Abstract
Several unidentified specimens of Bipolaris deposited in the Queensland Plant Pathology Herbarium (BRIP) that were previously recognised by Dr. John L. Alcorn as taxonomically interesting were re-examined. The morphology of conidia and conidiophores, as well as phylogenetic inference from the analyses of three loci (ITS, GAPDH and TEF1α) supported the classification of eight novel Bipolaris species, which were originally isolated from leaf spots on grasses (Poaceae).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Bipolaris Shoemaker (1959) has traditionally been treated as part of the helminthosporioid complex, so-called because the conidia and conidiophores morphologically resemble species of Helminthosporium Link (1809). Bipolaris was originally established to accommodate species that formed fusoid conidia with two or more septa that exhibited bipolar germination, but also included some species with curved conidia and hyaline apical cells (Shoemaker 1959).
Until the late 1990s, the classification and identification of Bipolaris species was based entirely on morphological characteristics (Sivanesan 1987). This proved problematic, as conidia and conidiophores are highly variable within species. In recent years, the generic limits for the helminthosporioid fungi (including Curvularia, Drechslera, Exserohilum, Johnalcornia and Porocercospora) have been more clearly defined with the aid of molecular sequence data (Ahmadpour et al. 2012; da Cunha et al. 2012; Madrid et al. 2014; Manamgoda et al. 2012, 2014; Tan et al. 2014). Subsequent analyses of DNA sequence data have established the synonymy between Bipolaris (typified by B. maydis) and its sexual morph, Cochliobolus Drechsler (1934) (typified by C. heterostrophus) (Manamgoda et al. 2012; Rossman et al. 2013). The rules of nomenclature for fungi only allow one name for each genus, instead of different names for different morphs in the fungal life cycle (McNeill et al. 2012). Although Cochliobolus is the older name, Bipolaris is more frequently used by plant pathologists in disease reports and widely applied in the taxonomic literature. The name Bipolaris was subsequently proposed for conservation against the earlier name Cochliobolus (Rossman et al. 2013).
Species of Bipolaris are commonly associated with leaf spots, leaf blights and root rots on hosts in the Poaceae (Ellis 1971; Sivanesan 1987; Manamgoda et al. 2011, 2014). Some species that are considered serious pathogens are those on high-value commodity cereal crops, such as B. maydis on maize, B. oryzae on rice (Sunder et al. 2014) and B. sorokiniana on wheat (Acharya et al. 2011). Several species have multiple grass hosts, including other cereals and weeds, which presents additional problems related to crop rotation and disease management (Iftikhar et al. 2009; Strange and Scott 2005; Sunder et al. 2014). Furthermore, many Bipolaris species are saprobes or pathogens on hosts in the families Anacardiaceae, Araceae, Euphorbiaceae, Fabaceae, Malvaceae, Rutaceae and Zingiberaceae (Ellis 1971; Manamgoda et al. 2011, 2014). There are approximately 47 species of Bipolaris (Manamgoda et al. 2014), of which 29 occur in Australia (Alcorn 1982, 1990, 1996; DAF Biological Collections 2016; Sivanesan 1985, 1987). Most of these species were associated with hosts in the Poaceae, with the exceptions of B. cactivora and B. incurvata, which were only recorded on hosts in the families Cactaceae and Arecaceae, respectively (DAF Biological Collections 2016; Forsberg 1985; Fröhlich et al. 1997; Shivas 1995).
Accurate identification of Bipolaris species based on DNA sequences is dependent on the availability of ex-type cultures. In recent years, many DNA sequences from ex-type or reference cultures of Bipolaris species have been made available in GenBank (Manamgoda et al. 2012, 2014, Tan et al. 2014). In this study, 13 unidentified isolates of Bipolaris held in the Queensland Plant Pathology Herbarium (BRIP) were examined by molecular and morphological methods, and compared with ex-type and reference isolates. Most of the fungi were collected and isolated by Dr. John L. Alcorn as curator of the BRIP from the early 1960s through to the late 1990s. Ten new species of Bipolaris were revealed from the combined data analyses and morphological studies, and are herein introduced and described.
Materials and methods
Isolates and morphology
All isolates examined are listed in Table 1. The unidentified isolates of Bipolaris were obtained from BRIP, which retains cultures in a metabolically inactive state at −80 °C in a sterile solution of 15 % v/v glycerol. In order to observe conidia and conidiophores, living cultures were grown on sterilised leaf pieces of Zea mays placed on modified Sachs agar or sterilised wheat straws on water agar, incubated at 23 °C for 4 weeks, and exposed to near ultraviolet light source on a 12-h light/dark diurnal cycle (Sivanesan 1987). Conidia and conidiophores were mounted on glass slides in lactic acid (100 % v/v) and images captured with a Leica DFC500 camera attached to a Leica DM5500 B compound microscope with Nomarski differential interference contrast illumination. The images presented in Fig. 3d–e were taken from dried cultures, and Figs. 2e, i and 3a were taken from dried herbarium specimens. Conidial widths were measured at the widest part of each conidium. Means and standard deviations (SDs) were calculated from at least 20 measurements. Ranges were expressed as (minimum value–) mean−SD – mean+SD (–maximum value), with values rounded to 0.5 μm. Images of the herbarium specimens were captured by an Epson Perfection V700 scanner at 300 dpi resolution.
DNA isolation, amplification and phylogenetic analyses
The isolates were grown on PDA for 7 days at 23 °C. Mycelia were scraped off the PDA cultures and macerated with 0.5-mm glass beads (Daintree Scientific) in a TissueLyser (Qiagen). Genomic DNA was extracted with the Gentra Puregene DNA Extraction Kit (Qiagen), according to the manufacturer’s instructions.
The primers V9G (de Hoog and Gerits van den Ende 1998) and ITS4 (White et al. 1990) were used to amplify the internal transcribed spacer (ITS) region of the nrDNA. The primers gpd1 and gpd2 (Berbee et al. 1999) were used to amplify part of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. A partial region of the translation elongation factor 1-α (TEF1α) locus was amplified using the primers EF1983/EF12218R (Schoch et al. 2009). All loci were amplified with the Phusion High-Fidelity PCR Master Mix (New England Biolabs). The polymerase chain reaction (PCR) products were purified and sequenced by Macrogen Incorporated (Seoul, Korea).
All sequences generated were assembled using Geneious v. 9.1.5 (Biomatters Ltd.) and deposited in GenBank (Table 1, in bold). These sequences were aligned with selected sequences of Bipolaris species obtained from GenBank (Table 1) using the MAFFT alignment algorithm (Katoh et al. 2009) in Geneious. Curvularia lunata CBS 730.96 was included as the outgroup (Table 1). The sequences of each locus were aligned separately and manually adjusted as necessary. Alignment gaps were treated as missing character states, and all characters were unordered and of equal weight. The Markov chain Monte Carlo (MCMC) algorithm was used to create a phylogenetic tree based on Bayesian probabilities using MrBayes v.3.2.1 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) in Geneious. To remove the need for a priori model testing, the MCMC analysis was set to sample across the entire general time-reversible (GTR) model space with a gamma-distributed rate variation across the sites. Ten million random trees were generated using the MCMC procedure with four chains. The sample frequency was set at 100 and the temperature of the heated chain was 0.1. Burn-in was set at 25 %, after which the likelihood values were stationary. Maximum likelihood (ML) analysis was run using RAxML v.7.2.8 (Stamatakis and Alachiotis 2010) in Geneious and started from a random tree topology. The nucleotide substitution model used was GTR with a gamma-distributed rate variation. The concatenated alignment was deposited in TreeBASE (Study 19483). All novel sequences were deposited in GenBank (Table 1).
In order to determine the species limits, the criterion of genealogical concordance phylogenetic species recognition (GCPSR) was applied to the molecular data (Taylor et al. 2000). A combined analysis of three genes was used to determine the final species boundaries with the support of all single gene trees inferred. Unique fixed nucleotides are used to characterise genetic differences in the new species. For each species description, the closest phylogenetic neighbour was selected and these alignments were subject to single nucleotide polymorphism (SNP) analyses. These SNPs were determined for each aligned locus using the Find Variation/SNPs feature in Geneious. SNPs were determined based on a minimum variant frequency of 0.2. Taxonomic novelties were registered in MycoBank (http://www.mycobank.org; Crous et al. 2004).
Results
Phylogenetic analysis
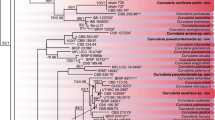
On average, 860 bp of the ITS region, 551 bp of the GAPDH gene and 876 bp of the TEF1α gene were sequenced from the BRIP isolates. For the phylogenetic analyses, the ITS and GAPDH were trimmed to 474 and 445 bp, respectively. The combined alignment deposited in TreeBASE is composed of 1733 characters from 46 isolates, of which 96 bp (20.3 %), 156 bp (35.1 %) and 99 bp (11.3 %) were variable for ITS, GAPDH and TEF1α, respectively. The ITS alignment was able to resolve 19 out of 38 Bipolaris species, including four of the new species (data not shown). Individually, both the GAPDH and TEF1α alignments were able to resolve 36 out of 38 Bipolaris species, including the ten new species described here (data not shown). None of the ITS, GAPDH or the TEF1α alignments were able to differentiate between the ex-holotype strain of B. coffeana and the recently designated ex-epitype strain of B. cynodontis (Manamgoda et al. 2014). A pairwise comparison of the unannotated sequences of B. coffeana and B. cynodontis showed 100 % identity in the ITS and TEF1α loci, and one SNP in the GAPDH locus, indicating a potential synonymy. Morphologically, B. coffeana can have conidiophores longer than B. cynodontis (up to 260 μm versus 170 μm), though the conidial dimensions of B. coffeana (32–75 × 11–14 μm) falls within the range described for B. cynodontis (30–75 × 10–16 μm). To avoid duplication, the novel taxa described below are, therefore, compared to B. cynodontis. The inferred phylogenetic tree based on the concatenated alignment resolved the 17 BRIP isolates into ten well-supported and unique clades, which are accepted in this study as novel species (Fig. 1).
Phylogenetic tree based on maximum likelihood analysis of the combined multilocus alignment. RAxML bootstrap values (bs) greater than 70 % and Bayesian posterior probabilities (pp) greater than 0.9 are given at the nodes (bs/pp). Novel species are in bold and highlighted in blue. Ex-type isolates are marked with a superscript T. The outgroup is Curvularia lunata
Taxonomy
Bipolaris austrostipae Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 2a–b)
Bipolaris austrostipae (ex-holotype BRIP 12490): a conidiophore with conidia; b conidiophore with conidium. Bipolaris shoemakeri (ex-holotype BRIP 15929): c conidiophore with a conidium; d conidia. Bipolaris axonopicola (ex-holotype BRIP 11740): e leaf spots on A. fissifolius; f conidiophores and conidia. Bipolaris subramanianii (ex-holotype BRIP 16226): g conidiophore, h conidia, i leaf spots on S. sphacelata. Bipolaris woodii (ex-holotype 12239): j conidiophore and conidia. Scale bars: e, i = 1 cm; a–d, f, h, j = 20 μm; g = 10 μm
MycoBank MB 817461
Etymology: Named after Austrostipa, the grass genus from which it was isolated.
Holotype: Australia, Queensland, Leyburn, from Austrostipa verticillata (Nees ex Spreng.) S.W.L. Jacobs & J. Everett, 11 May 1977, J.L. Alcorn, BRIP 12490 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, geniculate towards the apex, uniformly brown to dark brown, smooth, septate, up to 260 μm × 5–6 μm; basal cell swollen and darker than the other cells, up to 10 μm diam. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic. Conidiogenous nodes darkening and becoming verruculose. Conidia fusiform, straight to slightly curved, (55–) 70–77 (–86) × (11–) 14–15.5 (–20) μm, brown to dark brown, 6–9-distoseptate. Hilum thick and darkened.
Notes: Bipolaris austrostipae is only known from the type specimen on Austrostipa verticillata, which is an Australian perennial grass found predominantly in Queensland and New South Wales (Simon and Alfonso 2011). Bipolaris austrostipae is phylogenetically close to B. cynodontis (Fig. 1), and its conidial size falls within the range given for B. cynodontis (30–75 × 10–16 μm) (Sivanesan 1987). Bipolaris austrostipae differs from the ex-type culture of B. cynodontis in two loci: GAPDH 98 % match (Identities 432/443, Gaps 0/443); TEF1α positions 225 (C), 266 (G) and 717 (T).
Bipolaris axonopicola Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 2e–f)
MycoBank MB 817462
Etymology: Named after Axonopus, the grass genus from which it was isolated.
Holotype: Australia, Queensland, Peregian Beach, from leaf spot on Axonopus fissifolius (Raddi) Kuhlm., 6 June 1976, J.L. Alcorn, BRIP 11740 (includes ex-type culture).
Leaf spots on Axonopus fissifolius, narrowly ellipsoidal, up to 1 × 0.5 mm, reddish brown, larger spots with grey centres. Conidiophores mononematous, erect, straight to flexuous, rarely branched, geniculate towards the apex, uniformly pale brown to brown, smooth, septate, up to 250 μm × 5–9 μm; basal cell swollen and darker than the other cells, up to 18 μm diam. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic with undarkened circular scars. Conidiogenous nodes distinct, slightly verruculose below the node. Conidia fusiform to subcylindrical or obclavate, (40–) 55–60 (–71) × (10–) 11.5–12.5 (–14) μm, pale brown with the end cells slightly paler than the central cells, smooth, 5–10-distoseptate, apex rounded, base obconically truncate or rounded. Hilum darkened and sometimes thickened. Germination bipolar.
Culture characteristics: Colonies cover the entire plate; surface grey olivaceous with smoky grey patches, velutinous with abundant aerial mycelium.
Notes: Bipolaris axonopicola is only known from a single specimen on Axonopus fissifolius in south-east Queensland. Axonopus fissifolius is native to the Americas and was introduced to Australia as a pasture grass (Simon and Alfonso 2011). The conidial dimensions of B. axonopicola overlap with those of B. cynodontis (30–75 × 10–16 μm). Marignoni (1909) described Helminthosporium cynodontis (synonym of B. cynodontis) as having conidia 60–75 μm long and also illustrated them as slightly curved. Subsequently, many morphologically similar isolates with slightly curved conidia have been assigned to B. cynodontis from a wide range of hosts (Manamgoda et al. 2014), including A. fissifolius (Sivanesan 1987). Bipolaris axonopicola has straight conidia, which distinguishes it from B. cynodontis.
Bipolaris axonopicola is phylogenetically close to B. cynodontis and B. austrostipae (Fig. 1). Bipolaris axonopicola differs from B. cynodontis in three loci: ITS 99 % match (Identities 451/457, Gaps 2/457); GAPDH 97 % match (Identities 427/441, Gaps 0/441); and TEF1α 99 % match (Identities 865/873, Gaps 0/873). The straight conidia of B. axonopicola distinguishes it from the slightly curved conidia of B. austrostipae, in addition to differences in three loci: ITS 98 % match (Identities 450/457, Gaps 2/457); GAPDH 98 % match (Identities 431/441, Gaps 0/441); and TEF1α 99 % match (Identities 866/875, Gaps 0/875).
Bipolaris bamagaensis Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 3a–c)
Bipolaris bamagaensis (ex-holotype BRIP 16634): a necrotic leaves from U. subquadripara; b conidiophore; c conidia. Bipolaris simmondsii (ex-holotype BRIP 12030): d conidiophore; e conidia. Bipolaris sivanesaniana (ex-holotype BRIP 15847): f conidiophores; g conidia. Scale bars: a = 1 cm; b–g = 10 μm
MycoBank MB 817463
Etymology: Named after the locality, Bamaga, from where it was collected.
Holotype: Australia, Queensland, Bamaga, from necrotic leaf on Urochloa subquadripara (Trin.) R.D. Webster, 28 May 1981, J.L. Alcorn, BRIP 13577 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, geniculate towards the apex, pale brown to brown to subhyaline at the apex, smooth, septate, up to 370 μm × 4 μm, base sometimes swollen (7–9 μm). Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic with undarkened circular scars. Conidiogenous nodes dark, distinct and slightly verruculose. Conidia ellipsoidal, fusiform, straight to slightly curved, (40–) 50–55 (–70) × (10–) 12–13 (–17) μm, uniformly pale brown to brown, smooth, 3–7 (usually 5)-distoseptate. Hilum darkened and sometimes thickened.
Additional specimens examined. Australia, Queensland, Bamaga, from leaf on Dactyloctenium aegyptium (L.) Willd., 29 May 1981, J.L. Alcorn, BRIP 10711; Queensland, culture formed in vitro by crossing isolates BRIP 13577 and BRIP 10711, 26 June 1985, J.L. Alcorn, BRIP 14847; Queensland, on Yarrabah Road, Mackey Creek (near Gordonvale), from leaf blight on D. aegyptium, 1 May 1987, J.L. Alcorn, BRIP 15879; Queensland, culture formed in vitro by single-spored isolates of BRIP 15897, June 1987, J.L. Alcorn, BRIP 15934.
Notes: Bipolaris bamagaensis is known from specimens on Dactyloctenium aegyptium and Urochloa subquadripara with leaf necrosis. Although both grass hosts are found across Australia, B. bamagaensis has only been found in northern Queensland. Many Bipolaris species have been associated with Dactyloctenium, including B. clavata, B. cynodontis, B. luttrellii and B. maydis (Sivanesan 1987; Manamgoda et al. 2014), while only one species, B. urochloae, has been recorded on Urochloa (Manamgoda et al. 2014; Sivanesan 1987). There may be other records in the literature of Bipolaris species on Urochloa, as many Brachiaria species were transferred to Urochloa (Webster 1987).
Bipolaris bamagaensis formed its sexual morph in culture (BRIP 14847) when single-spored isolates from different cultures (ex-holotype BRIP 13577 and BRIP 10711), as well as from the same culture (BRIP 15879), were crossed (J.L. Alcorn herbarium notes). The sexual morph was not observed during this study, and, therefore, a description could not be provided. Morphologically, the conidiophores of B. bamagaensis in culture are much shorter than that observed for B. chloridis (up to 1.2 mm long), and the dimensions of the typically straight to slightly curved conidia fall within the range described for the mostly curved conidia of B. chloridis (30–100 × 10–20 μm). Bipolaris bamagaensis differs from B. chloridis in two loci: GAPDH positions 20 (C) and 62 (T); TEF1α positions 307 (A) and 312 (G).
Bipolaris shoemakeri Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 2c–d)
MycoBank MB 817466
Etymology. Named after Professor Robert Alan Shoemaker, an internationally respected mycologist and plant pathologist, who established Bipolaris for helminthosporioid species with fusoid conidia and bipolar germination, thereby differentiating it from Drechslera and Helminthosporium (Shoemaker 1959).
Holotype: Australia, Queensland, Mount Molloy, from leaf spot on Ischaemum rugosum var. segetum (Trin.) Hack., culture formed in vitro by crossing single-spored isolates, June 1987, J.L. Alcorn, BRIP 15929 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, uniformly pale brown to brown, smooth, septate, up to 1.8 mm × 6 μm. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale to subhyaline, smooth, mono- or polytretic with undarkened circular scars. Conidiogenous nodes distinct and slightly verruculose. Conidia fusiform, straight to slightly curved, (60–) 70–80 (–100) × (10–) 13.5–15 (–19) μm, pale brown to brown, smooth, 4–10 (usually 8)-distoseptate. Hilum darkened.
Additional specimen examined: Australia, Queensland, Mount Molloy, from leaf spot on Ischaemum rugosum var. segetum, 30 Apr. 1987, J.L. Alcorn, BRIP 15806.
Notes: Bipolaris shoemakeri was isolated from Ischaemum rugosum var. segetum, which is found mainly in the northern coastal region of Australia, and extends from India to Taiwan (Simon and Alfonso 2011). The ex-holotype culture (BRIP 15929) produced ascospores and was derived in vitro from self-crossed single-spored isolates of BRIP 15806 (J.L. Alcorn herbarium notes). The sexual morph was not observed during this study, and, therefore, a description could not be provided. Other species recorded on I. rugosum are B. cynodontis, B. oryzae and B. setariae (Sivanesan 1987; Manamgoda et al. 2014; Farr and Rossman 2016; Herbarium Catalogue 2016). Bipolaris shoemakeri has longer conidiophores (up to 1.8 mm) than B. cynodontis (up to 170 μm), B. oryzae (up to 600 μm) and B. setariae (200 μm). Bipolaris shoemakeri is phylogenetically close to B. secalis (Fig. 1). Morphologically, the very long, straight to flexuous conidiophores of B. shoemakeri differ from the shorter (up to 300 μm) and apically geniculate conidiophores of B. secalis. Bipolaris shoemakeri differs from B. secalis in three loci: ITS positions 103 (G) and 339 (indel); GAPDH positions 209 (T) and 446 (C); TEF1α positions 453 (C) and 816 (T).
Bipolaris simmondsii Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 3d–e)
MycoBank MB 817467
Etymology: Named after the Australian plant pathologist Dr. John Howard (Jack) Simmonds MBE, who listed the first helminthosporioid fungi found in Queensland (Simmonds 1966).
Holotype: Australia, Queensland, Peregian Beach, on leaf spot on Zoysia macrantha Desv., 14 Nov. 1976, J.L. Alcorn, BRIP 12030 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, sometimes geniculate towards the apex, uniformly yellowish brown, paler at the apex, smooth, septate, up to 240 μm × 8 μm, basal cell swollen, up to 18 μm diam. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic with circular scars. Conidiogenous nodes distinct and darkened. Conidia fusiform, straight or slightly curved, (70–) 78–116 (–130) × (12–) 13–17 (–18) μm, widest at the middle, yellowish brown to pale yellowish brown, paler at the ends, 7–10-distoseptate. Hilum darkened.
Notes: Bipolaris simmondsii is only known from the type specimen on Zoysia macrantha, an endemic temperate Australian grass. The ex-type isolate was sterile under the conditions it was grown. Fortunately, dried culture specimens from the original collection in 1976 had conidiophores and conidia that allowed morphological descriptions to be made. Bipolaris simmondsii is phylogenetically close to B. heveae, which has been associated with leaf spots on Zoysia japonica in Japan (Tsukiboshi et al. 2005). Bipolaris heveae has conidia that sometimes have a slightly protuberant hilum (3–4 μm), while B. simmondsii has an inconspicuous hilum. Bipolaris simmondsii differs from B. heveae in three loci: ITS positions 452 (indel), 453 (C) and 456 (T); GAPDH 98 % match (Identities 435/443, Gaps 0/443); TEF1α 9 (T), 102 (C), 307 (G), 453 (C), 655 (G), 735 (C) and 771 (C).
Bipolaris sivanesaniana Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 3f–g)
MycoBank MB 817468
Etymology: Named after Dr. Asaipillai Sivanesan, in recognition of his contributions to mycology and plant pathology, especially his seminal monograph on graminicolous helminthosporioid fungi (Sivanesan 1987).
Holotype: Australia, Queensland, Atherton, from Paspalidium distans (Trin.) Hughes, 1 May 1987, J.L. Alcorn, BRIP 15847 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, uniformly pale brown to brown, smooth, septate, up to 600 μm × 4–6 μm; basal cell swollen and darker than the other cells, up to 18 μm diam. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to subhyaline, smooth, mono- or polytretic with undarkened circular scars. Conidiogenous nodes distinct and swollen. Conidia fusiform, straight to slightly curved, (60–) 70–77 (–86) × (11–) 14–15.5 (–20) μm, pale brown to brown, 5–8-distoseptate. Hilum darkened and sometimes thickened.
Additional specimen examined: Australia, Queensland, Julatten, from Setaria sphacelata (Schumach.) Stapf & C.E. Hubb., 30 Apr. 1987, J.L. Alcorn, BRIP 15822.
Notes: Bipolaris sivanesaniana is known from Paspalidium distans and Setaria sphacelata in Queensland. This hints at a co-evolutionary relationship as the grass hosts, Setaria and Paspalidium, are closely related (Kellogg et al. 2009; Morrone et al. 2012). Bipolaris sivanesaniana is the only species described on P. distans, a native Australian perennial grass found in temperate and tropical regions of Asia and the Pacific. One other species, B. setariae, has been recorded on P. flavidum (Farr and Rossman 2016). Bipolaris sivanesaniana has longer conidiophores (up to 600 μm) than B. setariae (up to 200 μm long). Molecular phylogenetic comparison with B. setariae cannot be reliably made, as there are no available sequences for a type or authentic strain. Other Bipolaris species recorded on Setaria are B. bicolor, B. cynodontis, B. leersiae, B. maydis, B. oryzae, B. panici-milacei, B. sacchari, B. salviniae, B. setariae, B. sorokiniana, B. victoriae, B. yamadae and B. zeicola (Sivanesan 1987; Manamgoda et al. 2014; Farr and Rossman 2016; Herbarium Catalogue 2016), although some of these identifications have not been verified by DNA sequencing analyses.
Bipolaris sivanesaniana is phylogenetically close to B. oryzae and B. panici-milacei (Fig. 1). Morphologically, Bipolaris sivanesaniana has shorter conidia (60–86 μm) than B. oryzae (63–153 μm), and fewer septa (up to 8 versus 14). Bipolaris sivanesaniana has longer conidiophores than B. panici-milacei (up to 255 μm long). Bipolaris sivanesaniana differs from B. oryzae in two loci: ITS position 97 (C); TEF1α position 381 (C). Bipolaris sivanesaniana differs from B. panici-milacei in three loci: ITS position 97 (C); GAPDH position 182 (A); TEF1α position 342 (C).
Bipolaris subramanianii Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 2g–i)
MycoBank MB 817469
Etymology: Named after Professor C.V. Subramanian, in recognition of his contributions to mycology and plant pathology, especially his widely referenced monograph on hyphomycetes (Subramanian 1983).
Holotype: Australia, Queensland, Maclean Bridge, from leaf spot on Setaria sphacelata, 17 Mar. 1988, J.L. Alcorn, BRIP 16226 (includes ex-type culture).
Leaf spots on Setaria sphacelata, narrowly ellipsoidal, grey spots with brown margins, at first 1 × 0.5 mm, then expanding up to 5 cm in length with water-soaked appearance. Conidiophores mononematous, erect, straight to flexuous, never branched, uniformly brown to pale brown at the apex, smooth, septate, up to 830 μm × 5 μm; basal cell swollen and darker than the other cells, up to 13 μm diam. Conidiogenous nodes distinct and slightly swollen. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic with undarkened circular scars. Conidia straight to fusiform to subcylindrical, (70–) 90–99 (–130) × (9–) 11–12.5 (–15) μm, uniformly pale brown to subhyaline, smooth, 5–8-distoseptate, apex rounded, base obconically truncate. Hilum distinct and protuberant.
Notes: Bipolaris subramanianii is only known from the type specimen on Setaria sphacelata, which is a perennial African grass that has a worldwide distribution (Simon and Alfonso 2011). Other species recorded on S. sphaecelata are B. cynodontis, B. maydis and B. zeicola (DAF Biological Collections 2016; Farr and Rossman 2016; Herbarium Catalogue 2016); however, some of these records require verification by molecular methods. Bipolaris subramanianii has longer conidiophores (up to 830 μm) than B. cynodontis (up to 170 μm) and B. zeicola (up to 250 μm). Bipolaris subramanianii has longer conidia (70–130 μm) than B. cynodontis (30–75 μm). The conidia of B. subramanianii are typically straight to subcylindrical, whereas B. cynodontis and B. zeicola have slightly curved conidia that are broadest in the middle and taper towards the rounded ends. Bipolaris maydis has conidia that are distinctly curved.
Bipolaris subramanianii is phylogenetically close to B. shoemakeri and B. secalis (Fig. 1). The conidiophores of B. subramanianii are shorter than B. shoemakeri (up to 1.8 mm), but longer than B. secalis (up to 300 μm). The typically straight conidia of B. subramanianii are slightly longer and thinner than the slightly curved conidia of B. shoemakeri (70–80 × 13.5–15 μm). The conidia of B. subramanianii are uniformly paler in colour and have fewer septa than the conidia of B. secalis, which are mostly 10-distoseptate. Bipolaris subramanianii differs from B. shoemakeri by three loci: ITS 98 % match (Identities 452/461, Gaps 3/461); GAPDH positions 26 (T), 55 (T), 77 (G) and 209 (C); TEF1α positions 30 (G), 255 (C), 266 (G), 450 (A), 816 (C) and 843 (C). Bipolaris subramanianii differs from B. secalis by three loci: ITS 98 % match (Identities 453/460, Gaps 2/460); GAPDH positions 26 (T), 55 (T), 77 (G) and 446 (T); TEF1α positions 30 (G), 255 (C), 266 (G), 450 (A), 453 (C) and 843 (C).
Bipolaris woodii Y.P. Tan & R.G. Shivas, sp. nov. (Fig. 2j)
MycoBank MB 817470
Etymology: Named after Dr. Peter Wood, in recognition of his mentorship of microbiologists at the Queensland University of Technology, including the lead author.
Holotype: Australia, Queensland, Goondiwindi, from Paspalidium caespitosum C.E. Hubb., 25 Apr. 1977, J. Brouwer, BRIP 12239 (includes ex-type culture).
Conidiophores mononematous, erect, straight to flexuous, rarely branched, geniculate towards the apex, uniformly pale brown to brown, smooth, septate, up to 250 μm × 5–10 μm. Conidiogenous cells integrated, terminal or intercalary, with sympodial proliferation, pale brown to brown, smooth, mono- or polytretic with darkened circular scars. Conidiogenous nodes distinct, darkened and verruculose. Conidia fusiform, straight to slightly curved, (60–) 69–76 (–86) × (10–) 12.5–13.5 (–15) μm, brown, smooth, 7–10-distoseptate. Hilum darkened and sometimes thickened.
Notes: Bipolaris woodii is only known from a single specimen on Paspalidium caespitosum. This grass is a native species widely distributed across inland regions of eastern Australia (Simon and Alfonso 2011). Two other species recorded on Paspalidium are B. setariae on P. flavidum (Farr and Rossman 2016) and B. sivanesaniana described in this study from P. distans. Bipolaris woodii has shorter conidiophores (up to 250 μm) than B. sivanesaniana (up to 600 μm). Molecular phylogenetic comparison with B. setariae cannot be reliably made at this point in time as there are no available sequences for an ex-type or authentic strain of B. setariae.
Bipolaris woodii is phylogenetically close to B. microstegii, B. victoriae and B. zeicola (Fig. 1). Bipolaris woodii differs from B. microstegii in three loci: ITS 98 % match (Identities 452/461, Gap 4/461); GAPDH positions 83 (T), 111 (T) and 383 (T); TEF1α positions 138 (T), 265 (T) and 572 (C). Bipolaris woodii also differs in morphology, with shorter conidiophores than B. microstegii (up to 750 μm). Bipolaris woodii differs from B. victoriae in three loci: ITS 98 % match (Identities 454/461, Gaps 4/461); GAPDH positions 83 (T), 98 (T), 111 (T) and 383 (T); TEF1α positions 333 (C) and 573 (C). Bipolaris woodii has slightly smaller and darker conidia than B. victoriae (40–120 × 12–19 μm) (Sivanesan 1987). Bipolaris woodii differs from B. zeicola in three loci: ITS 98 % match (Identities 452/462, Gaps 5/462); GAPDH positions 83 (T), 111 (T), 383 (T) and 425 (C); TEF1α position 573 (C). Bipolaris woodii has a darkened and conspicuous hilum, and, thereby, differs from B. zeicola, which has an inconspicuous hilum.
Discussion
Phylogenetic analyses based on ITS and GAPDH sequences, either individually or concatenated, provided sufficient resolution for delimiting taxa within Bipolaris (Berbee et al. 1999; Manamgoda et al. 2012, 2014; Tan et al. 2014). Further, a four-locus dataset (ITS, GAPDH, LSU and TEF1α) provided stronger support for the description of new helminthosporioid species (Manamgoda et al. 2012; Tan et al. 2014). In this study, 13 isolates from the BRIP collection, recognised by Dr. John L. Alcorn as taxonomically interesting and potentially distinct, were analysed against reference sequences of cultures available from currently accepted Bipolaris species based on three loci, ITS, GAPDH and TEF1α. Analyses with LSU were omitted in the dataset as they provided little information to warrant inclusion. Nonetheless, LSU sequences have been deposited in GenBank to facilitate future studies (Table 1). The phylogenetic analyses of the combined three-locus dataset resolved the 13 BRIP isolates into eight novel Bipolaris species. It is not known whether the species are pathogens, endophytes or saprobes. The description of these species provides a foundation upon which additional sampling and accumulation of molecular data will improve knowledge of their host ranges and ecological roles.
The ITS locus is the universal barcode marker for fungi (Schoch et al. 2012). The ITS alignment used in this study was able to resolve 19 out of 36 Bipolaris species, including four of the new species. However, some studies have used only ITS to identify and describe Bipolaris species (Ahmadpour et al. 2012; da Cunha et al. 2012). Most recently, taxonomists have accepted that a secondary locus is essential for the accurate identification of many taxa (Madrid et al. 2014; Manamgoda et al. 2012, 2015; Tan et al. 2014; Stielow et al. 2015). The protein-coding loci of GAPDH, TEF1α and RNA polymerase II second largest subunit (RPB2) have been reported to be phylogenetically informative in the analyses of helminthosporioid species, and complement species identification and classification studies (Crous et al. 2012, 2013; Madrid et al. 2014; Manamgoda et al. 2012, 2014, 2015; Tan et al. 2014). The GAPDH and TEF1α alignments used in this study were able to resolve 34 out of 36 Bipolaris species, including the eight new species described here. None of the ITS, GAPDH or the TEF1α alignments were able to differentiate between the ex-holotype strain of B. coffeana and the recently designated ex-epitype strain of B. cynodontis (Manamgoda et al. 2014). A comparison of the sequences of B. coffeana and B. cynodontis indicates a potential synonymy, which is supported by shared conidial characteristics. GAPDH and TEF1α were determined to be the most suitable single locus marker for species-level identification within Bipolaris. Madrid et al. (2014) found RPB2, followed by GAPDH, to be the most informative loci for helminthosporioid phylogeny. Analyses with RPB2 could not be included in this study as sequences were only available for ex-type isolates of three Bipolaris species. It is strongly suggested that the classification of new taxa in Bipolaris be accompanied by the official fungal barcode, ITS and a secondary locus, GAPDH, TEF1α or RPB2.
References
Acharya K, Dutta AK, Pradhan P (2011) Bipolaris sorokiniana (Sacc.) Shoem.: the most destructive wheat fungal pathogen in the warmer areas. Aust J Crop Sci 5:1064–1071
Ahmadpour A, Heidarian Z, Donyadoost-Chelan M, Javan-Nikkhah M, Tsukiboshi T (2012) A new species of Bipolaris from Iran. Mycotaxon 120:301–307
Alcorn JL (1982) New Cochliobolus and Bipolaris species. Mycotaxon 15:1–19
Alcorn JL (1990) Additions to Bipolaris, Cochliobolus and Curvularia. Mycotaxon 39:361–392
Alcorn JL (1996) Cochliobolus heliconiae sp. nov. (Ascomycota). Aust Syst Bot 9:813–817
Berbee ML, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004) MycoBank: An online initiative to launch mycology into the 21st century. Stud Mycol 50:19–22
Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G (2004) MycoBank: An online initiative to launch mycology into the 21st century. Stud Mycol 50:19–22
Crous PW, Shivas RG, Wingfield MJ, Summerell BA, Rossman AY, Alves JL, Adams GC, Barreto RW, Bell A, Coutinho ML, Flory SL, Gates G, Grice KR, Hardy GEStJ, Kleczewski NM, Lombard L, Longa CMO, Louis-Seize G, Macedo F, Mahoney DP, Maresi G, Martin-Sanchez PM, Marvanová L, Minnis AM, Morgado LN, Noordeloos ME, Phillips AJL, Quaedvlieg W, Ryan PG, Saiz-Jimenez C, Seifert KA, Swart WJ, Tan YP, Tanney JB, Thu PQ, Videira SIR, Walker DM, Groenewald JZ (2012) Fungal Planet description sheets: 128–153. Persoonia 29:146–201
Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Yáñez-Morales M, Zúñiga-Estrada L, Cruywagen EM, De Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJN, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SND, Chen S, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Śliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack WP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ (2013) Fungal Planet description sheets: 154–213. Persoonia 31:188–296
da Cunha KC, Sutton DA, Fothergill AW, Cano J, Gené J, Madrid H, De Hoog S, Crous PW, Guarro J (2012) Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J Clin Microbiol 50:4061–4066
DAF Biological Collections (2016) Plant Pathology Herbarium & Insect Collection, Brisbane, Queensland, Australia. http://collections.daff.qld.gov.au. Accessed 11 May 2016
Ellis MB (1971) Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew
deHoog GS, Gerrits van den Ende AHG (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41:183–189
Farr DF, Rossman AY (2016) Fungal Databases, Systematic Mycology and Microbiology Laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/. Accessed 11 May 2016
Forsberg LI (1985) Foliar diseases of nursery-grown ornamental palms in Queensland. Australas Plant Pathol 14:67–71
Fröhlich J, Hyde KD, Guest DI (1997) Fungi associated with leaf spots of palms in north Queensland, Australia. Mycol Res 101:721–732
Herbarium Catalogue (2016) Royal Botanic Gardens, Kew. http://www.herbimi.info/herbimi/home.htm. Accessed 11 May 2016
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Iftikhar S, Asad S, Munir A, Sultan A, Ahmad I (2009) Hosts of Bipolaris sorokiniana, the major pathogen of spot blotch of wheat in Pakistan. Pak J Bot 41:1433–1436
Katoh K, Asimenos G, Toh H (2009) Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537:39–64
Kellogg EA, Aliscioni SS, Morrone O, Pensiero J, Zuloaga F (2009) A phylogeny of Setaria (Poaceae, Panicoideae, Paniceae) and related genera based on the chloroplast gene ndhF. Int J Plant Sci 170:117–131
Madrid H, da Cunha KC, Gené J, Dijksterhuis J, Cano J, Sutton DA, Guarro J, Crous PW (2014) Novel Curvularia species from clinical specimens. Persoonia 33:48–60
Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51:3–42
Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, Chukeatirote E, Shivas RG, Tan YP, Hyde KD (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris–Cochliobolus–Curvularia complex. Fungal Divers 56:131–144
Manamgoda DS, Rossman AY, Castlebury LA, Crous PW, Madrid H, Chukeatirote E, Hyde KD (2014) The genus Bipolaris. Stud Mycol 79:221–288
Manamgoda DS, Rossman AY, Castlebury LA, Chukeatirote E, Hyde KD (2015) A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): human and plant pathogens. Plytotaxa 212:175–198
Marignoni GB (1909) Micromiceti di Schio. Prima contribuzione alla flora micologica della provincia di Vicenza. Manifattura nazionale etichette, Italy
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GE, Wiersema JH, Turland NJ (eds) (2012) International code of nomenclature for algae, fungi, and plants (Melbourne code). Regnum vegetabile 154. Koeltz Scientific Books, Königstein
Morrone O, Aagesen L, Scataglini MA, Salariato DL, Denham SS, Chemisquy MA, Sede SM, Giussani LM, Kellogg EA, Zuloaga FO (2012) Phylogeny of the Paniceae (Poaceae: Panicoideae): integrating plastid DNA sequences and morphology into a new classification. Cladistics 28:333–356
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rossman AY, Manamgoda DS, Hyde KD (2013) Proposal to conserve the name Bipolaris against Cochliobolus (Ascomycota: Pleosporales: Pleosporaceae). Taxon 62:1331–1332
Schoch CL, Crous PW, Groenewald JZ, Boehm E, Burgess TI, de Gruyter K, de Hoog G, Dixon L, Grube M, Gueidan C (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW, Miller AN (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A 109:6241–6246
Shivas RG (1995) New records of plant pathogens in the Kimberley region of northern Western Australia. Australas Plant Pathol 24:188–201
Shoemaker RA (1959) Nomenclature of Drechslera and Bipolaris, grass parasites segregated from ‘Helminthosporium’. Can J Bot 37:879–887
Simmonds JH (1966) Host index of plant diseases in Queensland. Queensland Department of Primary Industries, Brisbane, 111 pp
Simon BK, Alfonso Y (2011) AusGrass2. http://ausgrass2.myspecies.info/. Accessed 10 May 2016
Sivanesan A (1985) New species of Bipolaris. Trans Br Mycol Soc 84(3):403–421
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap 158:1–261
Stamatakis A, Alachiotis N (2010) Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26:i132–i139
Stielow JB, Lévesque CA, Seifert KA, Meyer W, Iriny L, Smits D, Renfurm R, Verkley GJM, Groenewald M, Chaduli D, Lomascolo A, Welti S, Lesage-Meessen L, Favel A, Al-Hatmi AMS, Damm U, Yilmaz N, Houbraken J, Lombard L, Quaedvlieg W, Binder M, Vaas LAI, Vu D, Yurkov A, Begerow D, Roehl O, Guerreiro M, Fonseca A, Samerpitak K, van Diepeningen AD, Dolatabadi S, Moreno LF, Casaregola S, Mallet S, Jacques N, Roscini L, Egidi E, Bizet C, Garcia-Hermoso D, Martín MP, Deng S, Groenewald JZ, Boekhout T, de Beer ZW, Barnes I, Duong TA, Wingfield MJ, de Hoog GS, Crous PW, Lewis CT, Hambleton S, Moussa TAA, Al-Zahrani HS, Almaghrabi OA, Louis-Seize G, Assabgui R, McCormick W, Omer G, Dukik K, Cardinali G, Eberhardt U, de Vries M, Robert V (2015) One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35:242–263
Strange RN, Scott PR (2005) Plant disease: a threat to global food security. Annu Rev Phytopathol 43:83–116
Subramanian CV (1983) Hyphomycetes: taxonomy and biology. Academic Press, New York
Sunder S, Singh R, Agarwal R (2014) Brown spot of rice: an overview. Ind Phytopathol 67:201–215
Tan YP, Madrid H, Crous PW, Shivas RG (2014) Johnalcornia gen. et. comb. nov., and nine new combinations in Curvularia based on molecular phylogenetic analysis. Australas Plant Pathol 43:589–603
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol 31:21–32
Tsukiboshi T, Chung WH, Yoshida S (2005) Cochliobolus heveicola sp. nov. (Bipolaris heveae) causes brown stripe of bermudagrass and Zoysia grass. Mycoscience 46:17–21
Webster RD (1987) The Australian Paniceae (Poaceae). J. Cramer, Berlin
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgements
The authors wish to acknowledge Dr. John L. Alcorn (former curator of the BRIP) for his support, as well as his foresight in preserving all the cultures examined in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editors: Roland Kirschner and Pedro W. Crous
This article is part of the Special Issue “Biodiversity of Hyphomycetes—Special Issue in honour of Dr. Subramanian”.
Rights and permissions
About this article
Cite this article
Tan, Y.P., Crous, P.W. & Shivas, R.G. Eight novel Bipolaris species identified from John L. Alcorn’s collections at the Queensland Plant Pathology Herbarium (BRIP). Mycol Progress 15, 1203–1214 (2016). https://doi.org/10.1007/s11557-016-1240-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1240-6