Abstract
Purpose
Transoral robotic surgery (TORS) has the potential to improve some inherent disadvantages of transoral laser microsurgery (TLM). Here, we retrospectively assessed the application of the Medrobotics Flex system for the resection of supraglottic carcinomas compared to TLM.
Methods
84 patients underwent surgery for supraglottic carcinomas with the Flex robotic system (n = 19, T-stage distribution in %: T1 42, T2 47, T3 11, T4 0) or TLM (n = 65, T-stage distribution in %: T1 40, T2 44, T3 14, T4 2). Clinical and oncologic parameters were compared.
Results
All surgeries were successfully completed with the Flex system and tracheostomy rate was 13%. For patients with adequate follow-up, 24-month disease-free survival was 71.4% (n = 5/7) after TORS compared to 64.9% (n = 24/37) after TLM. Local recurrence rates were 0% for TORS and 11% for TLM.
Conclusions
Initial results for supraglottic carcinoma resection using the Medrobotics Flex system are encouraging with excellent local tumor control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past three decades, transoral laser microsurgery (TLM) has been established as an excellent treatment option for surgically resectable supraglottic carcinomas [1,2,3,4,5]. Large retrospective analyses have shown favorable oncologic outcomes compared to non-surgical approaches, as well as reduced morbidity compared to open surgical procedures [3, 6,7,8,9,10].
The introduction of transoral robotic surgery (TORS) for pharyngeal and laryngeal tumor resection has the potential to build on the inherent advantages of the transoral approach while addressing some of its limitations. In particular, the use of rigid endoscopes and laryngoscopes during TLM limits the exposure of the anatomical region and restricts the surgeon to a straight line of view.
TORS can be valuable to improve visualization and exposure of tumors located in difficult-to-reach regions of the pharynx and larynx, such as hypopharynx and supraglottis, and potentially facilitate en bloc resection. While the seminal works of Steiner et al. led to the general acceptance of the piecemeal resection technique for laryngeal tumors [2, 11], the concept relies on excellent microscopic tissue visualization to distinguish tumor from tumor-free regions, particularly after having removed parts of the lesion. Visualization and orientation can be challenging when restricted to a straight line of view and may require repeated re-positioning of the endoscope, particularly for larger tumors. Multiple authors have therefore applied the da Vinci robotic system (Intuitive Surgical, Sunnyvale, CA) for the resection of supraglottic tumors and were able to show encouraging results, including good intraoperative tumor exposure [12], lower post-operative morbidity compared to open surgery [13], as well as acceptable oncologic outcomes [14], the latter being comparable to results known from TLM.
The Medrobotics Flex robotic system was specifically designed for head and neck surgery. The robotic system is built on a flexible spine and supports flexible instruments and cutting devices for transoral surgery. It consists of small closed-loop mechanisms that can be bent locally in both transversal directions. At the tip of the robot, a 3D camera is installed. The system can be commanded to bend when navigating in the cavity and to freeze when inserting instruments such as graspers, cutters, or laser fibres. For the latter, the Flex robotic system offers two laterally mounted accessory ports through which the flexible tools can be inserted and tactile feedback from the operating tip to the surgeon’s hand is transmitted. As previously shown [15, 16], all regions of the pharynx and larynx can be reached with the system. Technical advancements, such as the introduction of a novel retractor, have further improved intraoperative exposure [17].
In this study, we evaluated the application of the Medrobotics Flex system for the resection of supraglottic laryngeal cancers in comparison to cases performed via TLM. The simultaneous availability of both technologies at our institution allowed for a direct, albeit retrospective, comparison at a single center and can potentially help further specify indications for TORS in general, and the Medrobotics Flex system in particular.
Methods
Previously untreated patients with histologically confirmed supraglottic squamous cell carcinoma diagnosed between 2007 and 2017 were included in this retrospective study. Initial diagnosis, staging, and treatment, as well as follow-up for all patients were performed at the same tertiary referral center, allowing full access and comparability for all relevant oncologic parameters. All patients who had undergone parts of the aforementioned diagnostic or therapeutic steps elsewhere were excluded. The study was performed after approval by our institutional ethics committee.
Surgeries and adjuvant treatment
The type of surgery performed (TORS or TLM) was in part determined by the availability of the Medrobotics Flex system, and the availability of Flex system-trained surgeons. In practice, dates for Flex-assisted procedures were set months in advance and patients with newly diagnosed cases of transorally resectable supraglottic carcinomas during the 4 weeks prior to the set dates were treated accordingly. Patients were counseled about the potential advantages and disadvantages of the respective technologies; thereafter informed consent was obtained. All procedures in both groups were performed by surgeons with ample experience in the respective type of surgery. Procedures with the Medrobotics Flex system were performed by two specifically trained surgeons.
Treatment decisions were made by an interdisciplinary tumor board based on tumor stage and location, as well as lymph node status as assessed by sonography and via CT, or MRI. Importantly, members of the tumor board did not decide which specific resection method, i.e. TLM, or the Flex system would be used. Neck dissection was commonly performed for tumors clinically staged as T2 and above, or if patients presented with clinically suspicious lymph nodes. Decisions regarding the necessity for tracheostomy were not considered part of the treatment plan since all tracheostomies were performed solely for temporary peri- and post-operative airway protection, and not as a permanent measure. Therefore, the necessity for tracheostomy was determined individually, and sometimes intraoperatively, depending on tumor size and exact location.
For cases using the Flex system, the visual exposure of all potential regions of interest was assessed by the operating surgeon. Regions of interest in the oropharynx (tonsils, base of tongue, posterior and lateral pharyngeal wall), as well as the hypopharynx (posterior and lateral pharyngeal wall, piriform sinus), and the larynx (epiglottis, arytenoid cartilages, vocal cords) were assessed prior to the operating procedure.
Simultaneous unilateral or bilateral neck dissection was performed according to tumor stage and based on the recommendation of the interdisciplinary tumor board. Similarly, post-operative adjuvant (chemo-) radiation was recommended by the tumor board according to the post-operative histological results, depending on tumor stage and lymph node status. Patients who did not need adjuvant therapy according to the tumor board were re-evaluated and re-biopsied 6–8 weeks after surgery under general anesthesia to determine whether they were tumor-free.
Post-operative clinical follow-up
At our institution, all tumor patients are routinely followed up for 5 years after initial treatment at regular intervals thus ensuring timely detection of potential recurrence. Follow-up intervals are every 8 weeks during the first year, every 3 months during the second year, every 6 months during the third year and every 12 months for the fourth and fifth year after primary treatment. Importantly, between every follow-up visit at the center, an additional follow-up examination is performed by the patients’ resident otolaryngologist. Every follow-up includes a thorough clinical exam, as well as an ultrasound scan of the neck. If deemed necessary, a CT or MRI scan is added.
Due to the relative novelty of the Flex robotics system, we chose to assess oncologic outcomes for patients who had at least a 2-year follow-up period after initial treatment. As recently shown by others, 2-year disease-specific survival (DSS) rates are a valid predictor of 5-year DSS [18]. Patients who were treated within the past 2 years or without regular follow-up records were not included in the oncologic outcomes’ assessment.
Main reasons for interrupted clinical follow-up within the first 2 years after initial treatment were non-related severe diseases and patient incompliance.
Patient charts were reviewed to gather patient information, including demographic data, as well as oncologic parameters, such as staging results, treatment regimens, and follow-up results.
Results
Patients
In total, 234 patients were identified who were diagnosed with a supraglottic laryngeal carcinoma at our institution between 2007 and 2017. The therapeutic approach was primarily determined by clinical and radiologic staging results and ensuing tumor board recommendation. In cases where a primary surgical approach and primary chemoradiation were considered equally valid, patients’ preference was taken into consideration. 3.0% (n = 7/234) patients were treated at other centers after initial diagnosis and no further information regarding treatment was available. 8.1% (n = 19/234) of patients were diagnosed with distant metastases and received palliative therapy. 35.4% (n = 83/234) of patients underwent primary chemoradiation and 17.5% (n = 41/234) of patients underwent total laryngectomy. 35.9% of patients (n = 84/234) were treated via a transoral surgical approach, i.e. TLM or TORS. In total, we identified 65 patients who underwent TLM and 19 patients who underwent TORS procedures using the Medrobotics Flex system for supraglottic laryngeal carcinomas between 2007 and 2017. Patient and disease characteristics are shown in Table 1. Patients were evenly matched regarding age and T-stage.
Surgeries and adjuvant treatment
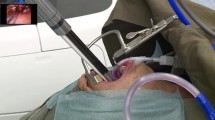
All surgeries—either with TORS or TLM—could be completed via the transoral approach. TORS using the Medrobotics Flex system provided an excellent visualization of the surgical field (Fig. 1a, d); the relevant regions of interest in the oropharynx, hypopharynx, and larynx could be reached with the flexible surgical instruments.
a Excellent visualization of the supraglottic carcinoma of the right aryepiglottic fold at the beginning of the Flex surgical procedure. b Intraoperative view during tumor resection. The entire tumor can be visualized throughout the procedure. The superior laryngeal artery is clipped, an enlarged view is shown in c. d View of the operative field after completion of tumor resection
While in the TLM group tumors were typically resected in piecemeal technique, in the TORS group, 57.9% (n = 11/19) of tumors were resected en bloc, i.e. in a single piece. Out of the remaining eight tumors, seven were resected in two pieces, one was resected in three pieces. The seven tumors which were resected in two pieces were located on the laryngeal plane of the epiglottis and the epiglottis had to be resected in total. In these cases, the epiglottis was intentionally split into two halves through the middle to which allowed an improved approach to the caudal resection margin, i.e. the epiglottis base.
In the TORS group, 42% (n = 8/19) of patients received adjuvant treatment with radiation or combined chemo-radiation according to the post-operative tumor board decision, compared to 36.9% (n = 24/65) of TLM patients. All patients who did not receive immediate post-operative adjuvant radiation underwent an endoscopic re-evaluation 6 weeks after their primary surgical treatment. Two out of 11 patients in the TORS group showed tumor remnants on re-biopsy and a second resection procedure with the Flex system was performed. Thereafter, both patients were tumor-free on further follow-up.
Neck treatment for both groups is shown in Table 2. All patients were discussed in an interdisciplinary tumor board prior to scheduling of the procedure, i.e. TLM or TORS. As to be expected, the portion of patients undergoing neck dissection was therefore similar in both groups, which were evenly matched regarding tumor stage. Notably, despite the majority of patients in both groups presenting with early T-stage tumors (T1 and T2 for TORS 68.4%, for TLM 57.0%), more than two-thirds of patients in both groups underwent neck dissection according to tumor board recommendation, thus emphasizing the aggressiveness of supraglottic carcinomas.
All patients were pre-operatively informed that a temporary tracheostomy would only be performed if necessary. In patients with locally advanced tumors (i.e. T3, T4), tracheostomy was commonly performed at the beginning of the surgical procedure. In patients with smaller primary tumors, tracheostomy in both groups was only performed, if deemed necessary due to strong swelling and/or intraoperative bleeding. Fewer patients required temporary tracheostomy in the TORS group, compared to the TLM group (TORS 15.8%, TLM 36.9%, Table 2).
Post-operative bleeding
The rate of post-operative bleeding requiring hemostasis under general anesthesia was 15.7% (n = 3/19) for TORS and 10.8% (n = 7/65) for TLM. Differences between the groups were statistically not significant.
Post-operative swallowing
For TORS patients, detailed data for post-operative swallowing and duration of post-operative feeding via a nasogastric tube were available for 14 out of 19 patients. Four patients (all stage T1) did not require a feeding tube, one patient refused a feeding tube and received parenteral nutrition for 5 days after surgery. The remaining nine patients were fed via a nasogastric tube for an average of 6.9 ± 4.5 days.
Oncologic outcome
Disease-free survival was calculated for patients who had undergone follow-up for at least 24 months, i.e. 37 out of 65 TLM patients and 7 out of 19 TORS patients. In the TLM group, recurrence (i.e. local, regional, distant) occurred in 35.1% (n = 13/37) of patients. Details are depicted in Table 3. Of these 13 patients, two refused adjuvant therapy despite recommendation by the interdisciplinary tumor board. Therefore, disease specific survival for patients undergoing the full recommended treatment regimen was 68.6% (n = 24/35).
In the TORS group, a complete 24-month follow-up was available for seven patients. 28.6% (n = 2/7) of patients suffered from disease recurrence (details shown in Suppl. Table 1). There were no cases of local recurrence in the TORS group. Comparative data for 24-month disease-free survival are shown in Fig. 2. In the TLM group, the majority of patients with disease recurrence presented with advanced stage disease at the time of surgery. It is noteworthy to mention that in total only one case of local recurrence was diagnosed for TORS/TLM in early stage cancer, i.e. stages I and II.
Discussion
The goal of this retrospective analysis was to assess whether the Flex robotic system can serve as viable addition to the well-established TLM approach for the resection of supraglottic carcinomas. At our institution, we have access to both technologies and can therefore directly compare surgical handling as well as therapeutic outcomes. With Flex surgery dates being set well in advance of the procedures and prior to actual patient accrual, selection bias between the two modalities was reduced to a certain degree but cannot be fully dismissed. Keeping this limitation in mind, the results of this retrospective analysis for a novel device do not yet allow for a definitive comparison but are nevertheless promising: all cases performed using the Flex system were successfully completed via the transoral approach and excellent visualization of the surgical region of interest was possible. The ability to approach a lesion from multiple angles due to the flexible robotic arms carrying the camera as well as the surgical instruments, addresses the inherent limitations of transoral laser microsurgery with its straight line of view. Our preliminary oncologic results further support the notion that the Flex system can be a valuable resection tool, particularly for supraglottic tumors, as shown by the high degree of local tumor control. This may be facilitated by the potential for en bloc tumor resections which become more feasible with the endoscopic system compared to TLM. In our study, most TORS-resected tumors were resected and sent to pathology in one or two pieces. Important to keep in mind though that definitive resection margin status can only be determined by follow-up over time for both groups due to tissue coagulation effects caused by the laser. In the TORS group, we experienced two cases in which secondary surgery was required after the initial TORS procedure due to positive results on re-biopsy 6 weeks post-operatively. In both cases, a tumor resection was successfully completed via a second TORS surgery using the Flex system. Of course, when assessing oncologic outcomes, the limitations of this study need to be taken into careful consideration. As commonly the case with analyses of novel surgical technologies for a highly specific indication [19, 20], patient numbers are relatively low and do not yield statistical significance. Also, importantly, in our study, the two groups were not matched completely evenly regarding tumor stage at the time of surgery. In the TORS group, more patients had early stage tumors (stage I and II disease 68.4%), than patients undergoing TLM (stage I and II disease 57.0%). Nevertheless, the results of the 24-month follow-up are highly encouraging: all patients with available 24-month follow-up data undergoing TORS did not suffer from local disease recurrence during follow-up. This included a patient with a T3-tumor and overall stage IVa disease, confirming the notion that TORS can be a viable treatment option not only for early stage disease, but also for selected cases of locally advanced disease [21, 22]. Again, the low number of patients with adequate follow-up periods needs to be kept in mind when assessing oncologic outcomes and at this point the available data does not transcend the state of a feasibility assessment. However, given the novelty of the device and the specific region of application, these preliminary data hold value.
When assessing disease-specific survival data for all patients in this study, refusal of recommended adjuvant therapy by two patients in the TLM group must be considered. In patients following the recommended therapeutic regimen, recurrence-free two-year survival was 68.2% across all stages for TLM, which is on par with reported rates in larger-scale retrospective studies [10, 18]. Notably, in our patient collective individuals from both groups with early-stage disease, i.e. overall stages I and II, suffered from regional recurrence (i.e. lymph node metastases), and even distant metastases during the follow-up period. While these findings may be somewhat coincidental and attributable to the small sample size of this analysis, they demonstrate the aggressiveness of the disease and should be taken into consideration during therapy planning and clinical follow-up, in particular.
In our study, only three patients (15.8% of all patients), both with overall stage T3 tumors and stage IV disease, underwent tracheostomy, while none of the patients with early stage tumors required tracheostomy. In all three cases, tracheostomy was performed as a safety measure in case of post-operative swelling or bleeding, and not as a primary measure to improve visualization of the region of interest. Other TORS studies have reported markedly higher tracheostomy rates, ranging from 24% [23], up to 100% [14], despite predominantly early tumor stages, in many cases to enable adequate visualization. Despite being a generally safe procedure, potential long-term risks of tracheostomy, including pneumonia, tracheal stenosis, and malignoma recurrence must be taken into consideration [24, 25]. While the Flex system’s specific design for transoral application and slim instruments certainly contribute favorably to improved visualization, the data also emphasize the importance of patient selection for TORS and expertise in device handling to avoid additional patient morbidity. Supraglottic lesions in particular can be difficult to reach and visualize, both via TLM and with TORS approaches, [26], therefore the successful completion of all procedures without additional morbidity to improve visualization is promising.
In our study, the majority of patients with T1 tumors did not require a nasogastric feeding tube postoperatively. These findings, albeit in a very limited sample size, compare favorably with published data for other TORS systems [27], and again support the assumption that the Flex system can potentially be a less invasive tool for transoral tumor resection.
In our TORS patients, we experienced a post-operative bleeding rate of 15.7%. These patients underwent a second procedure under general anesthesia for hemostasis after surgery. The rate is higher than the rate for patients undergoing TLM in our study and similar to reported rates for previously reported TORS studies [20, 28]. Intraoperative bleeding during tumor resection could be reliably controlled with a rigid cautery suction device inserted transorally next to the Flex scope, i.e. the same technique as commonly used during TLM. In cases where larger tumors have to be resected, the superior laryngeal artery is routinely located and clipped. Here too, currently, a conventional, i.e. rigid clipping device is used which is inserted transorally next to the Flex scope. While the latest camera generation of the Flex robotic system allows for excellent visualization of the artery (Fig. 1b, c), intraoperative handling of bleeding remains challenging, since no cautery or clipping device specifically designed for the Flex robotic system is currently available. Notably, in two out of the three cases requiring a second surgery for hemostasis, bleeding occurred despite the superior laryngeal artery having been clipped during the initial tumor resection procedure. Improved instrumentation for the Flex robotic system could potentially further lower complication rates and is one of the key aspects to be addressed by the device manufacturers in the future. Ideally, the benefits of visualization with the flexible endoscopic system should be applicable to all aspects of surgery and not limited to the resection procedure.
To the best of our knowledge, this is the first study to assess oncologic outcomes of transoral robotic surgery with the Medrobotics Flex system for the resection of supraglottic carcinomas. The system allowed excellent intraoperative visualization leading to a high degree of local tumor control. While some challenges remain, our preliminary findings suggest that the technology can potentially be a valuable addition to existing surgical modalities and that further studies are warranted. Based hereon, we have initiated a multi-center prospective trial.
References
Steiner W (1988) Experience in endoscopic laser surgery of malignant tumours of the upper aero-digestive tract. Adv Otorhinolaryngol 39:135–144
Steiner W (1993) Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol 14(2):116–121
Ambrosch P, Kron M, Steiner W (1998) Carbon dioxide laser microsurgery for early supraglottic carcinoma. Ann Otol Rhinol Laryngol 107(8):680–688. https://doi.org/10.1177/000348949810700810
Zeitels SM, Koufman JA, Davis RK, Vaughan CW (1994) Endoscopic treatment of supraglottic and hypopharynx cancer. Laryngoscope 104(1 Pt 1):71–78
Vilaseca I, Bernal-Sprekelsen M, Luis Blanch J (2010) Transoral laser microsurgery for T3 laryngeal tumors: prognostic factors. Head Neck 32(7):929–938. https://doi.org/10.1002/hed.21288
Iro H, Waldfahrer F, Altendorf-Hofmann A, Weidenbecher M, Sauer R, Steiner W (1998) Transoral laser surgery of supraglottic cancer: follow-up of 141 patients. Archives of otolaryngology–head & neck surgery 124 (11):1245–1250
Mendenhall WM, Parsons JT, Mancuso AA, Stringer SP, Cassisi NJ (1996) Radiotherapy for squamous cell carcinoma of the supraglottic larynx: an alternative to surgery. Head Neck 18(1):24–35. https://doi.org/10.1002/(SICI)1097-0347(199601/02)18:1%3c24:AID-HED4%3e3.0.CO;2-0
Spaulding CA, Krochak RJ, Hahn SS, Constable WC (1986) Radiotherapeutic management of cancer of the supraglottis. Cancer 57(7):1292–1298
Roh JL, Kim DH, Park CI (2008) Voice, swallowing and quality of life in patients after transoral laser surgery for supraglottic carcinoma. J Surg Oncol 98(3):184–189. https://doi.org/10.1002/jso.21101
Canis M, Martin A, Ihler F, Wolff HA, Kron M, Matthias C, Steiner W (2013) Results of transoral laser microsurgery for supraglottic carcinoma in 277 patients. Eur Arch Oto-Rhino-Laryngol 270(8):2315–2326. https://doi.org/10.1007/s00405-012-2327-6
Harris AT, Tanyi A, Hart RD, Trites J, Rigby MH, Lancaster J, Nicolaides A, Taylor SM (2018) Transoral laser surgery for laryngeal carcinoma: has Steiner achieved a genuine paradigm shift in oncological surgery? Ann R Coll Surg Engl 100(1):2–5. https://doi.org/10.1308/rcsann.2017.0190
Weinstein GS, O'Malley BW Jr, Snyder W, Hockstein NG (2007) Transoral robotic surgery: supraglottic partial laryngectomy. Ann Otol Rhinol Laryngol 116(1):19–23. https://doi.org/10.1177/000348940711600104
Park YM, Byeon HK, Chung HP, Choi EC, Kim SH (2013) Comparison of treatment outcomes after transoral robotic surgery and supraglottic partial laryngectomy: our experience with seventeen and seventeen patients respectively. Clin Otolaryngol 38(3):270–274. https://doi.org/10.1111/coa.12101
Park YM, Kim WS, Byeon HK, Lee SY, Kim SH (2013) Surgical techniques and treatment outcomes of transoral robotic supraglottic partial laryngectomy. Laryngoscope 123(3):670–677. https://doi.org/10.1002/lary.23767
Lang S, Mattheis S, Hasskamp P, Lawson G, Guldner C, Mandapathil M, Schuler P, Hoffmann T, Scheithauer M, Remacle M (2017) A european multicenter study evaluating the Flex robotic system in transoral robotic surgery. Laryngoscope 127(2):391–395. https://doi.org/10.1002/lary.26358
Mattheis S, Hasskamp P, Holtmann L, Schafer C, Geisthoff U, Dominas N, Lang S (2017) Flex robotic system in transoral robotic surgery: the first 40 patients. Head Neck 39(3):471–475. https://doi.org/10.1002/hed.24611
Hasskamp P, Lang S, Holtmann L, Stuck BA, Mattheis S (2016) First use of a new retractor in transoral robotic surgery (TORS). Eur Arch Oto-Rhino-Laryngol 273(7):1913–1917. https://doi.org/10.1007/s00405-015-3719-1
Ambrosch P, Gonzalez-Donate M, Fazel A, Schmalz C, Hedderich J (2018) Transoral laser microsurgery for supraglottic cancer. Front Oncol 8:158. https://doi.org/10.3389/fonc.2018.00158
Alon EE, Kasperbauer JL, Olsen KD, Moore EJ (2012) Feasibility of transoral robotic-assisted supraglottic laryngectomy. Head Neck 34(2):225–229. https://doi.org/10.1002/hed.21719
Lallemant B, Chambon G, Garrel R, Kacha S, Rupp D, Galy-Bernadoy C, Chapuis H, Lallemant JG, Pham HT (2013) Transoral robotic surgery for the treatment of T1–T2 carcinoma of the larynx: preliminary study. Laryngoscope 123(10):2485–2490. https://doi.org/10.1002/lary.23994
Smith RV (2014) Transoral robotic surgery for larynx cancer. Otolaryngol Clin North Am 47(3):379–395. https://doi.org/10.1016/j.otc.2014.03.003
Gorphe P (2018) A contemporary review of evidence for transoral robotic surgery in laryngeal cancer. Front Oncol 8:121. https://doi.org/10.3389/fonc.2018.00121
Razafindranaly V, Lallemant B, Aubry K, Moriniere S, Vergez S, Mones ED, Malard O, Ceruse P (2016) Clinical outcomes with transoral robotic surgery for supraglottic squamous cell carcinoma: experience of a French evaluation cooperative subgroup of GETTEC. Head Neck 38(Suppl 1):E1097–1101. https://doi.org/10.1002/hed.24163
Schmutz A, Dieterich R, Kalbhenn J, Voss P, Loop T, Heinrich S (2018) Protocol based evaluation for feasibility of extubation compared to clinical scoring systems after major oral cancer surgery safely reduces the need for tracheostomy: a retrospective cohort study. BMC Anesthesiol 18(1):43. https://doi.org/10.1186/s12871-018-0506-8
Fattahi T, Vega L, Fernandes R, Goldman N, Steinberg B, Schare H (2012) Our experience with 171 open tracheostomies. J Oral Maxillofac Surg 70(7):1699–1702. https://doi.org/10.1016/j.joms.2011.07.015
Mattheis S, Mandapathil M, Rothmeier N, Lang S, Dominas N, Hoffmann TK (2012) Transoral robotic surgery for head and neck tumors: a series of 17 patients. Laryngo Rhino Otol 91(12):768–773. https://doi.org/10.1055/s-0032-1327663
Park YM, Kim WS, Byeon HK, De Virgilio A, Jung JS, Kim SH (2010) Feasiblity of transoral robotic hypopharyngectomy for early-stage hypopharyngeal carcinoma. Oral Oncol 46(8):597–602. https://doi.org/10.1016/j.oraloncology.2010.05.003
Olsen SM, Moore EJ, Koch CA, Price DL, Kasperbauer JL, Olsen KD (2012) Transoral robotic surgery for supraglottic squamous cell carcinoma. Am J Otolaryngol 33(4):379–384. https://doi.org/10.1016/j.amjoto.2011.10.007
Funding
No specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The retrospective study was approved by the ethical committee of the University of Duisburg-Essen.
Conflict of interest
SL and SM received speaker fees and travel reimbursement from Medrobotics Corporation.
Ethical approval
The retrospective study was approved by the ethical committee of the University of Duisburg-Essen.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, T., Lang, S., Haßkamp, P. et al. The Flex robotic system compared to transoral laser microsurgery for the resection of supraglottic carcinomas: first results and preliminary oncologic outcomes. Eur Arch Otorhinolaryngol 277, 917–924 (2020). https://doi.org/10.1007/s00405-019-05767-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-019-05767-0