Abstract
Purpose
An anatomically realistic ultrasound liver phantom with tissue-specific distinct signal properties is needed for training of novices in diagnostic and interventional procedures. The main objective of this work was development and testing of a new durable liver ultrasound training phantom for use with a hybrid simulator.
Methods
A liver ultrasound phantom was fabricated in four main phases: materials selection, segmentation of CT images and realization of 3D models, vessel and lesion realization, and final assembly with silicone casting. Silicone was used as basic material due to its durability and stability over time. Several additives were analyzed and mixed with the polymer to reproduce the echogenicity of three simulated soft tissue types: parenchyma, lesions, and veins.
Results
Cysts and vessel trees appear anechoic in the B mode ultrasound images when realized with pure silicone. The liver parenchyma, hypoechoic, and hyperechoic lesions were realized with different concentrations of graphite and Vaseline oil to increase their relative echogenicity. These materials were successful for creation of an ultrasound liver phantom containing simulated blood vessels and lesions.
Conclusion
The phantom reproduces the human liver morphology and provides vessels and lesions ultrasound images with recognizable differences in echogenicity. The speed of sound in the simulated materials is inaccurate, but the problem can be overcome via software adjustment in a hybrid simulator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
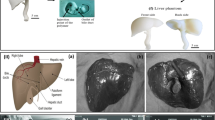
Throughout the last decade, the increased attention to patient safety and risk reduction has raised the interest in simulation as an important step in clinical training. In particular, in the diagnostic and interventional Ultrasound (US) procedures, an appropriate training allows the novices to develop and improve the medical practice. Novices generally start with probe manipulation training on volunteers and then proceed on patients. For safety reasons, training for interventional procedures such as US guided biopsy or ablation are generally performed on simulators. The use of simulators has brought several benefits such as improvements in the educational experience, increase of patient safety, and multiple and ongoing training opportunities [1]. Three kinds of simulators are commercially available: virtual reality (VR), physical phantoms and hybrid [2]. A previous work of our team concerned the realization of a hybrid simulator that combines physical mannequin and virtual reality visualization: the clinician scans an ultrasound physical phantom and the graphic interface of the simulator shows the 3D internal anatomy of the mannequin and the ultrasound probe with the relative scan plane. An additional view shows the traditional 2D US image (Fig. 1).
Hybrid simulator: the left image represents a biopsy simulation task on a commercially available phantom (CIRS phantom). The picture in the middle shows the US probe, the US scan plane mixed with the 3D representation of phantom internal anatomy in order to accelerate the biopsy learning phase. In the right image, the traditional US view is displayed
Early studies showed that this US simulation approach speeds up the probe manipulation training [3]. Nowadays, some types of phantoms for US scanner and training purposes are commercially available (Cirs, Blue phantom, Kyoto Kagaku). Unfortunately most of the commercial simulators are characterized by a very simplified anatomy and a coarse morphology and they are relatively expensive. Examples are the Ultrasound examination training model \(\hbox {ABDFAN}^{\textregistered }\) from Kyoto Kagaku (about 9,500$) and the \(\hbox {Zerdine}^{\textregistered }\)-based CIRS phantom (about 3,000$). Many items are necessary to set up a simulation course involving a very high cost [4]. Furthermore, they do not survive to repeated destructive tasks such as biopsy. For this reason, many groups use home-made phantoms developed with very common materials such as turkey, tofu or gelatin [5]. The main problem is the instability over time: They have a very short lifetime which can be extended with some additives, but it is not comparable to polymeric materials.
For the reasons discussed above, this research focused on the development of a phantom able to overcome all the cited problems and designed to be used in hybrid simulators [2]. Moreover, the intended physical simulator is patient-specific offering high detailed internal anatomy. A fabrication process for liver parenchyma and vessels’ tree has been studied and implemented, starting from real patient computed tomography (CT) data.
Materials and methods
The strategy used for the phantom realization consists of four main steps:
-
1.
The materials selection: Several tests have been performed in order to obtain specific mixtures which ensure the desired characteristics for the different tissues. The parenchyma, the portal and vena cava systems, and three kinds of lesions were studied.
-
2.
CT images processing and mold printing: Starting from real patient CT images, the 3D models have been extracted. In a CAD, step molds are designed to reproduce the segmented structures. By rapid prototyping techniques, the 3D models and molds have been printed in ABS material (Acrylonitrile butadiene styrene) by 3D printer.
-
3.
Vessels and lesions realization: By different techniques, different structures have been realized with the selected silicone mixtures.
-
4.
Final assembly: Phantom construction and veins and lesions insertion [6].
Materials selection
Several materials have been investigated in order to achieve the realization of a liver phantom able to be stable over time and reproduce the results of a human liver US test, while ensuring low production costs. The materials used are totally harmless and do not require a temperature-controlled environment.
According to the relating literature, phantoms are usually made of hydrogel materials, which guarantee low costs and good performances in terms of echogenicity under US scanner [7, 8]. Nevertheless, the need to obtain a liver phantom capable of operating for many years is a break-point requirement. Hydrogel phantoms have, in fact, an estimated usable life of 2/3 years and a consequent warranty time of one year only. Moreover, whenever they undergo invasive training (i.e., biopsy training), their life is significantly shortened. On the contrary, rubber-based phantoms ensure stability over 15 years and come with at least 10 years of warranty. Among the most common materials employed for home-made phantom fabrication there are agarose, gelatin, polyvinyl alcohol (PVA-C) and polyacrylamide (PAA) belonging to the hydrogel family, and the polyvinyl chloride (PVC) of the plastic category [9–11]. All the mentioned materials have strengths and weakness. For instance agarose and gelatin are the most widely used varieties of soft tissue because of the easy fabrication, flexibility and good acoustical performance. They are characterized by a fast and harmless preparation but they can maintain their properties for a limited period of time. The main disadvantage is the fast degradation because of microbial invasion [12] and dehydration [13]. Unlike the agarose, the PVA-C preserves its characteristics for several months when stored in freezing water but it has a very time-consuming preparation due to a 24-hour long freezing cycles [14]. The PAA allows to build different shapes but it needs additional procedures to achieve a smooth surface. As argued by Zell [15] these procedures can be dangerous during the acrylamide polymerization, carcinogenic and neurotoxic monomer. Under particular conditions (heat and UV light), the polyacrylamide can depolymerize resulting in a small amount of the monomer after production.
In order to guarantee the discussed requirements, the silicone (inorganic polymer composed of a silicon-oxygen chain and organic side-groups I) has been selected as phantom base material. In particular, RTV (Room Temperature Vulcanizing) platinum-catalyzed silicones, consist of two compounds: a catalyst and a cross linker. Compared to the hydrogels, the silicones are not subject to a high rate of dehydration and, for this reason, they do not show a drastic reduction of accuracy under US scanners. The loss of accuracy is even more marked when materials are exposed to specific temperatures, but again, this effect is only registered in hydrogels and not in silicones. The main drawback using silicone comes from their acoustical properties which do not exactly match the requirements to reproduce biological tissues. The resulting dimensional problem cannot be overcome if the phantom is used as a physical simulator. On the contrary, in the hybrid simulators, a software adjustment can be easily made correcting the resulting dimension of the phantom 3D model [2]. However, data in the literature show that silicone acoustical properties can be also modulated by means of additives [16].
As a first step, PAAG (Polyacrylamide Gel) has been tested in order to enhance the silicone echogenicity. The pure silicone is in fact totally anechoic to the US scanner. Mixing manually the gel with the silicone, until a homogeneous mixture is obtained, and letting them polymerizing together, it is possible to achieve an echogenic grade which is proportional to the applied amount of gel. The process has been carried out in ambient conditions with \(23-25\,^{\circ }\hbox {C}\) of temperature and 56 % of average humidity. The silicone becomes proportionally bright to the US scanner when combined with a range between 10 and 50 % by weight of PAAG. Unfortunately, this mixture is unstable and loses its properties in few weeks. The silicone, in fact, is strongly hydrophobic and tends to separate from the gel part, whose content is mostly made of water. The hydration occurs in the same temporal window when the samples are sealed with two different materials: Dragon Skin 10 \(\hbox {Medium}^{\textregistered }\) (platinum cure silicone rubber) and Psycho \(\hbox {Paint}^{\textregistered }\) (platinum silicone paint base) all from Smooth-On Inc (Pennsylvania).
A second experiment has been performed using graphite (\(17\,\upmu \hbox {m}\) as in Anderson et al. [17]) and talcum as scattering agents, as shown in Fig. 2. As stated in Anderson et al. [17] the echogenicity is proportional to the amount of powder. The picture displays the silicone US behavior when it is mixed, respectively, with 3 and 5 % of graphite and of talcum (by weight);
Some mechanical and acoustical problems have arisen during the US evaluation of the samples above discussed. First of all, the powders increase the hardness of the material making the silicone sample harder than biological tissue and giving an unnatural probe manipulation [18]. Furthermore, the powders leave sediment in the lower layer resulting in unrealistic responses of US analysis. The latter issue is due to the silicone viscosity and the inability to stir perfectly the two substances. The inability to achieve a perfect mixture of silicone and graphite generate additional problems when structures, such as silicone cylinders or spheres, are sunk in the solution. When the polymerization is complete, the powder aggregations and deposits cause several artifacts in the US images.
In order to solve the acoustical problem described above, Vaseline Oil (pure liquid paraffin) and the \(\hbox {Thinner}^{\textregistered }\) additive (Smooth-On Inc., Pennsylvania) have been added to the mixture. The Vaseline oil and the thinner have both the effect to reduce the viscosity of platinum cure silicone rubber products [19]. The latter is, however, different from the Vaseline oil since it is a non-reactive silicone fluid. In order to improve the mixture from a mechanical point of view, an additional component has been used. The \(\hbox {Slacker}^{\textregistered }\), which is another Smooth-On non-reactive silicon fluid, has been added to make the silicone rubber softer and to alter its rebound properties (Smooth-on Inc., Pennsylvania) [20]. The Slacker allows to modifying the degree of tackiness of the cured silicone (the degree is proportional to the amount of Slacker added) and provides to the silicone a human tissue-like self-sealing property (ability to close again after needle insertion).
After several tests, the following conclusions can be drawn regarding the amount of the different components that can be stirred in the mixture:
-
The amount of powders mixed with silicone cannot exceed 5 % of the total weigh of the mixture. Indeed, they enhance the scattering agent and the attenuation as a consequence, resulting in a shading of the underlying structures [21].
-
The Vaseline oil enhances the silicone echogenicity only for percentages higher than 10 % (by weight).
-
Oil percentages higher than 30 % cannot be totally absorbed.
-
A little powder percentage amount (about 3 % of talcum or graphite) makes the silicone able to be miscible with a greater amount of Oil.
-
If any powder is added, Oil tends to separate from the silicone after few days.
-
The maximum percentage of Slacker miscible together with silicone is 20 %. Addition of Vaseline oil and thinner reduces this percentage down to 10 %. The limit is imposed because of the high level of reached tackiness.
Taking into account the conclusions listed above, the parenchyma reproducing synthetic tissue has been realized using the following mixture (all the percentages are reported by weight):
-
Silicone: \(\hbox {Ecoflex}^{\textregistered }\) 00-10 (Smooth-On Inc., Pennsylvania): 53 %.
-
Graphite: 5 % (echogenicity enhancer).
-
Thinner: 15 % (to reduce the viscosity of platinum cure silicone rubber).
-
Vaseline oil: 20 % (to better amalgamate the graphite powder).
-
Slacker: 7 % (to ensure self-sealing properties).
During the preparation procedures, the final mixture has been degassed, before curing, by means of a dedicated vacuum chamber in order to eliminate any trapped air bubble and avoid the risk of US artifacts. The degassing process lasts 10 min every 200 ml of material.
The Ecoflex silicone has been chosen as base material due to its mechanical properties. Comparing the Young’s modulus of different types of silicone, it was found that Ecoflex0010 is the one closest to the human liver. Although the liver modulus can vary in a wide range, the average value is between 20 and 60 KPa [22]. Measuring the Ecoflex with the Instron instrument (5 % of deformation and 15 mm/min of deformation velocity), it was found a modulus value of about 50 KPa.
Since a reduction in the non-silicone components causes a proportional decrease of echogenicity, the mixture used for the hypoechoic lesions is:
-
Silicone: Ecoflex 00-10: 88 %.
-
Graphite: 2 %.
-
Vaseline oil: 10 %.
The hyperechoic lesions cannot be realized with the inverse procedure, enhancing the echogenicity with a graphite percentage greater than 5 %, because this is the limit value to avoid the shadowing of the underlying structures. Another strategy has been used, taking advantages of another Smooth-On silicone property. The Dragon Skin \(\hbox {FxPro}^{\textregistered }\) (Smooth-On Inc., Pennsylvania) is a soft and stable platinum silicone rubber whose curing and working time is quite low, making impossible the air bubbles elimination by means of vacuum chamber. These air bubbles generate a vacancy defect where the Vaseline oil is free to penetrate and fill in the gap. The Oil will be then trapped because of the polymerization, making the silicone lesion hyperechoic. The anechoic lesions have been realized with pure silicone.
CT images processing and mold printing
Once the materials have been selected, the fabrication technique proceeds with the CT images acquisition and processing. This step allows the realization of a patient-specific phantom able to reproduces the morphology of a real patient liver [23]. All the needed structures have been extracted from CT images by means of 3D segmentation software which allows the discrimination of different tissues. For the segmentation and rendering the ITK-Snap software (neighborhood connected region growing algorithm) has been used, in particular a version modified by EndoCAS integrating the “EndoCAS segmentation Pipeline” [24]. Figure 3 shows a software screenshot and the vessel trees 3D render.
By rapid prototyping techniques, the 3D models have been printed in ABS material. Figure 4 shows the liver mold and the vessel lumen (vena cava and portal vein).
Vessels and lesion realization
Unfortunately, the three dimensions branched structure makes the realization of the ABS vessels mold unfeasible due to the inability to extract the silicone, once polymerized, without breaking the mold itself, which would be a very costly process. The problem has been eluded printing the lumen of the vessel in ABS material (Fig. 5a).
Later, it has been covered by another rubber material which, once consolidated, becomes the actual mold (Fig. 5b).
The ABS lumen will be extracted (cutting the rubber external shell), leaving empty areas in the mold. The hollow areas will be fill in, at a later step, with pure silicone (Ecoflex 0010) and let polymerize. In Fig. 5c, the portal vein made of silicone is shown.
a ABS realization of vena cava printed by means of 3D printer. b Realization of the mold by pouring the melted ComposiMold on the ABS pattern. After the solidification the ABS will be cut, opened and reassembled, removing the ABS core. Then the empty areas will be fill in with silicone. c Resulting silicone vena cava ready to be inserted in the phantom
The used covering material is the \(\hbox {ComposiMold}^{\textregistered }\) (Manchester), a rubber-based substance which provides several benefits: It is an eco-friendly and low cost material, totally compatible with silicone materials and able to be reused many times by microwave melting. It solidifies at room temperature in few minutes and does not provoke interference to the silicone polymerization. This latter characteristic is very important and hard to find. For instance, another attempt has been made with other silicone rubbers, as the \(\hbox {GSP}400^{\textregistered }\) (Prochima, Pesaro Urbino), a moldable silicone rubber. When pure silicone gets in contact with GSP 400, it becomes sticky and softer after the polymerization.
Final phantom assembly
The last stage of the work has been the final assembly. The position of lesions and the vein systems has been fixed by means of lines, as shown in Fig. 6. The crucial point was to ensure that all the parts would remain in the right position to guarantee the authentic anatomical positioning of a human liver. That was obtained by keeping the lines tensioned from outside. The Fig. 6 shows the final assembly.
As discussed earlier in this paper, the samples have been progressively evaluated using an US scanner, which actually allows a qualitative analysis only. The final part of this work intends to provide also a quantitative measurement of the materials properties, testing their speed of sound. The measurement instrument consists of two transducers placed in a distilled water bath: a transmitter (piezoelectric type) and a detector (hydrophone). The cylindrical samples have been held between the transducers by means of an ABS support. As shown in Fig. 7, the samples lie with the faces perpendicular to the US longitudinal beam.
This technique provides a relative measurement employing distilled water as reference item. Speed of sound is obtained from the time difference between the pulse transit times with and without samples. The signal frequency is 1MHz and the signal peak-to-peak amplitude is 100 mV. The speed of sound of the sample has been obtained with the following formula:
where \(c_w\) is the water speed of propagation, \(\Delta x\) the thickness of the sample and \(\Delta t\) the calculated time [15].
Speed of sound measuring instrument. A distilled water bath is shown in the left image. The right picture displays the transmitter (piezoelectric type) and the detector (hydrophone). The cylindrical samples are held between the transducers by means of an ABS support with the faces perpendicular to the US longitudinal beam
The available system allows to measure the attenuation coefficient at the only frequency of 1 MHz. The assessment of the attenuation coefficient, although limited with the only frequency of 1 MHz as input parameter, meets the purpose of this paper to provide only an order of magnitude for phantom materials. The formula is shown below [15].
where \({A}_{{s}}\) is the amplitude of the US pulse in the sample and \({A}_{{w}}\) is the same parameter referred to the water. \(R\) is the acoustical reflection coefficient at the interface between water and material and \(z\) is the impedance which depends on velocity and density.
The attenuation coefficient in a real liver is around 0.7 dB/cm for 1 MHz frequency. For the same measurement conditions, this parameter is 1.5 dB/cm for pure silicone (Ecoflex0010) and 2.2 for the mixture chosen as parenchyma.
Results
The main goal of this research has been achieved realizing a patient-specific liver phantom, designed for an ultrasound and biopsy hybrid simulator, made of inexpensive and harmless materials that are stable over time [2]. Several tests have been performed in order to find the silicone-based mixture able to better simulate the parenchyma, the vessels and three different lesions. The external and internal morphology faithfully represent a real liver: the fabrication procedure allows to obtain real patient 3D models and, from them, to build the molds, maintaining the human morphology. Graphite has been used in order to enhance the scattering agent while thinner and Vaseline oil allow a better signal transmission. Thinner and slacker have been added to improve the homogeneity and decrease the viscosity, solving the acoustical and mechanical problems arisen with the use of graphite. The final mixtures are given below and are expressed by weight:
-
parenchyma: Silicone \(\hbox {Ecoflex}^{\textregistered }\) 00-10 (Smooth-On Inc., Pennsylvania) 53 %; graphite 5 % (as echogenicity enhancer); thinner 15 % (to reduce the viscosity of platinum cure silicone rubber); Vaseline oil 20 % (to better amalgamate the graphite powder); slacker 7 % (to ensure self-sealing properties).
-
hypoechoic lesion: silicone (88 %), graphite (2 %), Vaseline Oil (10 %).
-
hyperechoic lesion: silicone (without air bubble elimination process) dipped in Vaseline oil.
-
anechoic lesion: pure silicone (Ecoflex 00-10).
-
vena cava and portal vein: pure silicone (Dragon Skin Medium).
Figure 8 shows the phantom external morphology and the vena cava and portal vein back entrances.
The vessel trees and the lesions are clearly visible under US scanning. Figure 9 compares hyperechoic, anechoic, and hypoechoic real lesions to the three respective masses in the phantom.
Anechoic, Hyperechoic, Hypoechoic lesions. In the first column are displayed the phantom lesions while, in the second, three examples of real masses. The picture in the first row represents the anechoic mass which is totally ascribable to a cyst, confirmed comparing it to the picture of a real mass in the upper right corner (first row, second column). Similarly, proceeding by rows, the second picture to the left is attributable to the angioma (right) and in the last row the hypoechoic lesion in the phantom simulates optimally a real HCC—Hepato Cellular Carcinoma—(right)
In conclusion, the realized patient-specific liver phantom has been compared with a real human liver and also with a different phantom currently available in commerce. The comparison table with the real human liver is shown below [25] (Table 1).
On the other side, a comparison with a commercially available phantom had the scope to assess its usability and functionality. 15 clinicians (10 expert radiologists and 5 residents) were asked to test the realized patient-specific liver phantom and the commercial \(\hbox {CIRS}^{\textregistered }\) phantom.
The procedure consisted in 15 minutes of US scanning for each mannequin; the clinicians could freely scan them and proceed with a biopsy of a lesion.
As last step, a Likert qualitative questionnaire was given them to collect their opinion on the mechanical and acoustical aspects.
The test is shown in Fig. 10 and the results are exhibited in Table 2.
Biopsy simulation: needle’s insertion procedure (left). The needle is perfectly visible on the US scan plane and the target is easily reached (center). After the removal of the biopsy needle, there is no track of it in the parenchyma thanks to the tackiness and self-healing properties of the material
The physicians gave their opinion in relation to the feedback pressure realism and the echogenicity of all the phantom elements as well as the vessels position. The test has been performed placing the US probe directly on the phantom and visualizing the resulting US image. The scoring range was from 1 (negative evaluation) up to 5 (positive evaluation). Scores lower than 3 were given to both phantoms only in the feedback pressure realism parameter. This limit will not be difficult to overcome because the phantom can be easily placed in a chest human-like mannequin realized with the proper mechanical features. Furthermore, both phantoms received the score 1 referring to the arterial tree. In fact, is not embedded in neither of the two.
As last result, the quantitative evaluation of the materials acoustical properties are given as follow: all the phantom structures show the speed of sound of approximately 1,000–1,080 m/s and the attenuation coefficient of 1.5/2.2 dB/cm at 1 MHz frequency.
Discussion
Phantoms are important instruments for medical simulation and training and consequently for the patient safety. The objective of this research is the realization of a patient-specific physical phantom, able to be used in a hybrid simulator [2]. In particular, our team has worked on the realization of a liver phantom in order to simulate a real liver behavior under US scanner. The idea comes from the limits imposed by the current phantoms which are made of unstable and costly material, which also require an expensive maintenance. The base material used to build the phantom is the silicone, which remains stable over time and under particular conditions. Some additives have been tested and mixed with the silicone in order to obtain the proper level of echogenicity: graphite turns out to be the best. Other elements have been added to the mixture in order to achieve additional specifications. The cost of these phantoms is related to the production process and the materials employed: the greatest contribution comes from the 3D printer used for the mold realization. However, this cost will be faced once at the beginning of the process. Then, each phantom has a very low cost depending on the materials. Here, in the case of silicone, each item requires about 100$.
Fifteen physicians have been asked to test our liver phantom and a commercially available one, and fill out a questionnaire. Very good results have been achieved, unless for a slight difference in the feedback pressure realism. The problem can be easily solved placing the phantom inside a human-like chest mannequin which could also have some ribs located in the correct position: this would provide to the physician a better orientation and an easier visualization of the structures. Considering that in all the phantom structures the speed of sound is approximately 1,000 m/s, while in the real liver it is about 1,540 m/s, a traditional US examination results in an image distortion: a radial distortion for convex probes and a scaling along the deep direction for linear probes. Concerning our research and purposes, this is not a problem because the phantom will be used in hybrid simulators. In fact, by means of software routines, the image dimension can be easily adjusted and correctly displayed. However, since the speed of sound is an important item, the addition of substances able to modify this parameter will be subject of forthcoming studies.
References
Kunkler K (2006) The role of medical simulation: an overview. Int J Med Robot 2:203–210
Condino S, Carbone M, Ferrari V, Faggioni L, Peri A, Ferrari M et al (2010) How to build patient-specific synthetic abdominal anatomies. An innovative approach from physical toward hybrid surgical simulators. Int J Med Robot Comp 7:202–213
Freschi C, Parrini S, Dinelli N, Ferrari M, Ferrari V (2014) Hybrid simulation using mixed reality for interventional ultrasound imaging training. Int J CARS. doi:10.1007/s11548-014-1113-x
Hunt A, Ristolainen A, Ross P, Opik R, Krumme A, Kruusmaa M (2013) Low cost anatomically realistic renal biopsy phantoms for interventional radiology trainees. Europ J Radiol 82:594–600
Sultan SF, Shorten G, Iohom G (2013) Simulators for training in ultrasound guided procedures. Med Ultrasonogr 15:125–131
Shevchenko N, Schwaiger J, Markert M., Flatz W, Lueth TC (2011) Evaluation of a resectable ultrasound liver phantom for testing of surgical navigation systems. In: Proceedings of the 2011, IEEE Eng Med Biol Soc, pp 916–919
D’Souza WD, Madsen EL, Unal O, Vigen KK, Frank GR, Thomadsen BR (2011) Tissue mimicking materials for a multi-imaging modality prostate phantom. Med Phys 28:688–700
Madsen EL, Zagzebski JA, Banjavie RA, Jutila RE (1978) Tissue mimicking materials for ultrasound phantoms. Med Phys 5:391–394
Carbone M, Condino S, Mattei L, Forte P, Ferrari V, Mosca F (2012) Anthropomorphic ultrasound elastography phantoms-characterization of silicone materials to build breast elastography phantoms. In: IEEE Eng Med Biol Soc (ed IEEE)
Casciaro S, Conversano F, Musio S, Casciaro E, Demitri C, Sannino A (2009) Full experimental modelling of a liver tissue mimicking phantom for medical ultrasound studies employing different hydrogels. J Mat Sci Mater Med 20:983–989
Madsen EL, Hobson MA, Shi H, Varghese T, Frank GR (2005) Tissue-mimicking agar/gelatin materials for use in heterogeneous elastography phantoms. Phys Med Biol 50:5597–5618
Culjat MO, Goldenberg D, Tewari P, Singh RS (2010) A review of tissue substitutes for ultrasound imaging. Ultrasound Med Biol 36:861–873
Hungr N, Long JA, Beix V, Troccaz JA (2012) Realistic deformable prostate phantom for multimodal imaging and needle-insertion procedures. Med Phys 39:2031–2041
Chiarelli P, Lanat A, Carbone M (2010) High frequency poroelastic waves in hydrogels. J Acoust Soc Am 127:1197
Zell K, Sperl JI, Vogel MW, Niessner R, Haisch C (2007) Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys Med Biol 52:N475–484
Maggi LE, von Kruger MA, Pereira WCA, Monteiro E (2009) Development of silicon-based materials for ultrasound biological phantoms. In: ULTSYM, pp 1962–1965
Anderson PG, Rouze NC, Palmeri ML (2011) Effect of graphite concentration on shear-wave speed in gelatin-based tissue-mimicking phantoms. Ultrasound Imaging 33:134–142
Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML et al (2002) Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol 28:467–474
Madsen EL, Zagzebski JA, Frank GR (1982) Oil-in-gelatin dispersions for use as ultrasonically tissue-mimicking materials. Ultrasound Med Biol 8:277–287
Gerstenmaier JF, McCarthy CJ, Brophy DP, Cantwell CP (2013) Evaluation of the particulate concentration in a gelatin-based phantom for sonographically guided lesion biopsy. J Ultrasound Med 32:1471–1475
Nava A, Mazza E, Furrer M et al (2008) In vivo mechanical characterization of human liver. Med Image Anal 12(2):203–216
Willaert WI, Aggarwal R, Van Herzeele I, Cheshire NJ, Vermassen FE (2012) Recent advancements in medical simulation: patient-specific virtual reality simulation. World J Surg 36:1703–1712
Ferrari V, Carbone M, Cappelli C, Boni L, Melfi F, Ferrari M et al (2012) Value of multidetector computed tomography image segmentation for preoperative planning in general surgery. Surg Endosc 26:616–626
Branca PF, Fabiano B, D’Orazio A, Marinozzi F, Rubergni S (2008) Fondamenti di bioingegneria clinica, vol 2. Ecotomografia, Springer
Acknowledgments
The authors would like to thank Pietro Miloro for the precious contribution in the speed of sound measurements. This work has been financed by Arpa Foundation and by Opera (Advanced OPERAting room) Project (Tuscany Regional Funds: PAR FAS 2007–2013 Azione 1.1 P.I.R. 1.1.B.).
Conflict of interest
Alessia Pacioni, Marina Carbone, Cinzia Freschi, Rosanna Viglialoro, Vincenzo Ferrari, Mauro Ferrari declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacioni, A., Carbone, M., Freschi, C. et al. Patient-specific ultrasound liver phantom: materials and fabrication method. Int J CARS 10, 1065–1075 (2015). https://doi.org/10.1007/s11548-014-1120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-014-1120-y