Abstract
Purpose
Currently, self-retaining laryngoscopic surgery is not suitable for some patients, and there are dead zones relating to surgical field exposure and operation. The quality of the surgery can also be affected by the long periods of time required to complete it. Teleoperated continuum robots with flexible joints are expected to solve these issues. However, at the current stage of developing transoral robotic surgery systems, their large size affects the precision of surgical operative actions and there are high development and treatment costs.
Methods
We fabricated a flexible joint based on selective laser melting technology and designed a shallow neural network-based kinematic modeling approach for a continuum surgical robot. Then, human model and animal experiments were completed by master–slave teleoperation to verify the force capability and dexterity of the robot, respectively.
Results
As verified by human model and animal experiments, the designed continuum robot was demonstrated to achieve transoral laryngeal surgical field exposure without laryngoscope assistance, with sufficient load capability to finish the biopsy of vocal fold tissue in living animals.
Conclusion
The designed continuum robotic system allows the biopsy of vocal fold tissue without laryngoscope assistance. Its stiffness and dexterity indicate the system’s potential for applications in the diagnosis and treatment of vocal fold nodules and polyps. The limitations of this robotic system as shown in the experiments were also analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
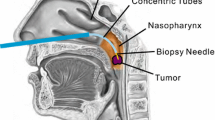
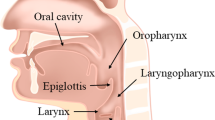
Clinically, the procedure to radically excise lesions via a self-retaining-assisted laryngoscopy is effective and widely used in the treatment of laryngeal diseases such as vocal cord polyps and glottic cancer [1,2,3]. However, the powerful action of the self-retaining laryngoscopy on the oropharyngeal and laryngeal tissues after fixation may result in complications such as teeth fracture, hematoma, swelling, mucosal injury, bleeding and nerve injuries [4, 5]. Key to a successful surgery is good exposure of the surgical field [6, 7]. As shown in Fig. 1a, the Jackson/traditional sniffing position is the common position for surgery [8, 9]. However, due to individual differences in patients’ anatomy, such as difficulties with the mouth opening, bull necks, large tongues, short jaws or prominent upper teeth, there may only be partial exposure of the vocal cord tissues or there could be failure to reveal the anterior coalition [10]. This can make it impossible to perform the surgery or there can be related complications if the surgery goes ahead [11]. In any case, transoral laryngeal surgery, with its narrow operating space and narrow surgical field, as well as its proximity to important anatomical structures, requires a high degree of operator precision. On the one hand, the force applied by the surgeon stabilizing the laryngoscope decays rapidly over time, which is related to operator fatigue and decreased effort [12]. On the other hand, hand tremors on the part of the other surgeon due to long instruments [13] can also seriously affect the quality of the surgery. This also leads to a crowded working environment with at least two surgeons crowded around the patient [14].
For those reasons, transoral robotic surgery (TORS) as illustrated in Fig. 1b which can be teleoperated by surgeons, presents a valuable new approach in head and neck surgery [15]. The continuum surgical robotic system has been widely studied and used in both single incision laparoscopic surgery [16, 17] and natural orifice transluminal endoscopic surgery [18] due to its advantages such as a high dexterity and small size [19]. Not only that, many clinical applications have shown that TORS may be a viable treatment option for early-stage laryngeal cancer [20].
Currently, only a few commercially available continuum surgical robotic systems have been reported for use in TORS. The DaVinci SP (Intuitive Surgical Inc., CA, USA) [21, 22] is a typical example. Compared to DaVinci Si/Xi, its two robotic arms and the endoscopic arm can be inserted through a single port of 25 mm in diameter, which results in minimum collisions between the robotic arms [23]. Another example, the Flex Robotic System (Medrobotics, MA, USA), has been shown to have more suitable features for TORS compared to the DaVinci surgical robot in related studies [24,25,26], but its surgical instruments still require manual operation by an operator. As such, it lacks a master–slave control system, and in a few cases, the operator has still been unable to visualize and/or access the vocal cords, with insufficient exposure of the targeted lesions [27]. It has also been shown that post-operative bleeding rate is higher after TORS with the Flex system [28]. In most cases, the use of surgical robots vastly increases the treatment costs [29, 30]. In addition to this, expensive annual service requirements, extensive additional training for surgeons and expensive accessories limit the application of these surgical robotic systems in developing countries [31, 32].
There are also some laryngeal surgical robots in the prototype stage. Olds et al. [14] designed a simple clinically usable robot to manipulate flexible endoscopes, named the Robotic EndoLaryngeal Scope System. This master–slave teleoperated robotic system solves the problems of operator fatigue, and the busy working environment of two surgeons crowded around the patient during transoral endoscopy, at an overall cost of approximately $30,000. Friedrich et al. [33] designed a tubular continuum robot for transoral surgery, and performed experiments on a porcine larynx model. However, the experiments still required an additional commercially available endoscope for visual exposure, and the porcine larynx model experiment did not verify the dexterity of the continuum robot for TORS. Gu et al. [34] designed a transoral laryngeal robotic system based on a parallel flexible mechanism. Cadaver trials were conducted in which the patient’s mouth was kept open using only a retractor. The cross section of the distal part of the robot was less than the size of a circle with a diameter of 20 mm. Kundrat et al. [35, 36] developed the MicroRALP system which consists of a tele-controlled laser instrument for non-contact endolaryngeal laser surgery with outer diameter of 11 mm. Since its laser optics are already integrated inside the continuum robot system instead of inserting in the surgical instrument channel, this robotic system does not have the ability to change instruments during surgery.
In this paper, we present our design of a low-cost continuum robot with both master–slave teleoperation and handheld capabilities, which integrates an endoscope with light-emitting diodes (LEDs) and a 2.1-mm surgical instrument channel that can accommodate instruments such as biopsy forceps and electrome. The continuum robot has a diameter of 6.0 mm with a controllable bending angle and can be used to perform partial transoral laryngeal surgery without the assistance of a laryngoscope. Human model experiments and animal experiments were designed to verify its dexterity and effectiveness, and the results of these are presented in the following sections.
Material and methods
Robot design
Figure 2a shows the prototype of the continuum robotic system. The whole system mainly consists of a three-degrees-of-freedom (DOF) continuum module, a motor drive module, a six-DOF manipulator (UR5e, Universal Robots, Denmark), a master controller (Geomagic Touch, 3D Systems Inc., SC, USA), personal computer (PC) and a monitor. As shown in Fig. 2b, the flexible joint of the continuum module is manufactured based on the helical structure of our previous study [37]. The continuum module has three-DOF, which are two-DOF for bending and one-DOF for surgical instrument delivery; these are independently controlled by three servo motors (RE16, Maxon Motor AG, Switzerland) of the drive module. The drive module can be assembled on the six-DOF manipulator. The flexible joint used in this paper is manufactured by a selective laser melting technique using Ti-6Al-4V, which is balanced in terms of stiffness and flexibility. A camera channel and a surgical instrument channel are designed in the flexible joint, and the surgical instruments are delivered by friction wheels, while the bending of the flexible joint is driven by four cables. In the robot system, camera and LEDs, the servo motor and main hand controller are connected to the PC through a USB interface, while the manipulator is connected to and controlled by the PC through a TCP/IP interface.
Sometimes, when the surgery is simple and the operation time is short, manually operated instruments not only provide direct force feedback to the operator but are also more conveniently and easy to manipulate [38], shortening the preparation and loading times of the surgery. Considering these factors, the continuum module in this robotic system is designed to be individually removable, which not only facilitates the rapid installation of a different size or function of continuum module while the drive module remains unchanged, but also makes it possible to replace the drive module with hand knobs to make it possible for the surgeon to directly move and bend the continuum module. The parameters of the robot are shown in Table 1.
Learning-based kinematics
In the master–slave system, the motion of the input device, which is controlled by the operator, should be transmitted to the motion of the continuum robot. As such, it is essential to analyze the kinematic model of the master controller and the continuum robot, and to perform kinematic mapping. For our robotic system, the forward kinematic \({f_{FK}}\) and inverse kinematic \({g_{IK}}\) can be expressed as (1).
where \(q = \left[ {{q_x},{q_y}} \right] \in {{\mathbb {R}}^2}\) represents the displacements of the motor-driven cables and \(p = \left[ {{p_x},{p_y}} \right] \in {{\mathbb {R}}^2}\) represents the tip displacements of the continuum module. It should be noted that the continuum module only has two bending degrees of freedom, and its displacement \(p_z\) in the z-direction is a fixed function of \(p_x\) and \(p_y\). To make the master–slave mapping relationship more direct and easier for the surgeon to operate, only two parameters \((p_x, p_y)\) are considered.
In this robotic system, the surgeon obtains the desired position of the end of the continuum \({\hat{p}} = km = \left[ {k{m_x},k{m_y}} \right] \) by controlling the displacement of the master device after the motion scale parameter k. After calculating the inverse kinematic model, the desired motor position can be expressed as \({\hat{q}} = {g_{IK}}\left( {{\hat{p}}} \right) \) . The continuum module can be driven to the specified position by passing \({\hat{q}}\) into the motor controllers.
In our previous study [37], static and kinematic models of the flexible joint were derived, but these were based on the condition that the inner channel of the flexible joint was regular and circular, and did not take into account the effects of friction and backlash. If finite element analysis is used, this is computationally intensive and cannot take into account the manufacturing errors of a flexible joint and the assembly errors of a robot. In recent years, the study of learning-based kinematic modeling has been increasing, a method that not only saves a lot of modeling time and effort but that can take into account the systematic errors of the robot during the learning process, to obtain a higher modeling accuracy [39].
Therefore, we designed a learning-based forward and inverse kinematic modeling approach for a continuum module based on shallow neural networks. As shown in Fig. 3, to obtain a fast execution speed when the kinematic model is deployed, our neural network model has just one hidden layer with 50 neurons, and the activation function is a sigmoid, which is trained using the least-squares method.
As shown in Fig. 4, a kinematic model data-acquisition platform was designed to acquire training data. The platform mainly consists of a motor drive module, a continuum module, an NDI magnetic field generator and three EM tracers (Aurora, Northern Digital Inc., Canada), two of which are used for the determination of the base coordinate frame and the other for the acquisition of the flexible joint’s tip position.
Based on the data acquisition platform of Fig. 4, we repeated the process until we had collected enough data: first, we generated two random numbers in the range of −7 to 7 and gave commands to two motors to drive the cables to the corresponding positions. After that, we waited for the end of the driving process and collected the readings from the motor encoder and the EM tracers at the same time as a set of training data. In the experiments of this paper, we collected more than 1,200 sets of data. After training, we obtained the training error results of the kinematic models, as shown in Table 2, and the error distribution of the (a) forward kinematic and (b) inverse kinematic in motor-driven space, as is presented in Fig. 5.
From Table 2 and Fig. 5, it can be seen that for the forward kinematic model, the standard error of predicting the end position of the continuum robot by the displacement of the motors was 1.36 mm, and the maximum error was around 4.0 mm. The error was larger when the two motor displacements were of a similar magnitude, which may be caused by nonlinear torsional deformation of the flexible joint due to the simultaneous driving of the pull wire in both directions. For the inverse kinematic model, the majority of errors remained within a small range of around -0.4-0.4 mm. For TORS with visual feedback, the surgeon can continuously adjust the tip position of the continuum robot based on the endoscopic image, so this level of error is acceptable.
Experimental setup
The experimental scenes of the human model and animal experiments are shown in Fig. 6. In the human model experiment, the model was an adult isometric upper body made by rubber. Its head could be detached for observation of the surgical effect. In the animal experiment, the animal used was a 20-month-old male Bama miniature pig weighing 55 kg. The experimental protocol was approved by the Animal Ethics Committee of Shanghai General Hospital. The experimental pig fasted for 12 hours before surgery. The induction anesthetic dose was 5 ml Zoletil 50 with intramuscular injection. The maintenance anesthesia time was between 30 and 60 minutes; an indwelling needle was placed in the ear margin vein of the experimental pig, and the maintenance anesthetic dose, which was given intravenously, was 33–50% of the initial dose. After successful general anesthesia, the experimental pig was fixed on the animal experimental bed in a supine position, and the operating area was routinely disinfected.
The surgeon remotely controlled the continuum robot and the six-DOF positioning manipulator using a Geomagic Touch as the master device, with the support of a PC interface. The 2.1-mm instrument channel in the continuum robot allowed for the delivery of electrome or biopsy forceps for TORS. Due to the flexible bending angle of the surgical instruments, the procedure could be performed without the assistance of a laryngoscope, requiring only a retractor to keep the mouth of the animal or human model open.
Results
Results of human model experiment
Figure 7 presents endoscopic and external views of the human model during the experimental procedure, in which the surgeon remotely operated the continuum robotic system to expose the vocal folds of the human model without the assistance of a laryngoscope. Similar to the animal experiment, the six-DOF manipulator was first manually adjusted near the mouth of the human model. Then, the surgeon gradually fine-tuned and fed the positioning manipulator arm through the PC control interface and the master controller. The surgeon needed to adjust the bending angle and direction of the continuum manipulator according to the endoscopic image. After reaching the position to be exposed, the surgeon controlled both the positioning manipulator and the continuum robot for retraction through the endoscopic image.
The human model experiment demonstrated that the designed continuum arm was flexible enough to perform partial transoral laryngeal surgery. After manual adjustment of the six-DOF positioning manipulator, the operator subsequently performed the experiment in teleoperation for a total time of approximately 90 s. The objective of exposing the field of vocal fold tissue was quickly achieved. The performance of the designed continuum robot indicated that it has sufficient flexibility to be used in the diagnosis of vocal fold nodules and polyps.
Results of animal experiment
The human model experiments did not verify whether the continuum robot had sufficient load capacity for transoral laryngeal surgery. Therefore, we performed animal vocal fold tissue biopsy experiments to verify the stiffness of the continuum robot. Figure 8 shows the process of excising the vocal cord tissue, with the surgeon remotely operating the continuum robot system and biopsy forceps under an endoscopic view during the animal experiment. Firstly, the six-DOF manipulator was manually adjusted to the vicinity of the mouth of the experimental pig. Then, the surgeon gradually fine-tuned and fed the positioning manipulator through the PC and master controller. When the vocal fold tissue was exposed under the endoscopic view, the biopsy forceps were controlled using the buttons on the master controller, while the view was adjusted through the position of the master controller. After the biopsy forceps clamped the vocal fold tissue, the surgeon controlled the biopsy forceps and excised the vocal cord tissue, then used the buttons of master device to retract the biopsy forceps. After confirming the completion of the biopsy operation, the surgeon rewound the entire continuum robot using the master device. The total duration of the process was approximately five minutes. The pig was awakened about 30 minutes after surgery and started to eat six hours after surgery.
The surgeon completed the vocal fold tissue biopsy operation in a relatively short time. Some studies [40, 41] have shown that vocal fold nodules and polyps may histologically be confined to the superficial layer of the lamina propria, which means that they may be more conveniently excised than normal tissues. This supports the notion that the designed continuum robot has sufficient load capability to perform the excision of vocal fold nodules or polyps in living animals.
Discussion
In this paper, a continuum robot system for TORS was designed. We proposed a shallow neural network-based kinematic modeling algorithm for the continuum surgical robot, which eliminated the complex modeling process of the master–slave continuum robot. With visual feedback, the modeling accuracy could meet the requirements of transoral laryngeal surgery.
The human model experiment verified that the designed continuum robot could achieve the exposure of the transoral laryngeal surgical field without the assistance of a laryngoscope, via master–slave teleoperation, with sufficient dexterity. The animal experiment, meanwhile, indicated that the continuum robot has sufficient load capability for the biopsy of vocal cord tissues in living animals. There are thus potential applications for this robotic system in procedures on vocal cord nodules, polyps.
However, our robotic system has a smaller outer diameter among the same type of transoral laryngeal continuum surgical robots. However, in human model and animal experiments, we have also identified some limitations of this robotic system. Firstly, the continuum robot has only one working channel, and although this robotic system allows for surgical instrument changes during surgery, it does not enable surgical steps that require multiple surgical instruments such as simultaneous grasping and cutting. Secondly, the stearable instrument has not been designed and tested in the robotic system. The laryngeal surgical operations that can be performed are therefore limited.
Therefore, in future work, we will design more surgical instruments for this robot, such as therapeutic laser optics. Also, we will try to increase the number of working channels in order to facilitate the synergistic operation of multiple surgical instruments.
References
Wang S, Zhang C, Luo S, Cai S, Xu M (2020) Analysis of perioperative nursing effects of vocal cord polyp laser excision under self-retaining laryngoscope and microscope. Acta Microsc 29(5):2540–2547
Han F, Zhang L, Cheng S, Liang F (2017) Comparison of clinical applications of single-dose intravenous injection of mivacurium and cisatracurium in adult’s vocalcordpolyps resection under self-retaining laryngoscope. BIO Web Conf. 8:01051. https://doi.org/10.1051/bioconf/20170801051
Zou Y, Huang J, Zhang X, Liu Q, Li W, Peng S, Zhan S, Zeng Q (2004) Clinical application of laryngeal endoscopy in the minimally invasive surgery of laryngeal diseases. J Clin Otorhinolaryngol 18(3):152–154
Gugatschka M, Gerstenberger C, Friedrich G (2008) Analysis of forces applied during microlaryngoscopy: a descriptive study. Eur Arch Oto-Rhino-Laryngol 265(9):1083–1087. https://doi.org/10.1007/s00405-008-0586-z
Klussmann JP, Knoedgen R, Wittekindt C, Damm M, Eckel HE (2002) Complications of suspension laryngoscopy. Ann Otol Rhinol Laryngol 111(11):972–976. https://doi.org/10.1177/000348940211101104
Liang F, Chen R, Lin P, Han P, Cai Q, Huang X (2019) Two-handed tying technique in vocal fold mucosa microsuture for the treatment of Reinke’s edema. Eur Arch Oto-Rhino-Laryngol 276(7):2015–2022. https://doi.org/10.1007/s00405-019-05480-y
Lechien JR, Fakhry N, Saussez S, Chiesa-Estomba CM, Chekkoury-Idrissi Y, Cammaroto G, Melkane AE, Barillari MR, Crevier-Buchman L, Ayad T, Remacle M, Hans S (2020) Surgical, clinical and functional outcomes of transoral robotic surgery for supraglottic laryngeal cancers: a systematic review. Oral Oncol 109:104848. https://doi.org/10.1016/j.oraloncology.2020.104848
Alon EE, Wolf M (2019) Instrumentation in endoscopic laryngeal surgery. Op Tech Otolaryngol Head Neck Surg 30(4):243–248. https://doi.org/10.1016/j.otot.2019.09.004
Collins SR (2014) Direct and indirect laryngoscopy: equipment and techniques. Respir Care 59(6):850–864. https://doi.org/10.4187/respcare.03033
Vaughan CW (1993) Vocal fold exposure in phonosurgery. J Voice 7(2):189–194. https://doi.org/10.1016/S0892-1997(05)80350-9
Qin M, Liu Y, Wu K, Zhao Y, Ton B, Gao C, Li Y, Zhang L, Wang Y (2016) Use of self-retaining laryngoscope in difficult laryngealy exposure in laryngeal microsurgery. J Audiol Speech Pathol 24(2):135–137
Caldiroli D, Molteni F, Sommariva A, Frittoli S, Guanziroli E, Cortellazzi P, Orena EF (2014) Upper limb muscular activity and perceived workload during laryngoscopy: comparison of Glidescope® and Macintosh laryngoscopy in manikin: An observational study. Br J Anaesth 112(3):563–569. https://doi.org/10.1093/bja/aet347
Plinkert P, Löwenheim H (1997) Trends and perspectives in minimally invasive surgery in otorhinolaryngology-head and neck surgery. Laryngoscope 107(11):1483–1489. https://doi.org/10.1097/00005537-199711000-00011
Olds K, Hillel A, Kriss J, Nair A, Kim H, Cha E, Curry M, Akst L, Yung R, Richmon J, Taylor R (2012) A robotic assistant for trans-oral surgery: the robotic endo-laryngeal flexible (Robo-ELF) scope. J Robot Surg 6(1):13–18. https://doi.org/10.1007/s11701-011-0329-9
Lang S, Mattheis S, Hasskamp P, Lawson G, Güldner C, Mandapathil M, Schuler P, Hoffmann T, Scheithauer M, Remacle M (2017) A european multicenter study evaluating the flex robotic system in transoral robotic surgery. Laryngoscope 127(2):391–395. https://doi.org/10.1002/lary.26358
Burgner-Kahrs J, Rucker DC, Choset H (2015) Continuum robots for medical applications: a survey. IEEE Trans Robot 31(6):1261–1280. https://doi.org/10.1109/TRO.2015.2489500
Xu K, Zhao J, Fu M (2015) Development of the SJTU unfoldable robotic system (SURS) for single port laparoscopy. IEEE/ASME Trans Mechatron 20(5):2133–2145. https://doi.org/10.1109/TMECH.2014.2364625
Hwang M, Yang U-J, Kong D, Chung DG, Lim J-G, Lee D-H, Kim DH, Shin D, Jang T, Kim J-W, Kwon D-S (2017) A single port surgical robot system with novel elbow joint mechanism for high force transmission. Int J Med Robot Comput Assist Surg 13(4):1808. https://doi.org/10.1002/rcs.1808
Hong W, Xie L, Liu J, Sun Y, Li K, Wang H (2018) Development of a novel continuum robotic system for maxillary sinus surgery. IEEE/ASME Trans Mechatron 23(3):1226–1237. https://doi.org/10.1109/TMECH.2018.2818442
Hanna J, Brauer PR, Morse E, Judson B, Mehra S (2020) Is robotic surgery an option for early T-stage laryngeal cancer? Early nationwide results. Laryngoscope 130(5):1195–1201. https://doi.org/10.1002/lary.28144
Chan JYK, Wong EWY, Tsang RK, Holsinger FC, Tong MCF, Chiu PWY, Ng SSM (2017) Early results of a safety and feasibility clinical trial of a novel single-port flexible robot for transoral robotic surgery. Eur Arch Oto-Rhino-Laryngol 274(11):3993–3996. https://doi.org/10.1007/s00405-017-4729-y
Orosco RK, Tam K, Nakayama M, Holsinger FC, Spriano G (2019) Transoral supraglottic laryngectomy using a next-generation single-port robotic surgical system. Head Neck 41(7):2143–2147. https://doi.org/10.1002/hed.25676
Park YM, Kim DH, Kang MS, Lim JY, Choi EC, Koh YW, Kim SH (2019) The first human trial of transoral robotic surgery using a single-port robotic system in the treatment of laryngo-pharyngeal cancer. Ann Surg Oncol 26(13):4472–4480. https://doi.org/10.1245/s10434-019-07802-0
Remacle MMN, Prasad V, Lawson G, Plisson L, Bachy V, Van der Vorst S (2015) Transoral robotic surgery (TORS) with the Medrobotics Flex\(^{\rm TM}\) System: first surgical application on humans. Eur Arch Oto-Rhino-Laryngol 272(6):1451–1455. https://doi.org/10.1007/s00405-015-3532-x
Mattheis S, Hasskamp P, Holtmann L, Schäfer C, Geisthoff U, Dominas N, Lang S (2017) Flex Robotic System in transoral robotic surgery: the first 40 patients. Head Neck 39(3):471–475. https://doi.org/10.1002/hed.24611
Mattheis S, Hussain T, Höing B, Haßkamp P, Holtmann L, Lang S (2019) Robotics in laryngeal surgery. Op Tech Otolaryngol Head Neck Surg 30(4):284–288. https://doi.org/10.1016/j.otot.2019.09.012
Poon H, Li C, Gao W, Ren H, Lim CM (2018) Evolution of robotic systems for transoral head and neck surgery. Oral Oncol 87:82–88. https://doi.org/10.1016/j.oraloncology.2018.10.020
Hussain T, Lang S, Haßkamp P, Holtmann L, Höing B, Mattheis S (2020) The Flex robotic system compared to transoral laser microsurgery for the resection of supraglottic carcinomas: first results and preliminary oncologic outcomes. Eur Arch Oto-Rhino-Laryngol 277(3):917–924. https://doi.org/10.1007/s00405-019-05767-0
Cole AP, Leow JJ, Chang SL, Chung BI, Meyer CP, Kibel AS, Menon M, Nguyen PL, Choueiri TK, Reznor G, Lipsitz SR, Sammon JD, Sun M, Trinh Q-D (2016) Surgeon and hospital level variation in the costs of robot-assisted radical prostatectomy. J Urol 196(4):1090–1095. https://doi.org/10.1016/j.juro.2016.04.087
Wang W, Li J, Wang S, Su H, Jiang X (2016) System design and animal experiment study of a novel minimally invasive surgical robot. Int J Med Robot Comput Assist Surg 12(1):73–84. https://doi.org/10.1002/rcs.1658
Yi B, Wang G, Li J, Jiang J, Son Z, Su H, Zhu S, Wang S (2017) Domestically produced Chinese minimally invasive surgical robot system Micro Hand is applied to clinical surgery preliminarily in China. Surg Endosc 31(1):487–493. https://doi.org/10.1007/s00464-016-4945-3
Goldenberg D, Goldenberg MDF (2017) TORS with the Flex® robotic system. Atlas Head Neck Robot Surg 108:193–200. https://doi.org/10.1007/978-3-319-49578-1_19
Friedrich DT, Modes V, Hoffmann TK, Greve J, Schuler PJ, Burgner-Kahrs J (2018) Teleoperated tubular continuum robots for transoral surgery - feasibility in a porcine larynx model. Int J Med Robot Comput Assist Surg 14(5):1928. https://doi.org/10.1002/rcs.1928
Gu X, Li C, Xiao X, Lim CM, Ren H (2019) A compliant transoral surgical robotic system based on a parallel flexible mechanism. Ann Biomed Eng 47(6):1329–1344. https://doi.org/10.1007/s10439-019-02241-0
Kundrat D, Schoob A, Piskon T, Grässlin R, Schuler PJ, Hoffmann TK, Kahrs LA, Ortmaier T (2019) Toward assistive technologies for focus adjustment in teleoperated robotic non-contact laser surgery. IEEE Trans Med Robot Bion 1(3):145–157. https://doi.org/10.1109/TMRB.2019.2931438
Kundrat D, Graesslin R, Schoob A, Friedrich DT, Scheithauer MO, Hoffmann TK, Ortmaier T, Kahrs LA, Schuler PJ (2021) Preclinical performance evaluation of a robotic endoscope for non-contact laser surgery. Ann Biomed Eng 49(2):585–600. https://doi.org/10.1007/s10439-020-02577-y
Feng F, Hong W, Xie L (2020) Design of 3D-printed flexible joints with presettable stiffness for surgical robots. IEEE Access 8:79573–79585. https://doi.org/10.1109/ACCESS.2020.2991092
Kim J, Kwon S-I, Moon Y, Kim K (2021) A hand-held non-robotic surgical device to compensate for wire length in unpredicted paths. IEEE Access 9:60629–60639. https://doi.org/10.1109/ACCESS.2021.3073139
Feng F, Hong W, Xie L (2021) A learning-based tip contact force estimation method for tendon-driven continuum manipulator. Sci Rep 11(1):17482. https://doi.org/10.1038/s41598-021-97003-1
Benninger MS (2000) Microdissection or microspot CO2 laser for limited vocal fold benign lesions: a prospective randomized trial. Laryngoscope 110(211):1. https://doi.org/10.1097/00005537-200002001-00001
Wallis L, Jackson-Menaldi C, Holland W, Giraldo A (2004) Vocal fold nodule vs. vocal fold polyp: answer from surgical pathologist and voice pathologist point of view. J Voice 18(1):125–129. https://doi.org/10.1016/j.jvoice.2003.07.003
Author information
Authors and Affiliations
Contributions
The robotic system was developed by FF, WH and LX. YZ and KL performed the animal and human model experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported in part by the National Natural Science Foundation of China under Grants 62133009, 61973211, 51911540479, M-0221, in part by the Science and Technology Commission of Shanghai Municipality under Grants 21550714200 and in part by the Project of Institute of Medical Robotics of Shanghai Jiao Tong University.
Conflict of interest
All authors declare that there is no conflict of interest.
Ethics approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Chinese Regulations for the Administration of Affairs Concerning Experimental Animals. The study was approved by the Animal Ethics Committee of Shanghai General Hospital (No. 2019AW044).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, F., Zhou, Y., Hong, W. et al. Development and experiments of a continuum robotic system for transoral laryngeal surgery. Int J CARS 17, 497–505 (2022). https://doi.org/10.1007/s11548-022-02558-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-022-02558-7