MRI study in living PD patients is from 2005 (Junque et al. 2005). The authors reported significantly smaller total amygdala volumes in 16 PD-D patients compared to HCs (the amygdala volumes of the non-demented PD patients were lying in between the two groups). Beyer et al. (Beyer et al. 2007) also reported significant volume loss in bilateral amygdalae in 16 PD-D patients compared to 20 HCs. The non-demented PD group of 20 patients, 8 of whom had PD-MCI, did not show significant volumetric changes in their amygdalae compared to the other two groups. Weintraub et al. (Weintraub et al. 2011) and Melzer et al. (Melzer et al. 2012) studies reported similar findings to ours: smaller amygdala volumes both in PD-D and PD-MCI groups compared to both PD-CN and HC groups. The study of (Blair et al. 2019) showed that the subcortical structures, including basal forebrain, amygdala, and entorhinal cortex, had lower gray matter density in advanced PD patients (mean disease duration was eight years) compared to newly diagnosed PD patients (mean disease duration was 0.6 years) and normal controls. In contrast, no differences were found in the comparison of the neocortical regions, indicating that atrophy in advanced PD is confined mainly to subcortical gray matter structures. Moreover, advanced PD patients had reduced volumes in all of the five amygdala sectors than both early PD and control groups, which was most prominent in the olfactory superficial amygdala, followed by centromedial and laterobasal amygdala; amygdalo-striatal transition and hippocampo-amygdalar transition areas were the least affected (Blair et al. 2019). It seems that for the structural (volume) and functional (cognition and olfaction) changes to be detectable a certain threshold must be passed, which probably corresponds to Braak’s Stage 3 to 4.
The results of the present study generally confirm the overall shrinkage of the amygdala volumes in PD cognitive decline in line with a number of neuropathological and neuroimaging results summarized above. However, the detailed analyses in amygdala sectors and nuclei revealed clearly that this effect mainly stems from the volume loss in the olfactory regions of the amygdalae with a predominance in the left amygdala. The association of the sCLR sectoral volume but more strongly of the CAT nuclear volume with the subjective complaint of olfaction in PD patients further supports this finding at a functional level. It points to the importance of testing olfactory function parallel to evaluating cognition in PD. These results show that studies in larger PD samples focusing on the olfactory amygdala, specifically CAT volumes, might provide an important anatomical biomarker of cognitive impairment in PD. However, beyond being a marker of cognitive impairment, volumes of these structures may also indicate the involvement of the neighboring limbic structures, such as the transentorhinal cortex, hippocampal formation, and the basal forebrain, which should also be included in future investigations. It is very likely that if the olfaction had been tested objectively, some of the patients classified as normosmic based on their subjective complaints in this study would actually be hyposmic, since olfactory bulb involvement is one of the earliest pathologic alterations (Stage 1) that precedes amygdalar involvement (Stage 3). Self-awareness of impaired olfaction may demand overtly shrunk amygdalae, and this alteration may parallel the structural and functional changes in the above-mentioned neighboring limbic structures. Thus, the detected cognitive impairment is unlikely to be the consequence of amygdala shrinkage per se, but damaged limbic structures, which are not specifically investigated in this study. The likely affective/emotional consequence of the amygdala damage could be detected if a social cognitive instrument had been included in the neuropsychological battery.
Limitations
The major limitation of our study comes from the retrospective collection of data. Since the acquisition of neuropsychological data from the patients took place as a routine protocol, the patients were not objectively evaluated in terms of hyposmia and social cognition. Revealing the relationship of objective olfactory tests with neuroimaging data in future studies will be an important step forward. Although the current sample size is acceptable for a neuroimaging study, analyses on larger samples with precise measurement of the olfactory function might extend present findings to define the olfactory amygdala volumes as a clear anatomical biomarker of cognitive impairment in PD.
Finally, evaluating the OB and other primary olfactory cortices (anterior olfactory nucleus, olfactory tubercle, piriform cortex, and rostral entorhinal cortex) and the hippocampal formation, parahippocampal structures and examining their relationship with olfactory tests and detailed neuropsychological tests, including those tapping social cognition should be a future research project.
Conclusion
In conclusion, the volume of the olfactory amygdala, predominantly on the left side, was discriminatory between both cognitively intact and impaired but also hyposmic and normosmic PD groups. While this finding provides an important route for further investigations on the involvement of other limbic and olfactory structures associated with the olfactory amygdala in the course of the disease progression towards PD-D, it also provides evidence for the practical use of olfactory testing or questionnaires in predicting current and future cognitive impairment in PD.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AAA:
-
anterior amygdala area
- AB:
-
accessory basal nuclei of amygdala
- Ba:
-
basal nuclei of amygdala
- BLNG:
-
basolateral nuclear group of amygdala
- CAT:
-
cortico-amygdaloid transition area
- Ce:
-
central nucleus
- CMG:
-
centromedial group nucleus of amygdala
- Co:
-
cortical nuclei of amygdala
- HC:
-
healthy control
- La:
-
lateral nuclei of amygdala
- LB:
-
Lewy body
- LDA:
-
linear discriminant analysis
- LN:
-
Lewy neurite
- Me:
-
medial nuclei of amygdala
- OB:
-
Olfactory bulb
- PAC:
-
periamygdaloid cortex
- PD:
-
Parkinson’s disease
- PD-CI:
-
Parkinson’s disease with cognitive impairment
- PD-CN:
-
Parkinson’s disease with normal cognition
- PD-D:
-
Parkinson’s disease with dementia
- PD-MCI:
-
Parkinson’s disease with mild cognitive impairment
- PL:
-
paralaminar nuclei of amygdala
- sCLR:
-
superficial cortex-like region of amygdala
- UPDRS:
-
Unified Parkinson’s disease Rating Scale
References
Aarsland D, Bronnick K, Larsen JP et al (2009) Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72(13):1121–1126. https://doi.org/10.1212/01.wnl.0000338632.00552.cb
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol 20(3):633–639. https://doi.org/10.1111/j.1750-3639.2009.00369.x
Adler CH, Beach TG, Zhang N et al (2019) Unified Staging System for Lewy Body Disorders: Clinicopathologic Correlations and Comparison to Braak Staging. J Neuropathol Exp Neurol 78(10):891–899. https://doi.org/10.1093/jnen/nlz080
Amunts K, Kedo O, Kindler M et al (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210(5–6):343–352. https://doi.org/10.1007/s00429-005-0025-5
Arslan DB, Gurvit H, Genc O et al (2020) The cerebral blood flow deficits in Parkinson’s disease with mild cognitive impairment using arterial spin labeling MRI. J Neural Transm (Vienna) 127(9):1285–1294. https://doi.org/10.1007/s00702-020-02227-6
Azamat S, Arslan DB, Erdogdu E et al (2021) Detection of visual and frontoparietal network perfusion deficits in Parkinson’s disease dementia. Eur J Radiol 144:109985. https://doi.org/10.1016/j.ejrad.2021.109985
Baba T, Kikuchi A, Hirayama K et al (2012) Severe olfactory dysfunction is a prodromal symptom of dementia associated with Parkinson’s disease: a 3 year longitudinal study. Brain 135(Pt 1):161–169. https://doi.org/10.1093/brain/awr321
Beach TG, Adler CH, Lue L et al (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117(6):613–634. https://doi.org/10.1007/s00401-009-0538-8
Bertrand E, Lechowicz W, Szpak GM et al (2004) Limbic neuropathology in idiopathic Parkinson’s disease with concomitant dementia. Folia Neuropathol 42(3):141–150. https://www.ncbi.nlm.nih.gov/pubmed/15535032
Beyer MK, Janvin CC, Larsen JP, Aarsland D (2007) A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 78(3):254–259. https://doi.org/10.1136/jnnp.2006.093849
Blair JC, Barrett MJ, Patrie J et al (2019) Brain MRI Reveals Ascending Atrophy in Parkinson’s Disease Across Severity. Front Neurol 10:1329. https://doi.org/10.3389/fneur.2019.01329
Boesveldt S, Verbaan D, Knol DL et al (2008) A comparative study of odor identification and odor discrimination deficits in Parkinson’s disease. Mov Disord 23(14):1984–1990. https://doi.org/10.1002/mds.22155
Bouchard TP, Malykhin N, Martin WR et al (2008) Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol Aging 29(7):1027–1039. https://doi.org/10.1016/j.neurobiolaging.2007.02.002
Braak H, Braak E, Yilmazer D et al (1994) Amygdala pathology in Parkinson’s disease. Acta Neuropathol 88(6):493–500. https://doi.org/10.1007/BF00296485
Braak H, Del Tredici K, Bratzke H et al (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol, 249 Suppl 3, III:1–5. https://doi.org/10.1007/s00415-002-1301-4
Braak H, Del Tredici K, Rub U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Braak H, Ghebremedhin E, Rub U et al (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134. https://doi.org/10.1007/s00441-004-0956-9
Buchanan TW, Tranel D, Adolphs R (2003) A specific role for the human amygdala in olfactory memory. Learn Mem 10(5):319–325. https://doi.org/10.1101/lm.62303
Buter TC, van den Hout A, Matthews FE et al (2008) Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70(13):1017–1022. https://doi.org/10.1212/01.wnl.0000306632.43729.24
Churchyard A, Lees AJ (1997) The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson’s disease. Neurology 49(6):1570–1576. https://doi.org/10.1212/wnl.49.6.1570
Coughlin DG, Petrovitch H, White LR et al (2019) Most cases with Lewy pathology in a population-based cohort adhere to the Braak progression pattern but ‘failure to fit’ is highly dependent on staging system applied. Parkinsonism Relat Disord 64:124–131. https://doi.org/10.1016/j.parkreldis.2019.03.023
de la Monte SM, Wells SE, Hedley-Whyte T, Growdon JH (1989) Neuropathological distinction between Parkinson’s dementia and Parkinson’s plus Alzheimer’s disease. Ann Neurol 26(3):309–320. https://doi.org/10.1002/ana.410260302
De Olmos JS, Beltramino CA, Alheid G (2004) Amygdala and Extended Amygdala of the Rat: A Cytoarchitectonical, Fibroarchitectonical, and Chemoarchitectonical Survey. In G. Paxinos (Ed.), The Rat Nervous System (Third Edition) (pp. 509–603) Academic Press. https://doi.org/10.1016/B978-012547638-6/50020-1
Del Tredici K, Braak H (2012) Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord 27(5):597–607. https://doi.org/10.1002/mds.24921
Del Tredici K, Rub U, De Vos RA et al (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61(5):413–426. https://doi.org/10.1093/jnen/61.5.413
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22(12):1689–1707 quiz 1837. https://doi.org/10.1002/mds.21507
Entis JJ, Doerga P, Barrett LF, Dickerson BC (2012) A reliable protocol for the manual segmentation of the human amygdala and its subregions using ultra-high resolution MRI. NeuroImage 60(2):1226–1235. https://doi.org/10.1016/j.neuroimage.2011.12.073
Fischl B (2012) FreeSurfer. Neuroimage 62(2):774–781. https://doi.org/10.1016/j.neuroimage.2012.01.021
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355. https://doi.org/10.1016/s0896-6273(02)00569-x
Gallagher M, Schoenbaum G (1999) Functions of the amygdala and related forebrain areas in attention and cognition. Ann N Y Acad Sci 877:397–411. https://doi.org/10.1111/j.1749-6632.1999.tb09279.x
Goldman JG, Postuma R (2014) Premotor and nonmotor features of Parkinson’s disease. Curr Opin Neurol 27(4):434–441. https://doi.org/10.1097/WCO.0000000000000112
Harding AJ, Stimson E, Henderson JM, Halliday GM (2002) Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125(Pt 112431–2445. https://doi.org/10.1093/brain/awf251
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33(6):599–614. https://doi.org/10.1111/j.1365-2990.2007.00874.x
Hawkes CH, Del Tredici K, Braak H (2009) Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci 1170:615–622. https://doi.org/10.1111/j.1749-6632.2009.04365.x
Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 62(5):436–446. https://doi.org/10.1136/jnnp.62.5.436
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184. https://doi.org/10.1136/jnnp.55.3.181
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517(7534):284–292. https://doi.org/10.1038/nature14188
Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116(1):1–16. https://doi.org/10.1007/s00401-008-0406-y
Junque C, Ramirez-Ruiz B, Tolosa E et al (2005) Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord 20(5):540–544. https://doi.org/10.1002/mds.20371
Kadohisa M (2013) Effects of odor on emotion, with implications. Front Syst Neurosci 7:66. https://doi.org/10.3389/fnsys.2013.00066
Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK (2008) Controversies over the staging of alpha-synuclein pathology in Parkinson’s disease. Acta Neuropathol 116(1):125–128 author reply 129–131. https://doi.org/10.1007/s00401-008-0381-3
Kempster PA, O’Sullivan SS, Holton JL et al (2010) Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain 133(Pt 6):1755–1762. https://doi.org/10.1093/brain/awq059
Leverenz JB, Hamilton R, Tsuang DW et al (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18(2):220–224. https://doi.org/10.1111/j.1750-3639.2007.00117.x
Li X, Xing Y, Schwarz ST, Auer DP (2017) Limbic grey matter changes in early Parkinson’s disease. Hum Brain Mapp 38(7):3566–3578. https://doi.org/10.1002/hbm.23610
Litvan I, Aarsland D, Adler CH et al (2011) MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26(10):1814–1824. https://doi.org/10.1002/mds.23823
Litvan I, Goldman JG, Troster AI et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356. https://doi.org/10.1002/mds.24893
Mattila PM, Rinne JO, Helenius H, Roytta M (1999) Neuritic degeneration in the hippocampus and amygdala in Parkinson’s disease in relation to Alzheimer pathology. Acta Neuropathol 98(2):157–164. https://doi.org/10.1007/s004010051064
Melzer TR, Watts R, MacAskill MR et al (2012) Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 83(2):188–194. https://doi.org/10.1136/jnnp-2011-300828
Muller CM, de Vos RA, Maurage CA et al (2005) Staging of sporadic Parkinson disease-related alpha-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol 64(7):623–628. https://doi.org/10.1097/01.jnen.0000171652.40083.15
Nelson PT, Abner EL, Patel E et al (2018) The Amygdala as a Locus of Pathologic Misfolding in Neurodegenerative Diseases. J Neuropathol Exp Neurol 77(1):2–20. https://doi.org/10.1093/jnen/nlx099
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115(4):399–407. https://doi.org/10.1007/s00401-008-0346-6
Pessoa L (2010) Emotion and cognition and the amygdala: from “what is it?“ to “what’s to be done?“. Neuropsychologia 48(12):3416–3429. https://doi.org/10.1016/j.neuropsychologia.2010.06.038
Pigot K, Rick J, Xie SX et al (2015) Longitudinal study of normal cognition in Parkinson disease. Neurology 85(15):1276–1282. https://doi.org/10.1212/WNL.0000000000002001
Price J, Russchen FT, Amaral DG (1987) The Limbic Region II. In: Björklund HT (ed) Handbook of Chemical Neuroanatomy, 5 edn. Elsevier, pp 279–388
Price JL (1990) Olfactory System. In: Paxinos G (ed) The Human Nervous System. Academic Press, pp 979–998
Santangelo G, Vitale C, Picillo M et al (2015) Mild Cognitive Impairment in newly diagnosed Parkinson’s disease: A longitudinal prospective study. Parkinsonism Relat Disord 21(10):1219–1226. https://doi.org/10.1016/j.parkreldis.2015.08.024
Saygin ZM, Kliemann D, Iglesias JE et al (2017) High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. NeuroImage 155:370–382. https://doi.org/10.1016/j.neuroimage.2017.04.046
Schapira AH, Tolosa E (2010) Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat Rev Neurol 6(6):309–317. https://doi.org/10.1038/nrneurol.2010.52
Silveira-Moriyama L, Holton JL, Kingsbury A et al (2009) Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci Lett 453(2):77–80. https://doi.org/10.1016/j.neulet.2009.02.006
Solano-Castiella E, Schafer A, Reimer E et al (2011) Parcellation of human amygdala in vivo using ultra high field structural MRI. NeuroImage 58(3):741–748. https://doi.org/10.1016/j.neuroimage.2011.06.047
Spillantini MG, Crowther RA, Jakes R et al (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A 95(11):6469–6473. https://doi.org/10.1073/pnas.95.11.6469
Uysal-Canturk P, Hanagasi HA, Bilgic B et al (2018) An assessment of Movement Disorder Society Task Force diagnostic criteria for mild cognitive impairment in Parkinson’s disease. Eur J Neurol 25(1):148–153. https://doi.org/10.1111/ene.13467
van Mierlo TJ, Chung C, Foncke EM et al (2015) Depressive symptoms in Parkinson’s disease are related to decreased hippocampus and amygdala volume. Mov Disord 30(2):245–252. https://doi.org/10.1002/mds.26112
Vriend C, Boedhoe PS, Rutten S et al (2016) A smaller amygdala is associated with anxiety in Parkinson’s disease: a combined FreeSurfer-VBM study. J Neurol Neurosurg Psychiatry 87(5):493–500. https://doi.org/10.1136/jnnp-2015-310383
Weintraub D, Doshi J, Koka D et al (2011) Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 68(12):1562–1568. https://doi.org/10.1001/archneurol.2011.725
Williams-Gray CH, Foltynie T, Brayne CE et al (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 130(Pt 7:1787–1798. https://doi.org/10.1093/brain/awm111
Yilmazer-Hanke D (2012) Amygdala. In G. P. Jürgen K. Mai (Ed.), The Human Nervous System (Third ed., pp. 759–834) Academic Press. https://doi.org/10.1016/B978-0-12-374236-0.10022-7
Acknowledgements
Ulaş Ay acknowledges the support of Turkish Council of Higher Education for 100/2000 CoHE doctoral scholarship. We would like to acknowledge funding support from the TUBITAK grant #115S219 and the Istanbul University Research Projects Unit project #1567/42362. We would also like to Dilek Betul Arslan, Zeynep Tufekcioglu, Basar Bilgic, and Hasmet Hanagasi for their contributions to data collection. Some of the data in this paper were previously presented at the annual meeting of the Turkish Neurological Society in Antalya, Turkey, in Nov 2019, and we won first prize for an oral presentation. Therefore, we are also immensely grateful to the Turkish Neurological Society.
Funding
This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) grant #115S219 and the Istanbul University Research Projects Unit project #1567/42362. EE received a yearly doctoral stipend (57140539) from the DAAD (German Academic Exchange Service).
Author information
Authors and Affiliations
Contributions
Ulaş Ay: Conceptualization, Methodology, Software, Formal analysis, Visualization, Writing - Original Draft. Zerrin Yıldırım: Conceptualization, Methodology, Formal analysis, Writing - Original Draft. Emel Erdoğdu: Investigation, Resources, Data Curation. Ani Kıçik: Investigation, Resources, Data Curation. Esin Öztürk-Işık: Writing - Review & Editing, Supervision, Project administration, Funding acquisition. Tamer Demiralp: Methodology, Formal analysis, Writing - Review & Editing, Supervision, Funding acquisition. Hakan Gürvit: Methodology, Writing - Review & Editing, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests.
Ethics approval
Ethical approval was obtained from Istanbul Medical Faculty Clinical Research Ethics Committee of Istanbul University, Turkey
Participant consent
Participants were informed about the research, and their written consents were obtained before the research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Ulaş Ay and Zerrin Yıldırım contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ay, U., Yıldırım, Z., Erdogdu, E. et al. Shrinkage of olfactory amygdala connotes cognitive impairment in patients with Parkinson’s disease. Cogn Neurodyn 17, 1309–1320 (2023). https://doi.org/10.1007/s11571-022-09887-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-022-09887-y